Treatment of High-Ammonia-Nitrogen Wastewater with Immobilized Ammonia-Oxidizing Bacteria Alcaligenes sp. TD-94 and Paracoccus sp. TD-10
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Reagents
2.2. Preparation of Carriers and Immobilization of Cells
2.3. High-Ammonia-Nitrogen Wastewater Removal Experiments—Simulated Wastewater
2.3.1. Ammonia Nitrogen Removal Capacity of Immobilized Cells
2.3.2. Reusability and Stability of Immobilized Cells
2.4. High-Ammonia-Nitrogen Wastewater Removal Experiments—Actual Wastewater
2.5. Characterization and Analytical Methods
3. Results and Discussion
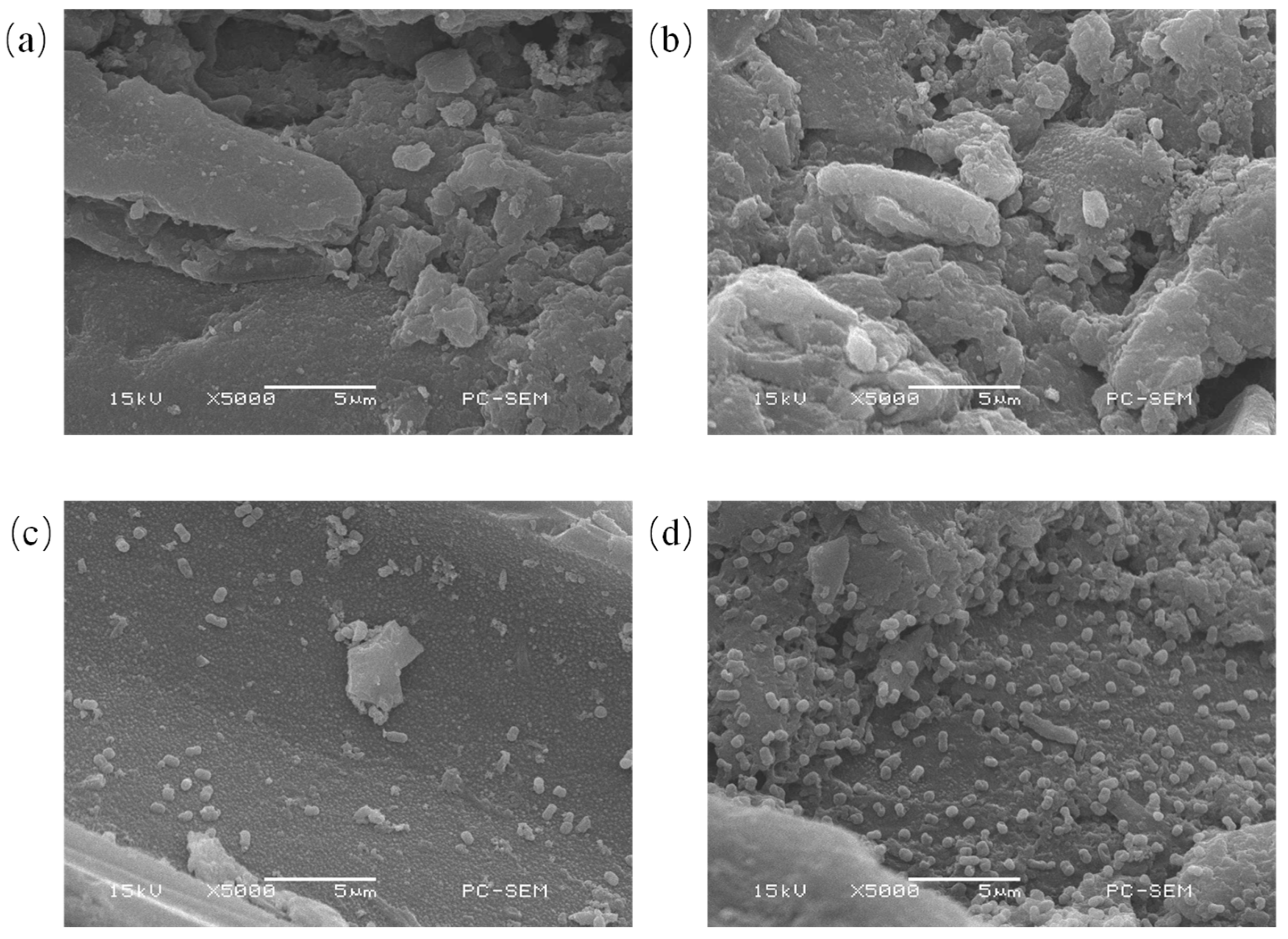
3.1. Scanning Electron Microscopy of Carriers and Immobilized Cells
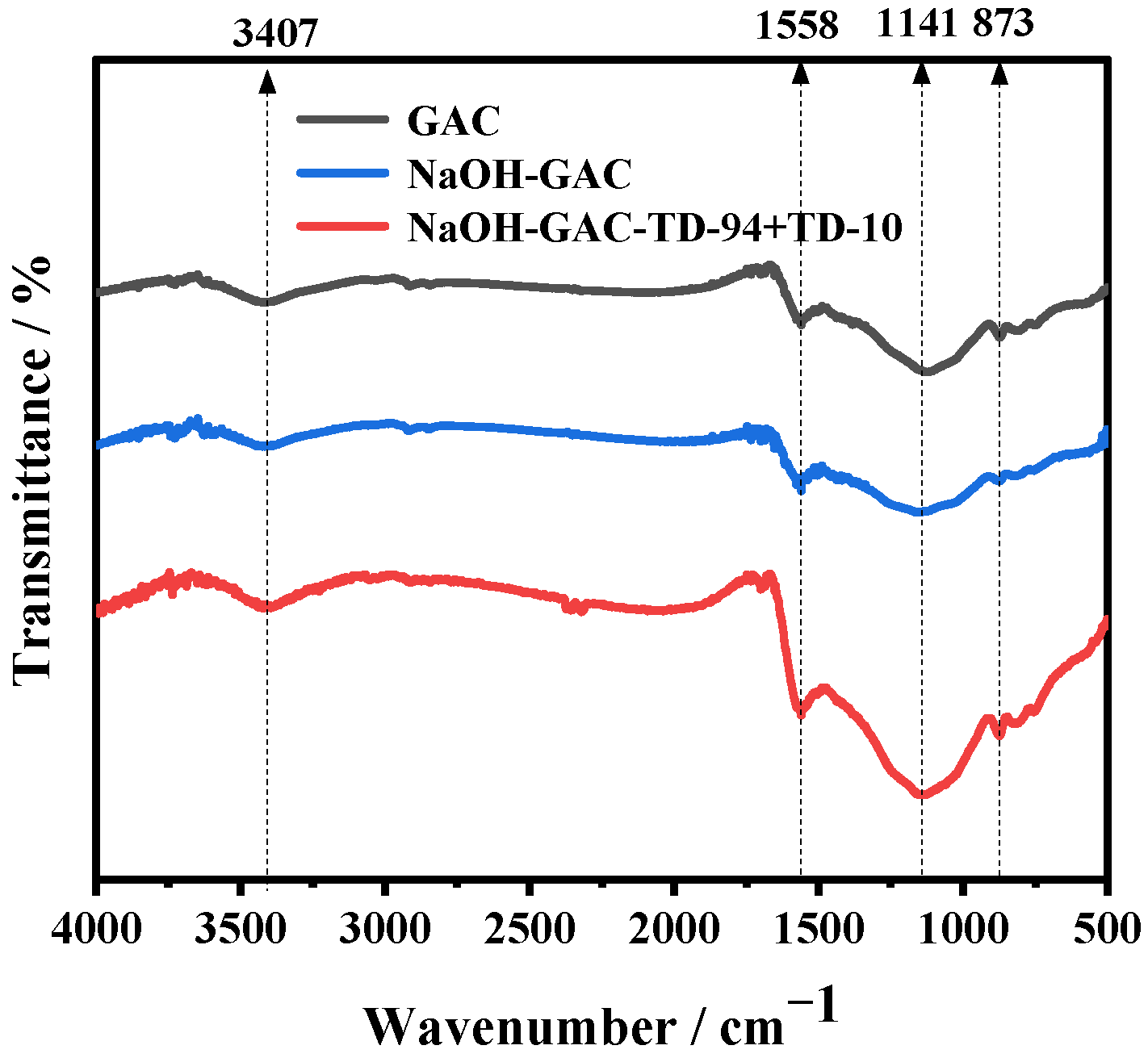
3.2. Fourier-Transform Infrared Analysis
3.3. Treatment of High-Ammonia-Nitrogen Wastewater Using Immobilized Cells
3.4. Stability of the Immobilized Cells
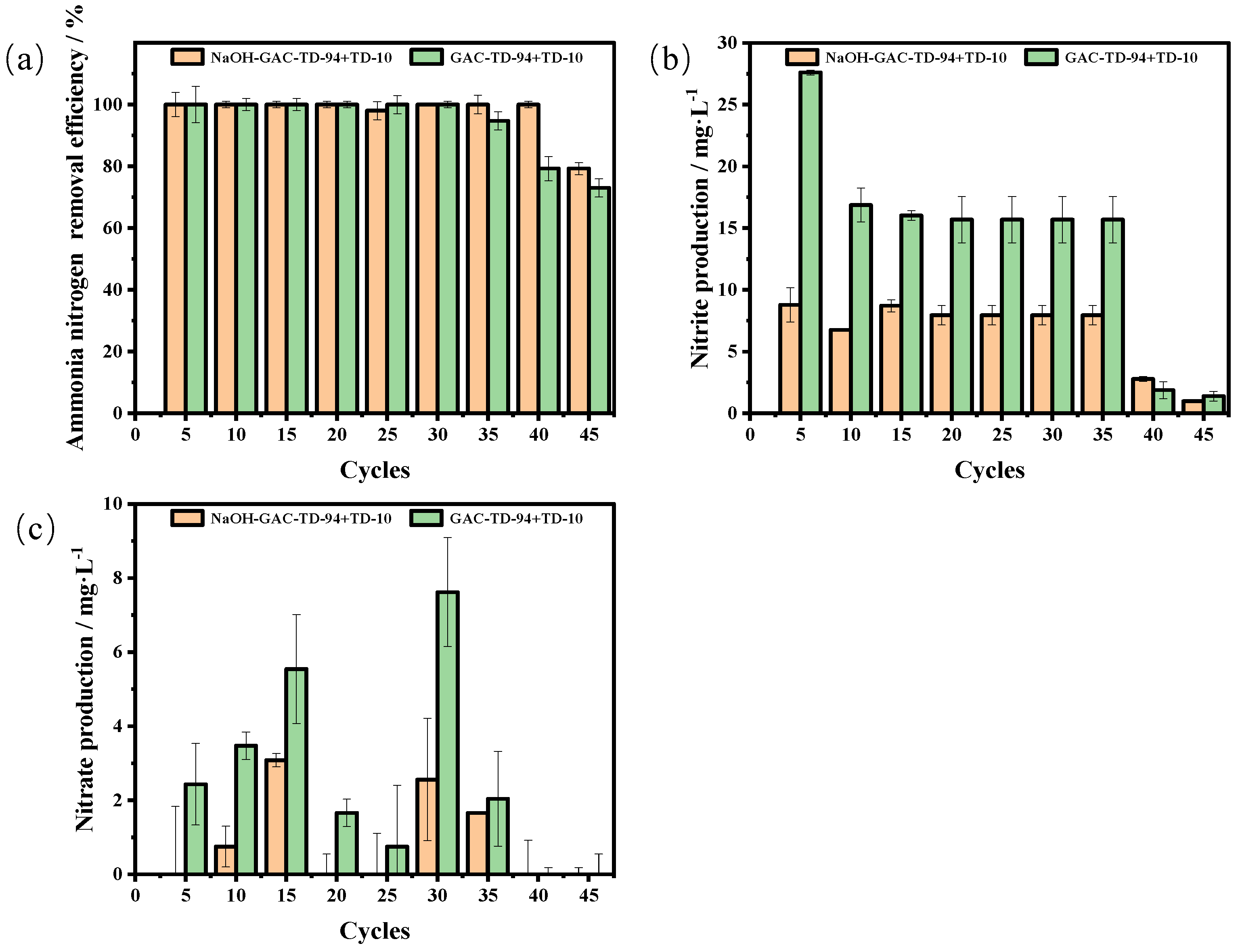
3.4.1. Reusability
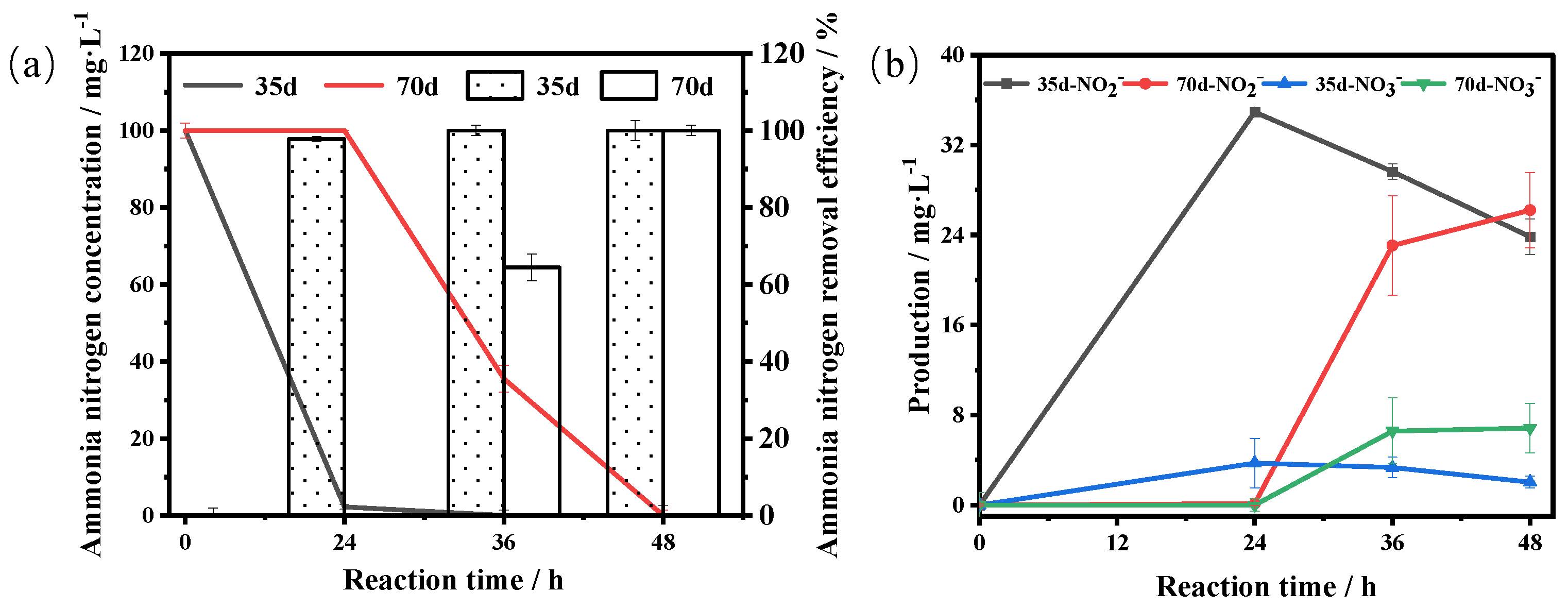
3.4.2. Storage Stability
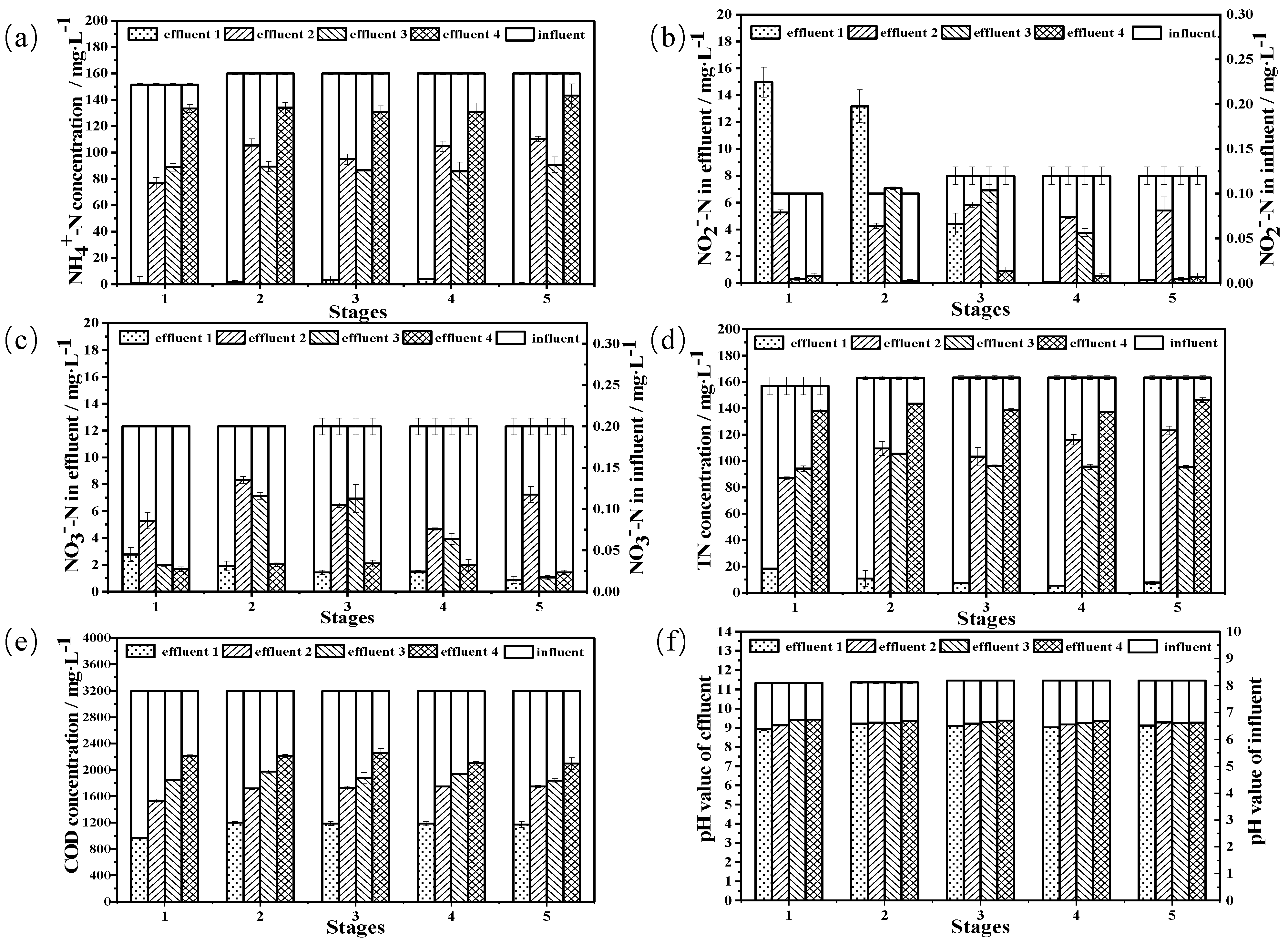
3.5. Treatment of High-Ammonia-Nitrogen Wastewater from a Petrochemical Enterprise Using Immobilized Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tortajada, C.; Fernandez, V. Towards Global Water Security: A Departure from the Status Quo? In Global Water Security; Water Resources Development and Management; Springer: Singapore, 2018; pp. 1–19. [Google Scholar]
- Tu, Y.; Chen, K.; Wang, H.; Li, Z. Regional Water Resources Security Evaluation Based on a Hybrid Fuzzy BWM-TOPSIS Method. Int. J. Environ. Res. Public Health 2020, 17, 4987. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Cao, S.; Wang, S.; Wu, C. Advanced nitrogen removal from wastewater by combining anammox with partial denitrification. Bioresour. Technol. 2015, 179, 497–504. [Google Scholar] [CrossRef]
