1. Introduction
Paper-based fluidic devices (microPADs) have been developed over the past eight years as a new platform for simple, portable and low cost diagnostic tests [
1,
2]. These devices have the potential to allow for the qualitative and quantitative analysis of a variety of analytes—pathogens, toxins, hazardous chemicals, or biomarkers—in resource-limited settings such as remote areas in developed and developing countries. MicroPADs share many common features with other types of paper-based diagnostic devices such as dipstick assays and lateral-flow assays in that the devices are inexpensive, easy to use, and the results of the assays are usually reported as color changes [
2,
3]. What sets microPADs apart from other types of paper-based devices is that they incorporate networks of channels patterned into one or multiple pieces of paper that can transport fluids via capillary wicking [
3]. These networks of channels can be used to perform multiple assays simultaneously or complex fluid handling steps so that more complex assays involving multiple steps could be performed on microPADs while still maintaining simplicity and low cost for the end user [
2]. Some of the challenges faced by microPADs as a diagnostic platform are that multiple pipetting steps of sample and reagent solutions are still required for multistep assays, reagents are often not stable for extended periods of time when stored in dry form on the devices under ambient conditions, and freshly prepared external calibration curves are still required for quantitative analysis of the results, which means calibration standard solutions would have to be transported into the field so that external calibration assays could be conducted side-by-side with any test [
4,
5,
6,
7,
8]. In an effort to overcome some of these limitations, we developed a convenient system for storing and then delivering reagent solutions to microPADs using the thermally-responsive hydrogel
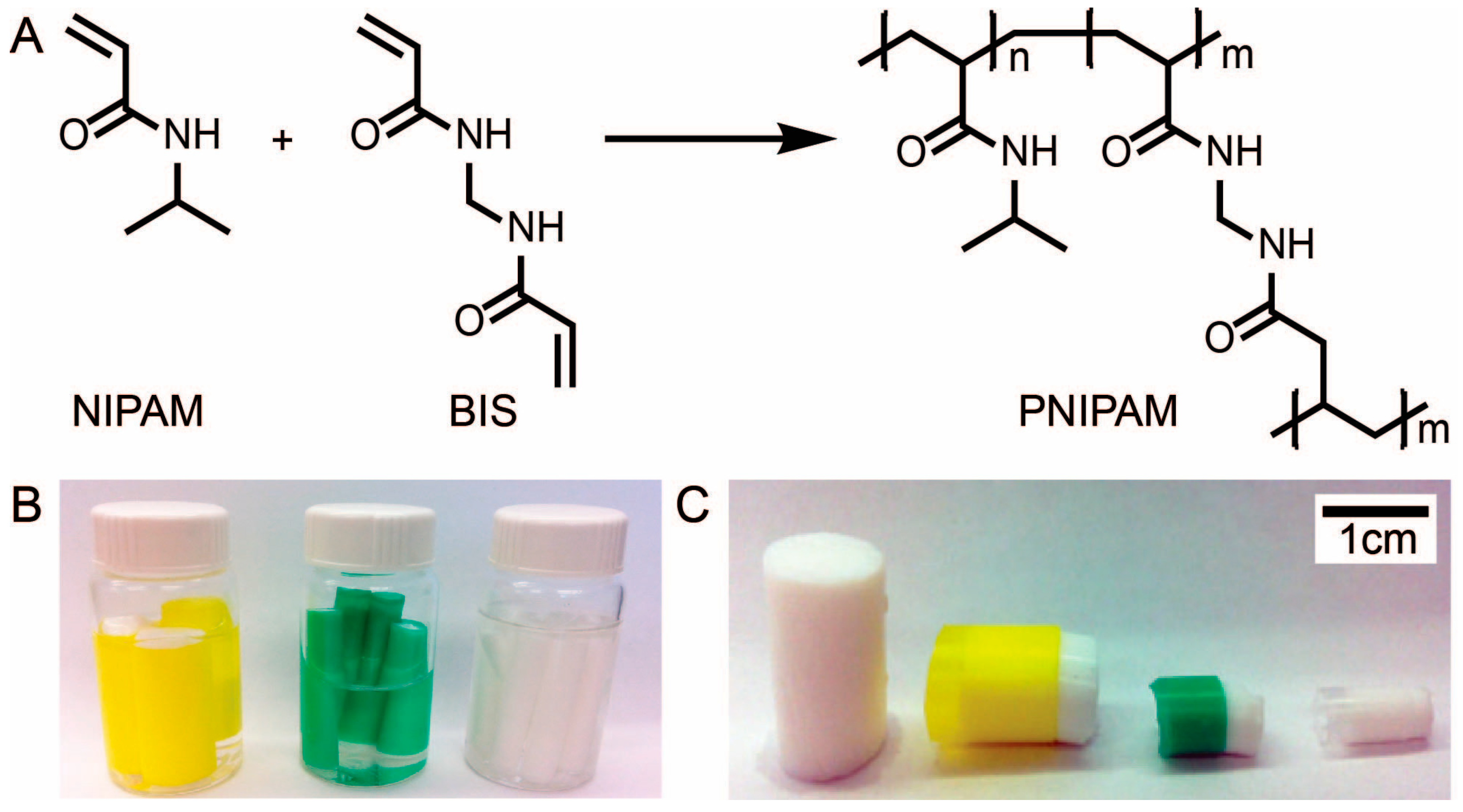
N,
N'-methylenebisacrylamide-cross-linked poly(N-isopropylacrylamide) (PNIPAM).
