Simultaneous High Performance Liquid Chromatography Assay of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in Humco™ Lavare Wound Base
Abstract
:1. Introduction
| Ingredient | %w/w in formulation | Ingredient | %w/w in formulation |
|---|---|---|---|
| Pentoxifylline | 5% | Gentamicin Sulfate | 0.2% |
| Mupirocin | 5% | Fluticasone Propionate | 1% |
| Itraconazole | 3.75% | Humco™ Lavare Wound Base | Quantity sufficient (q.s.) |
2. Experimental Section
2.1. Materials and Methods
2.1.1. Chromatographic Conditions
| Column: | Phenomenex® Gemini 150 × 4.6 mm C18 5µm Part # 00F-4435-E0 or equivalent (Torrance, CA, USA) |
| Guard column: | Phenomenex® SecurityGuard C18 Guard Column Part # KJ0-4282 |
| Column temperature: | 25 °C |
| Mobile Phase A: | 0.025 M Sodium phosphate dibasic, pH 3.0 with o-phosphoric acid |
| Mobile Phase B: | Acetonitrile |
| Gradient profile: | See Table 2 |
| Time (min) | %A | %B |
|---|---|---|
| 0 | 90 | 10 |
| 5 | 90 | 10 |
| 25 | 20 | 80 |
| 30 | 90 | 10 |
| 35 | 90 | 10 |
| Flow rate: | 1.0 mL/min |
| Injection volume: | 10 µL |
| Wavelength: | 220 nm |
| Seal/needle rinse: | 50/50 Acetonitrile/water |
| Run time: | 37 min |
| Typical retention times: | See Table 3 |
| Active | Approximate retention time (min) | Active | Approximate retention time (min) |
|---|---|---|---|
| Pentoxifylline | 12.2–12.9 | Fluticasone Propionate | 23.3–24.3 |
| Mupirocin | 17.0–18.0 | Itraconazole | 25.0–25.8 |
2.1.2. Materials and Equipment
| Pentoxifylline: | United States Pharmacopeia or reference standard grade |
| Fluticasone Propionate: | United States Pharmacopeia or reference standard grade |
| Mupirocin: | United States Pharmacopeia or reference standard grade |
| Itraconazole: | United States Pharmacopeia or reference standard grade |
| Sodium phosphate dibasic: | Reagent grade |
| HPLC grade water: | HPLC grade |