- Kumar, M.; Lin, J.G. Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal--Strategies and issues. J. Hazard. Mater. 2010, 178, 1–9. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, D.; Hua, X.; Xu, Y.; Guo, Z.; Liang, D. Ammonia nitrogen removal and recovery from acetylene purification wastewater by air stripping. Water Sci. Technol. 2017, 75, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Wang, K.; Yu, S.; Yuan, Y.; Qin, Y.; An, Y.; Zhao, X.; Zhou, Z. Membrane fouling behavior in membrane bioreactors for nitrogen-deficient wastewater pretreated by ammonium ion exchange. J. Membr. Sci. 2023, 665, 121087. [Google Scholar] [CrossRef]
- Zhou, H.; Ou, L. Adsorption of ammonia nitrogen in wastewater by tailing loaded manganese oxide material. Inorg. Chem. Commun. 2022, 144, 109886. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, S.; Li, H.; Liu, J.; Li, S.; Zhang, L. Treatment of ammonia-nitrogen wastewater by the ultrasonic strengthened break point chlorination method. J. Water Process Eng. 2022, 45, 102501. [Google Scholar] [CrossRef]
- Mao, X.; Xiong, L.; Hu, X.; Yan, Z.; Wang, L.; Xu, G. Remediation of ammonia-contaminated groundwater in landfill sites with electrochemical reactive barriers: A bench scale study. Waste Manag. 2018, 78, 69–78. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Chen, X.; Chen, J.; Feng, X.; Peng, X. Nitrogen removal via nitritation pathway for low-strength ammonium wastewater by adsorption, biological desorption and denitrification. Bioresour. Technol. 2018, 267, 541–549. [Google Scholar] [CrossRef]