Gels are unique polymer systems that are formed by cross-linking polymer chains to create a single large molecule [
9]. Gels are classified into three major types depending on their physical properties: hydrogels, organogels and xerogels [
9,
10]. As the name suggests, hydrogels are compatible with aqueous solutions, which makes these gels particularly attractive for work with microPADs. We chose to focus on a specific class of hydrogels called thermally-responsive hydrogels. These gels incur conformational changes when subjected to temperature changes, which can be used to tune the water content of the gels [
9,
10,
11,
12]. PNIPAM is a hydrogel with a lower critical solution temperature (LCST) meaning this gel will collapse and expel aqueous solutions from its matrix when heated above its LCST [
11,
12,
13]. This process is reversible, so a solution can be expelled from a PNIPAM gel at high temperatures, and the gel can then be loaded with a new solution by immersing the gel in the solution at low temperatures. In this way, PNIPAM gels can be used to store and then deliver reagent solutions to microPADs in response to temperature changes.
Hydrogels have long been associated with microfluidic devices. Microfluidic devices have been used to synthesize hydrogels, microfluidic devices have been fabricated out of hydrogels, and hydrogels have been incorporated into microfluidic devices to form valves, to form scaffolds for cell culture, and to trap or deliver analytes [
14,
15,
16,
17,
18,
19,
20]. An article published recently by Niedl and Beta in
Lab on a Chip introduced the use of hydrogels as fluid reservoirs for use with microPADs [
21]. We had been developing the same concept concurrently without any knowledge of Niedl and Beta’s work. While the work presented here and the work by Niedl and Beta both focus on the same general idea of combining hydrogels with microPADs, we have demonstrated different capabilities and applications, and, in many ways, the two articles complement each other. The article by Niedl and Beta focuses primarily on the use of hydrogels to deliver water to microPADs in order to drive sequential reactions with the reagents being pre-dried on the device. Our work involved a more detailed study of the use of hydrogels for storing and then delivering reagents in solution to microPADs.
The experimental plan guiding this work had four primary objectives: (i) optimization of the PNIPAM gels for use with microPADs, (ii) characterization of the delivery of aqueous solutions from PNIPAM to microPADs, (iii) characterization of the delivery of reagents from PNIPAM to microPADs, and (iv) characterization of the storage of reagents, specifically enzymes, in PNIPAM for extended periods of time under ambient conditions. In terms of optimizing the PNIPAM gels for use with microPADs, we looked to synthesize gels that were durable, could be handled easily and could also be fabricated with reproducible dimensions. The cross-linker to monomer ratio was varied to produce a gel with the desired mechanical properties, and different types of molds were explored to control the dimensions of the gels. To characterize the delivery of aqueous solutions from PNIPAM gels to microPADs, we explored the delivery of a single solution to a device and also the delivery of multiple solutions either simultaneously or in a sequence to a device. Once the fluid-delivery of PNIPAM to microPADs was characterized, the ability of PNIPAM to deliver reagents to microPADs was assessed. This work focused on four major classes of reagents relevant to point-of-care diagnostic assays: water-soluble small molecules, enzymes, antibodies and DNA. There were three primary goals for this portion of the project: (i) to determine if PNIPAM was able to deliver the reagents to microPADs, (ii) to determine if PNIPAM had any effect on the concentration of the reagent it was delivering by either retaining or excluding it from the gel matrix, and (iii) to determine if PNIPAM could deliver the reagent accurately and precisely enough to construct an external calibration curve for a colorimetric assay. Finally, PNIPAM was assessed as a storage system for enzymes by measuring the activity of the enzyme horseradish peroxidase after being stored in PNIPAM gels for extended periods of time.