| Acetonitrile: | HPLC grade |
| o-Phosphoric acid, 85%: | HPLC grade |
| Tetrahydrofuran: | HPLC grade |
| Humco™ Lavare wound base: | In-house supply |
| Syringe filter: | Thermo target 2 0.2 µm 30 mm Nylon media Syringe Filter Part #F2500-2 or equivalent |
2.1.3. Mobile Phase A Preparation
2.1.4. Diluent (50/50 Water/Acetonitrile)
2.1.5. Standard Preparation
2.1.6. Sample Preparation
2.2. Method Verification Elements
2.2.1. Specificity
2.2.2. Linearity
2.2.3. Accuracy
2.2.4. Precision
System Precision (Reproducibility)
Method Precision (Repeatability)
2.2.5. Range
3. Results and Discussion
3.1. System Suitability
| Active | Peak area % RSD (n = 6) | Peak area % RSD (overall) | Average overall retention time (min) | Retention time % RSD (overall) | Average theoretical plates | Average tailing | Average resolution |
|---|---|---|---|---|---|---|---|
| Pentoxifylline | 1% | 1% | 12.5 | 0.3 | 197,467 | 1.1 | n/a |
| Mupirocin | 1% | 1% | 17.6 | 0.2 | 334,115 | 1.1 | 3.9 |
| Fluticasone Propionate | 1% | 1% | 23.7 | 0.1 | 415,238 | 1.1 | 22.2 |
| Itraconazole | 1% | 1% | 25.4 | 0.1 | 348,768 | 1.1 | 10.2 |
| Specification | ≤2% | ≤2% | n/a | ≤2% | ≥2000 | ≤2.0 | >1.5 |
3.2. Specificity Results
3.2.1. Examining the Blank
3.2.2. Examining the Sample Matrix (Placebo)
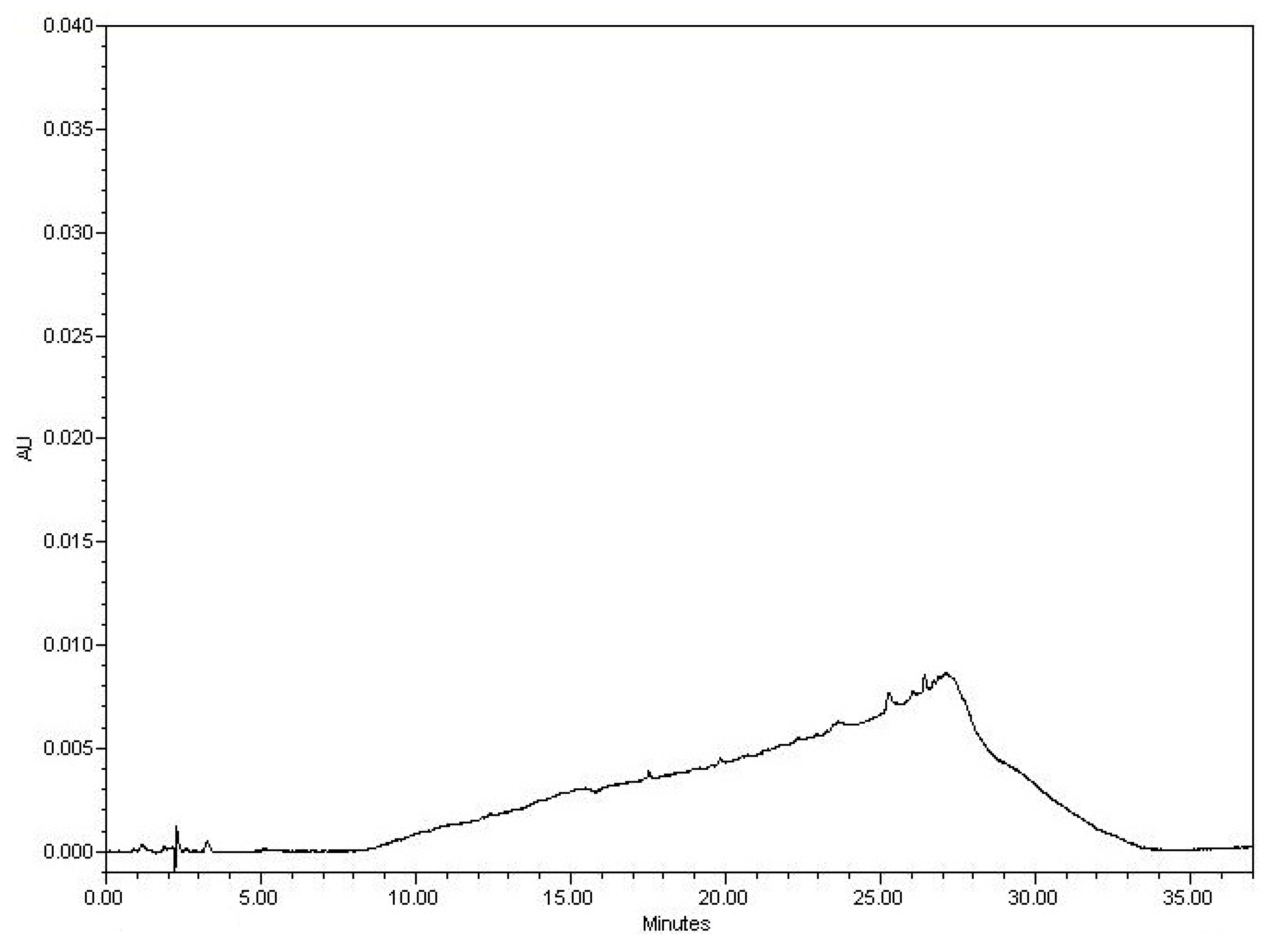
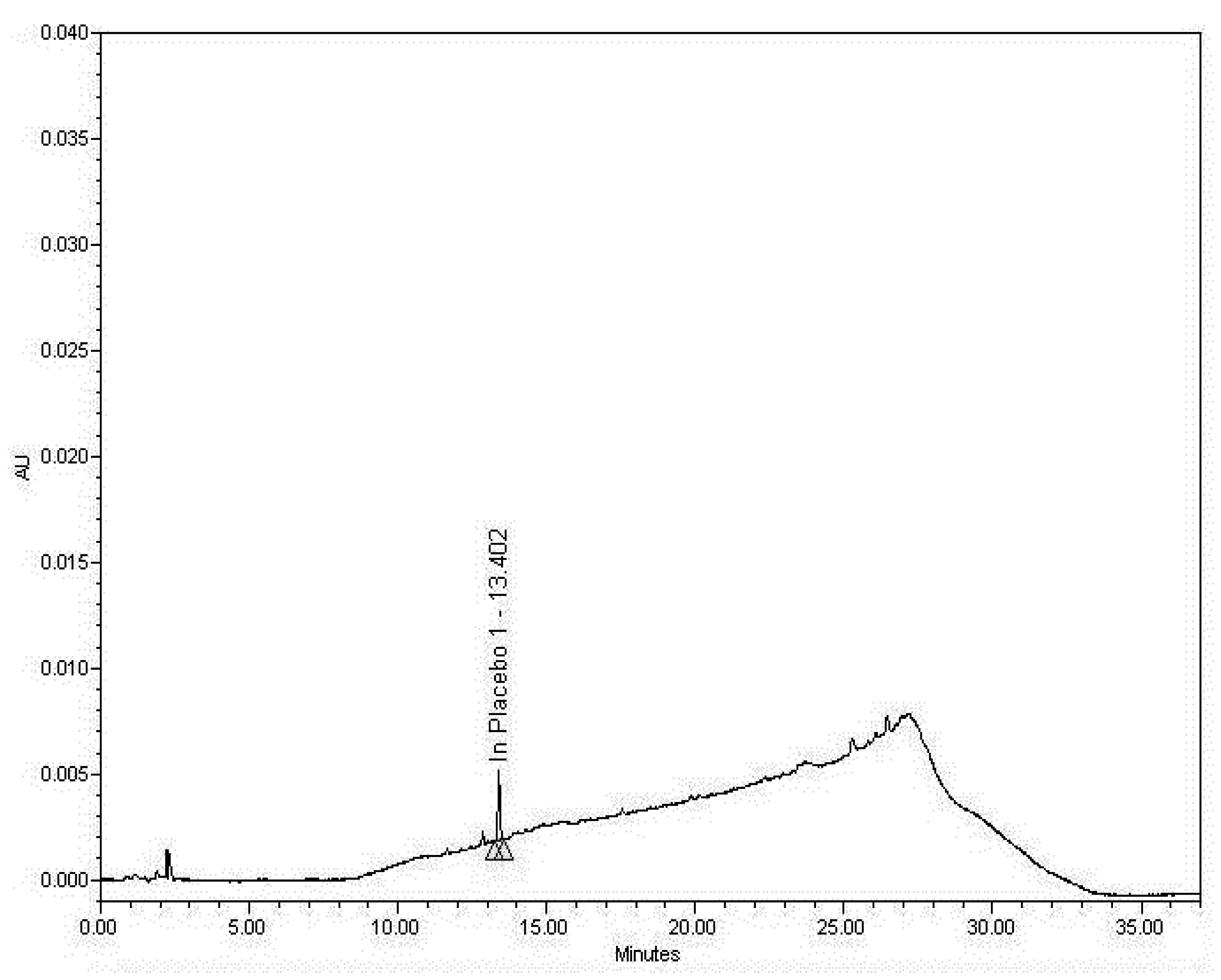


3.2.3. Specificity Discussion
3.3. Linearity Results
3.3.1. Experimental
| Compound | Standard weight (mg) | 50% Conc. (mg/mL) | 80% Conc. (mg/mL) | 100% Conc. (mg/mL) | 120% Conc. (mg/mL) | 150% Conc. (mg/mL) |
|---|---|---|---|---|---|---|
| Pentoxifylline | 100.7 | 0.1007 | 0.1611 | 0.2014 | 0.2417 | 0.3021 |
| Mupirocin | 100.4 | 0.1004 | 0.1606 | 0.2008 | 0.2410 | 0.3012 |
| Fluticasone Propionate | 21.9 | 0.0219 | 0.0350 | 0.0438 | 0.0526 | 0.0657 |