- Li, X.; Lu, M.Y.; Huang, Y.; Yuan, Y.; Yuan, Y. Influence of seasonal temperature change on autotrophic nitrogen removal for mature landfill leachate treatment with high-ammonia by partial nitrification-Anammox process. J. Environ. Sci. 2021, 102, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.T.; Lien, P.J.; Chen, C.C.; Chang, Y.M.; Kao, C.M. Bioremediation of 2,4,6-trinitrotoluene-contaminated groundwater using unique bacterial strains: Microcosm and mechanism studies. Int. J. Environ. Sci. Technol. 2016, 13, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Xi, H.; Zhou, X.; Arslan, M.; Luo, Z.; Wei, J.; Wu, Z.; Gamal El-Din, M. Heterotrophic nitrification and aerobic denitrification process: Promising but a long way to go in the wastewater treatment. Sci. Total Environ. 2022, 805, 150212. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Bi, X.; Xu, C. Ammonia-Oxidizing Archaea (AOA) Play with Ammonia-Oxidizing Bacteria (AOB) in Nitrogen Removal from Wastewater. Archaea 2018, 2018, 8429145. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Huang, W.; Zhou, J.; He, X.; Wang, J.; Wang, X.; Zhou, J. The variation on nitrogen removal mechanisms and the succession of ammonia oxidizing archaea and ammonia oxidizing bacteria with temperature in biofilm reactors treating saline wastewater. Bioresour. Technol. 2020, 314, 123760. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.T.; Miao, L.L.; Peng, J.S.; Ma, L.; Huang, Q.; Liu, Y.; Wu, M.R.; Ai, G.M.; Liu, S.J.; Liu, Z.P. Dirammox Is Widely Distributed and Dependently Evolved in Alcaligenes and Is Important to Nitrogen Cycle. Front. Microbiol. 2022, 13, 864053. [Google Scholar] [CrossRef]