2. Experimental Section
2.1. Reagents and Materials
All reagents were purchased from commercial sources unless otherwise stated. The following chemicals were used: N-isopropylacrylamide (NIPAM, Sigma Aldrich), N,N'-methylenebisacrylamide (BIS, Fisher Bioreagents), potassium persulfate (KPS, Sigma Aldrich), tetramethylethylenediamine (TEMED, Fisher Bioreagents), food coloring (Durkee), glucose (Sigma Aldrich), horseradish peroxidase (HRP, MP Biomedical), glucose oxidase (GOX, MP Biomedical), fluorescein tagged DNA (a 20 base pair-long oligonucleotide, Biosearch Technologies), fluorescein tagged rabbit anti-sheep IgG (Thermo Scientific), 2,2'-azino-bis(3-ethylbenzothioazoline-6-sulfonic acid) diammonium salt (ABTS, Alfa Aesar), and 1-StepTM ABTS (a proprietary solution of ABTS and H2O2, Thermo Scientific). A phosphate buffered saline solution (1XPBS) was prepared from 10XPBS (Fisher Scientific) with in-house nanopure water obtained from a nanopure dispenser (Thermo Scientific D13661). The following materials were obtained from commercially available sources and were used in the experiments: chromatography paper (Whatman No. 1), thermal laminating pouches (3 M, Scotch), and poly(ethylene) (PE) straws of three diameters: 11.03 mm (Karat), 7.24 mm (Crystal Ware), and 5.35 mm (Starbucks Coffee Company).
2.2. Fabrication of MicroPADs
MicroPADs were fabricated by the method of wax patterning [
22]. The patterns for the microPADs were designed in AutoCAD and then printed onto chromatography paper using a solid-ink printer (Xerox Phaser 8560). The paper was then baked in a convection oven (MTI Compact Forced Air) for 15 min at 145 °C and then cooled to room temperature under ambient conditions. The devices were stored in plastic petri dishes until they were used.
After patterning the channels, some devices were enclosed with lamination sheets. Enclosing the devices was advantageous because it protected the channels from contamination and minimized evaporation of fluids from the channels. Hot lamination was performed by inserting the microPAD in a thermal laminating pouch and running the pouch through a thermal laminator (APACHE AL-12P, set to the 250 °F setting).
2.3. Digital Image Colorimetry
The results from colorimetric assays conducted on microPADs were quantified via digital image colorimetry (DIC) [
7,
23,
24]. The devices were first scanned using a desktop scanner (Epson Perfection V300) using the following settings: 48-bit Color image type, 300 dpi resolution, and reflective document type. Images were analyzed using ImageJ 1.46r, open source analysis software. The images were split into three color-channels: red, green and blue. The green and blue channels were discarded. The red channel was inverted, and the color intensity of the test zones was measured using a microarray profile plugin [
25]. The mean intensity values obtained were analyzed in Excel.
2.4. Synthesis of PNIPAM
Poly(N-isopropylacrylamide) (PNIPAM) was synthesized via free radical solution polymerization. The synthesis was performed at room temperature using three purged vials. Molar ratios of monomer (NIPAM) to cross-linker (BIS) of 1:1, 3:1, 4:1, 5:1, 6:1, 7:1, 8:1, and 10:1 were tested. The following procedure is described for a monomer to cross-linker molar ratio of 5:1. Vial 1 contained NIPAM (1.209 g, 10.68 mmoles) and BIS (0.3294 g, 2.14 mmoles) in DI H2O (15 mL). Vial 2 contained the KPS (0.009 g, 0.03 mmoles), a radical initiator, in DI H2O (2.0 mL). Vial 3 contained TEMED (71 μL, 0.06 g, 0.47 mmoles), a radical generator accelerator, in DI H2O (2.0 mL). Each vial was capped with a rubber septum, which was fastened with copper wire and then purged with nitrogen gas (N2) for 20 min under atmospheric pressure. After purging, the contents of Vials 2 and 3 were added to Vial 1. Vial 1 was then taken off the nitrogen purge line, shaken thoroughly and allowed to react to completion at room temperature for 24 h. The polymerization was considered to be complete when an opaque gel had solidified in Vial 1. This occurred typically within 10 min of combining the contents of the three vials, but the gel was left over night to ensure that the polymerization had gone to completion.