| Itraconazole | 75.6 | 0.0756 | 0.1210 | 0.1512 | 0.1814 | 0.2268 |
3.3.2. Linearity of Actives
3.3.3. Linearity Discussion
| Active | Linearity % | Theoretical conc. (mg/mL) | Actual conc. (mg/mL) | % Recovery | r2 |
|---|---|---|---|---|---|
| Pentoxifylline | 50 | 0.1007 | 0.0992 | 98.5% | 0.9991 |
| 80 | 0.1611 | 0.1613 | 100.1% | ||
| 100 | 0.2014 | 0.2030 | 100.8% | ||
| 120 | 0.2417 | 0.2438 | 100.9% | ||
| 150 | 0.3021 | 0.2998 | 99.2% | ||
| Mupirocin | 50 | 0.1004 | 0.0993 | 98.9% | 0.9996 |
| 80 | 0.1606 | 0.1616 | 100.6% | ||
| 100 | 0.2008 | 0.2014 | 100.3% | ||
| 120 | 0.2410 | 0.2415 | 100.2% | ||
| 150 | 0.3012 | 0.3003 | 99.7% | ||
| Fluticasone Propionate | 50 | 0.0219 | 0.0218 | 99.6% | 0.9997 |
| 80 | 0.0350 | 0.0353 | 100.7% | ||
| 100 | 0.0438 | 0.0437 | 99.9% | ||
| 120 | 0.0526 | 0.0524 | 99.7% | ||
| 150 | 0.0657 | 0.0658 | 100.1% | ||
| Itraconazole | 50 | 0.0756 | 0.0748 | 99.0% | 0.9996 |
| 80 | 0.1210 | 0.1215 | 100.5% | ||
| 100 | 0.1512 | 0.1517 | 100.3% | ||
| 120 | 0.1814 | 0.1819 | 100.3% | ||
| 150 | 0.2268 | 0.2260 | 99.7% |
| Active | LOQ (mg/mL) | LOD (mg/mL) |
|---|---|---|
| Pentoxifylline | 0.00043 | 0.00018 |
| Mupirocin | 0.00040 | 0.00012 |
| Fluticasone Propionate | 0.00088 | 0.00029 |
| Itraconazole | 0.00030 | 0.00011 |
3.4. Accuracy Results
3.4.1. Accuracy of Actives
3.4.2. Accuracy Discussion
3.5. Precision Results
| Active | Accuracy% | Theoretical conc. (mg/mL) | Actual conc. (mg/mL) | % recovery | % RSD |
|---|---|---|---|---|---|
| Pentoxifylline | 50 | 0.1007 | 0.0989 | 98.2% | 0% |
| 80 | 0.1611 | 0.1615 | 100.3% | 1% | |
| 100 | 0.2041 | 0.2034 | 99.7% | 1% | |
| 120 | 0.2417 | 0.2434 | 100.7% | 1% | |
| 150 | 0.3021 | 0.2998 | 99.2% | 1% | |
| Mupirocin | 50 | 0.1004 | 0.0997 | 99.3% | 0% |
| 80 | 0.1606 | 0.1614 | 100.4% | 0% | |
| 100 | 0.2008 | 0.2008 | 100.0% | 1% | |
| 120 | 0.2410 | 0.2418 | 100.3% | 1% | |