- Medinets, S.; Skiba, U.; Rennenberg, H.; Butterbach-Bahl, K. A review of soil NO transformation: Associated processes and possible physiological significance on organisms. Soil Biol. Biochem. 2015, 80, 92–117. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Gao, M.; Mohammed, A.; Liu, Y. Responses of various carbon to nitrogen ratios to microbial communities, kinetics, and nitrogen metabolic pathways in aerobic granular sludge reactor. Bioresour. Technol. 2023, 367, 128225. [Google Scholar] [CrossRef]
- Guo, J.; Wu, X.; Liu, S.; Zhang, Y.; Cai, Z. Screening of three ammonia-oxidizing bacteria and construction of compounding agent CCZU C6 in high-efficiency ammonia-oxidizing. J. Water Process Eng. 2021, 40, 101862. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, S.; Yang, Y.; Li, L.; Wang, D. Study on Degradation of Oily Wastewater by Immobilized Microorganisms with Biodegradable Polyacrylamide and Sodium Alginate Mixture. ACS Omega 2019, 4, 15149–15157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naushad, M.; Khan, M.R.; ZA, A.L.; AlSohaimi, I.; Rodriguez-Reinoso, F.; Turki, T.M.; Ali, R. Removal of BrO3- from drinking water samples using newly developed agricultural waste-based activated carbon and its determination by ultra-performance liquid chromatography-mass spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 15853–15865. [Google Scholar] [CrossRef]
- Chatla, A.; Almanassra, I.W.; Jaber, L.; Kochkodan, V.; Laoui, T.; Alawadhi, H.; Atieh, M.A. Influence of calcination atmosphere on Fe doped activated carbon for the application of lead removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129928. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, W.; Wang, Y.; Zhang, D.; Qin, W.; Zhao, Y. Application of glucose for improving NH4+-N removal in micro-polluted source water by immobilized heterotrophic nitrifiers at low temperature. Chemosphere 2021, 278, 130459. [Google Scholar] [CrossRef]