In order to create samples of PNIPAM with a defined surface area, PE straws with diameters of 5.37 mm, 7.24 mm, and 11.03 mm were used as inexpensive molds for PNIPAM. The straws were placed into Vial 1 prior to purging and retrieved with molded PNIPAM once the reaction was complete. To produce varying lengths of sample, the length of the straws were varied as well as the size of the vial. Regardless of the size of the vial, molar ratios of the reagents were kept constant (i.e., NIPAM (5): BIS (1): TEMED (0.22): KPS (0.016)) and solvent volumes were scaled proportionally.
After 24 h, the reaction vial (Vial 1) was etched with a glass cutter above the level of the gel. The rubber septum was then removed, and the vial was immersed in a liquid nitrogen bath for approximately 45 s, ensuring that the entire etched path was exposed to liquid nitrogen. Immediately after liquid nitrogen exposure, the vial was shattered along the etch line using channel-lock pliers. The vial was covered with an aluminum foil sheet to contain the shattered glass. The straw-encased PNIPAM hydrogel samples were then retrieved from the PNIPAM mass.
The hydrogels were thermocycled to purify the polymer matrix by removing any residual reagents (
Figure S1). The thermocycling procedure involved the following steps: (i) the PNIPAM was submerged in a vial containing DI H
2O and heated for 10 minutes at 60 °C in a silicon oil bath, (ii) the water was decanted and the samples were rinsed in the vial with fresh portions of DI H
2O two times, (iii) the vial was filled with fresh DI H
2O and placed in an ice-water bath (0 °C) for 20 min, (iv) the vial was removed from the ice-water bath and allowed to warm to room temperature. The cycle was repeated a total of 6 times for each hydrogel.
2.5. Characterization of Fluid Delivery from PNIPAM
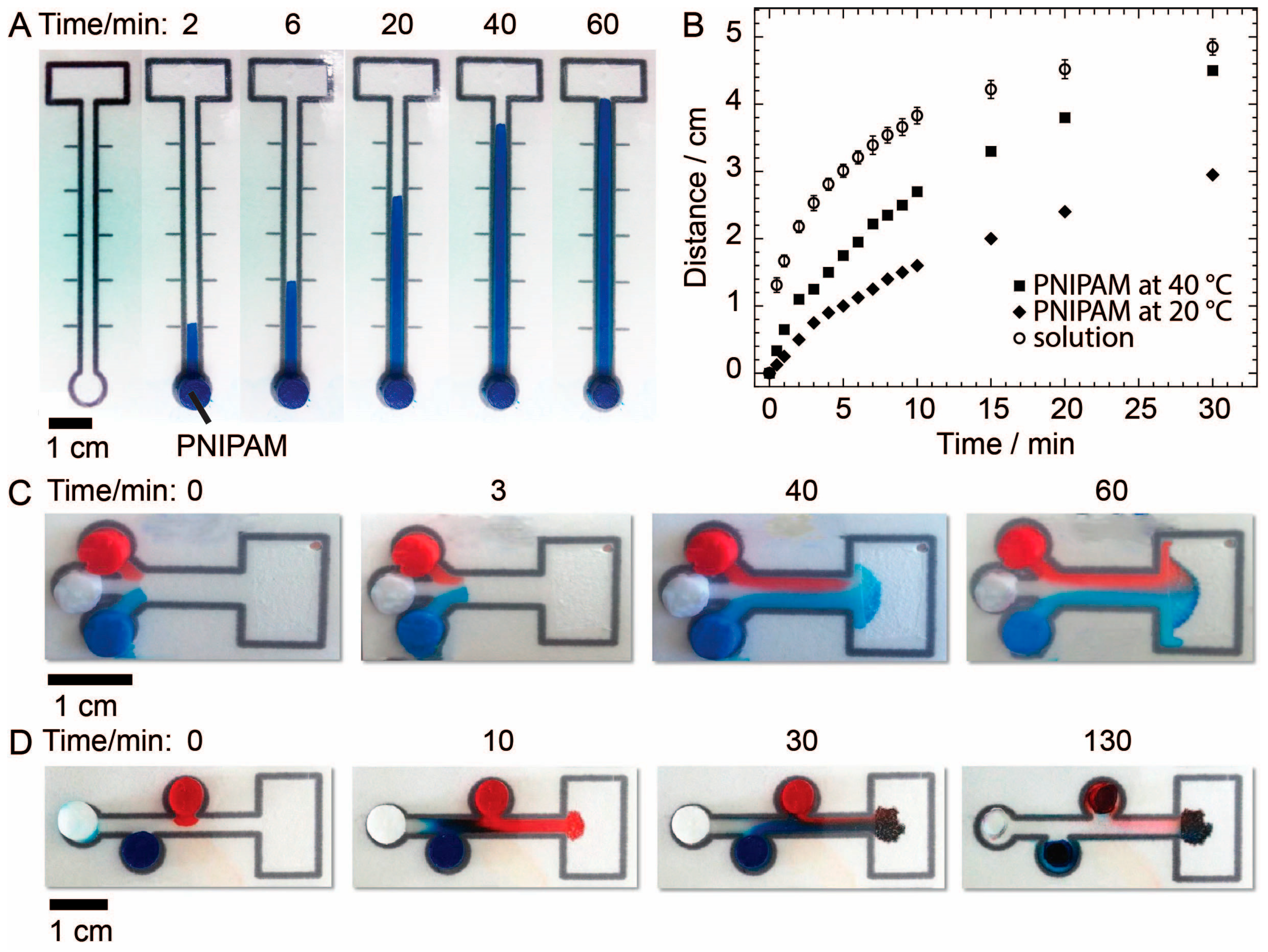
To determine the amount of fluid that could be released by PNIPAM upon heating, we heated samples of PNIPAM of each of the three diameters for 12 min on a digital hotplate set to 40 °C and recorded the initial and final mass of the samples. The duration of the heating step (12 min) was an arbitrary amount of time selected because we believe it is a reasonable time scale