| 150 | 0.3012 | 0.3004 | 99.7% | 1% | |
| Fluticasone Propionate | 50 | 0.0219 | 0.0216 | 98.4% | 0% |
| 80 | 0.0350 | 0.0354 | 100.9% | 2% | |
| 100 | 0.0438 | 0.0440 | 100.5% | 2% | |
| 120 | 0.0526 | 0.0526 | 100.1% | 1% | |
| 150 | 0.0657 | 0.0654 | 99.6% | 1% | |
| Itraconazole | 50 | 0.0756 | 0.0749 | 99.1% | 0% |
| 80 | 0.1210 | 0.1211 | 100.2% | 1% | |
| 100 | 0.1512 | 0.1517 | 100.4% | 1% | |
| 120 | 0.1814 | 0.1825 | 100.6% | 1% | |
| 150 | 0.2268 | 0.2258 | 99.5% | 1% |
3.5.1. System Precision Results
| Active | Peak Area % RSD; (n = 6 injections) |
|---|---|
| Pentoxifylline | 1% |
| Mupirocin | 1% |
| Fluticasone Propionate | 1% |
| Itraconazole | 1% |
3.5.2. System Precision Discussion
3.5.3. Method Precision Results
| Active | Peak area % RSD; (n = 3 preparations) |
|---|---|
| Pentoxifylline | 1% |
| Mupirocin | 1% |
| Fluticasone Propionate | 1% |
| Itraconazole | 1% |
3.5.4. Method Precision Discussion
3.6. Range Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Trental, Pentoxil (pentoxifylline) dosing, indications, interactions, and adverse effects. Available online: http://www.webmd.com/drugs/2/drug-3696/trental-oral/details (accessed on 13 September 2015).
- National Institutes of Health: Fluticasone Propionate Nasal Spray. Available online: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a695002.html (accessed on 3 November 2015).
- Fuller, A.T.; Mellows, G.; Woolford, M.; Banks, G.T.; Barrow, K.D.; Chain, E.B. Pseudomonic acid: An antibiotic produced by Pseudomonas fluorescens. Nature 1911, 234, 416–417. [Google Scholar] [CrossRef]