- Abdul Halim, A.; Abu Sidi, S.F.; Hanafiah, M.M. Ammonia Removal using Organic Acid Modified Activated Carbon from Landfill Leachate. Environ. Ecosyst. Sci. 2017, 1, 28–30. [Google Scholar] [CrossRef]
- Ren, Z.; Jia, B.; Zhang, G.; Fu, X.; Wang, Z.; Wang, P.; Lv, L. Study on adsorption of ammonia nitrogen by iron-loaded activated carbon from low temperature wastewater. Chemosphere 2021, 262, 127895. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhu, Y.; Zhang, Y.; Lin, H. Determination of ammonia nitrogen in natural waters: Recent advances and applications. Trends Environ. Anal. Chem. 2019, 24, e00073. [Google Scholar] [CrossRef]
- Wang, Q.H.; Yu, L.J.; Liu, Y.; Lin, L.; Lu, R.G.; Zhu, J.P.; He, L.; Lu, Z.L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.K.; Beg, Y.R.; Nishad, G.R. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta 2019, 191, 364–381. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, B.; Lin, J.; Naidu, R.; Chen, Z. Simultaneous adsorption and biodegradation (SAB) of diesel oil using immobilized Acinetobacter Venetianus Porous Material. Chem. Eng. J. 2016, 289, 463–470. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Gong, J.; Zhan, X.; Zeng, Y. Exploring a New Method to Study the Effects of Surface Functional Groups on Adsorption of CO2 and CH4 on Activated Carbons. Langmuir 2020, 36, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, H.; Zheng, Y.M.; Qu, J.; Chen, J.P. Systematic study of synergistic and antagonistic effects on adsorption of tetracycline and copper onto a chitosan. J. Colloid Interface Sci. 2010, 344, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Lin, A.; Ji, G.; Wang, D.; Wang, X. The effects of adsorbing organic pollutants from super heavy oil wastewater by lignite activated coke. J. Hazard. Mater. 