- Hughes, J.; Mellows, G. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Echerichia coli by pseudomonic acid. Biochem. J. 1978, 176, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H.; Rosselet, J.P.; Marquez, J.A.; Coniglio, C.T.; Charney, W.; Herzog, H.L.; Black, J. Gentamicin, 1a New Antimicrobial Complex from Micromonospora. J. Med. Chem. 1963, 6, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Janssen Pharmaceuticals Sporanox (Itraconazole Capsules) Package Insert. Available online: http://www.janssen.com/us/sites/www_janssen_com_usa/files/products-documents/039808-150915_sporanox_pi_code_update_only_12.pdf (accessed on 15 August 2015).
- Remington, J.P.; Allen, L.V. Remington: The Science and Practice of Pharmacy; Pharmaceutical Press: London, UK, 2013. [Google Scholar]
- <1225> Validation of Compendial Procedures; United States Pharmacopoeia. Available online: http://www.ofnisystems.com/wp-content/uploads/2013/12/USP36_1225.pdf (accessed on 15 August 2015).
- Validation of Analytical Procedures: Text and Methodology. Available online: http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html (accessed on 1 August 2015).
- Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics; Food and Drug Administration Center for Drug Evaluation Research: Silver Spring, MD, USA, 2015.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purvis, T. Simultaneous High Performance Liquid Chromatography Assay of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in Humco™ Lavare Wound Base. Chromatography 2015, 2, 642-654. https://doi.org/10.3390/chromatography2040642
Purvis T. Simultaneous High Performance Liquid Chromatography Assay of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in Humco™ Lavare Wound Base. Chromatography. 2015; 2(4):642-654. https://doi.org/10.3390/chromatography2040642
Chicago/Turabian StylePurvis, Troy. 2015. "Simultaneous High Performance Liquid Chromatography Assay of Pentoxifylline, Mupirocin, Itraconazole, and Fluticasone Propionate in Humco™ Lavare Wound Base" Chromatography 2, no. 4: 642-654. https://doi.org/10.3390/chromatography2040642