2016, 308, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mazarji, M.; Aminzadeh, B.; Baghdadi, M.; Bhatnagar, A. Removal of nitrate from aqueous solution using modified granular activated carbon. J. Mol. Liq. 2017, 233, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Fu, X.; Zhang, G.; Li, Y.; Qin, Y.; Wang, P.; Liu, X.; Lv, L. Study on performance and mechanism of enhanced low-concentration ammonia nitrogen removal from low-temperature wastewater by iron-loaded biological activated carbon filter. J. Environ. Manag. 2022, 301, 113859. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Wu, Z. Coupling effect of pH and dissolved oxygen in water column on nitrogen release at water–sediment interface of Erhai Lake, China. Estuar. Coast. Shelf Sci. 2014, 149, 178–186. [Google Scholar] [CrossRef]
- Chi, W.; Yang, H.; Zhang, S.; Zou, Z.; Wang, X.; Wang, S.; Liu, Z. Nitrogen removal performance and bacterial flora analysis of a partial nitrification-anaerobic ammonium oxidation-denitrification system treating rare earth element tailings wastewater. J. Environ. Chem. Eng. 2022, 10, 107961. [Google Scholar] [CrossRef]
- Lin, J.; Gan, L.; Chen, Z.; Naidu, R. Biodegradation of tetradecane using Acinetobacter venetianus immobilized on bagasse. Biochem. Eng. J. 2015, 100, 76–82. [Google Scholar] [CrossRef]
- Devi, N.; Patel, S.K.S.; Kumar, P.; Singh, A.; Thakur, N.; Lata, J.; Pandey, D.; Thakur, V.; Chand, D. Bioprocess Scale-up for Acetohydroxamic Acid Production by Hyperactive Acyltransferase of Immobilized Rhodococcus Pyridinivorans. Catal. Lett. 2021, 152, 944–953. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Liu, F.; Luo, P.; Xiao, R.; Zhang, M.; Wu, J. The immobilized Alcaligenes faecalis strain WT14 for removing high strength nitrate and reducing nitrite accumulation. Environ. Technol. 2022, 43, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Kalia, V.C.; Lee, J.K. Synthetic design of methanotroph co-cultures and their immobilization within polymers containing magnetic nanoparticles to enhance methanol production from wheat straw-based biogas. Bioresour. Technol. 2022, 364, 128032. [Google Scholar] [CrossRef]
- Cervantes, F.V.; Fernandez-Polo, D.; Merdzo, Z.; Miguez, N.; Garcia-Gonzalez, M.; Ballesteros, A.O.; Fernandez-Lobato, M.; Plou, F.J. Reuse of Immobilized Komagataella phaffii Cells for the Elimination of D-Glucose in Syrups of Bioactive Carbohydrates. ACS Food Sci. Technol. 2022, 2, 682–690. [Google Scholar] [CrossRef]
- Belgrano, F.D.S.; Diegel, O.; Pereira, N., Jr.; Hatti-Kaul, R. Cell immobilization on 3D-printed matrices: A model study on propionic acid fermentation. Bioresour. Technol. 2018, 249, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-S.; Yuan, X.; Peng, F.; Lou, W.-Y. Immobilization of engineered E. coli cells for asymmetric reduction of methyl acetoacetate to methyl-(R)-3-hydroxybutyrate. Bioresour. Bioprocess. 2022, 9, 19. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Tu, B. Immobilization of ammonia-oxidizing bacteria by polyvinyl alcohol and sodium alginate. Braz. J. Microbiol. 2017, 48, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, Z.; Ma, Z.; Chen, J.; Song, Z. Immobilization of Rhodopseudomonas palustris P1 on glass pumice to improve the removal of NH4+-N and NO2−-N from aquaculture pond water. Biotechnol. Appl. Biochem. 2020, 67, 323–329. [Google Scholar] [CrossRef]
- Chen, H.; Liu, K.; Yang, E.; Chen, J.; Gu, Y.; Wu, S.; Yang, M.; Wang, H.; Wang, D.; Li, H. A critical review on microbial ecology in the novel biological nitrogen removal process: Dynamic balance of complex functional microbes for nitrogen removal. Sci. Total Environ. 2023, 857, 159462. [Google Scholar] [CrossRef]

| Projects | COD/mg·L−1 | NH4+-N/mg·L−1 | Ca2+/mg·L−1 | Mg2+/mg·L−1 | Hardness/mg·L−1 | Alkalinity/mg·L−1 | pH |
|---|---|---|---|---|---|---|---|
| Value | 500–800 | 150–300 | 80–200 | 30–50 | 120–250 | 900–1400 | 7.5–8.5 |
| Group | Treatment |
|---|---|
| 1 | High-ammonia-nitrogen wastewater and modified activated carbon with immobilized cells |
| 2 | High-ammonia-nitrogen wastewater and free cells |
| 3 | High-ammonia-nitrogen wastewater and modified activated carbon |
| 4 | High-ammonia-nitrogen wastewater |
| Procedures | Biomass/pc·g−1 |
|---|---|
| 1 | 1.8 × 108 |
| 2 | 4.9 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, K.; Liu, X.; Chen, H.; Cai, Z. Treatment of High-Ammonia-Nitrogen Wastewater with Immobilized Ammonia-Oxidizing Bacteria Alcaligenes sp. TD-94 and Paracoccus sp. TD-10. Processes 2023, 11, 926. https://doi.org/10.3390/pr11030926
Zhang J, Chen K, Liu X, Chen H, Cai Z. Treatment of High-Ammonia-Nitrogen Wastewater with Immobilized Ammonia-Oxidizing Bacteria Alcaligenes sp. TD-94 and Paracoccus sp. TD-10. Processes. 2023; 11(3):926. https://doi.org/10.3390/pr11030926
Chicago/Turabian StyleZhang, Jingyun, Ke Chen, Xing Liu, Huiling Chen, and Zhiqiang Cai. 2023. "Treatment of High-Ammonia-Nitrogen Wastewater with Immobilized Ammonia-Oxidizing Bacteria Alcaligenes sp. TD-94 and Paracoccus sp. TD-10" Processes 11, no. 3: 926. https://doi.org/10.3390/pr11030926