for the operation of microPADs in the real world. The temperature of 40 °C was chosen because it is achievable using a HotHands®’s hand warmer, which is an inexpensive, commercially available and portable heat source, and it is conceivable that a health professional could use this type of product in the field to perform a paper-based diagnostic assay with PNIPAM as a fluid source. Several other instrument-free approaches for controlling temperature of paper microfluidic devices have also been described and could be used with PNIPAM [
26]. Using the same approach, we also studied the effect of heating time at 40 °C on the amount of fluid expelled by PNIPAM gels.
2.6. Loading Reagents into PNIPAM
To load reagents or dyes into the hydrogel, the gel was first heated to expel all fluid, then transferred to a vial with the desired reagent solution and allowed to equilibrate at room temperature for 24 h. The gel was heated by submerging it in DI H2O in a glass vial, and then placing the vial into a silicone oil bath set to 60 °C. The temperature of the oil bath was increased by 10 °C every 30 min to a final temperature of 80 °C, and the final temperature was held for 30 min.
2.7. Delivery of Fluids from PNIPAM to MicroPADs
PNIPAM gels (7.24-mm in diameter and 1-cm in length) were loaded with aqueous dye solutions and placed on microPADs to study fluid delivery to paper. One microPAD was used to measure the rate of wicking for fluids delivered to paper-based devices via PNIPAM at room temperature and at 40 °C. This device consisted of a single circular sample addition zone with a diameter of 7.3 mm leading into a 6-cm-long and 2-mm-wide channel with markings every centimeter along both sides of the channel. For comparison, the same experiment was carried out by adding 45 µL of solution in three 15-μL aliquots directly to the sample inlet using a micropipette. A second microPAD was used to demonstrate that PNIPAM could deliver fluids in laminar flow. This device had three circular sample addition zones all with a diameter of 7.3 mm that were situated at one end of the device and were equidistant from the point of entry into a single channel that led to a waste zone. A third microPAD was used to demonstrate that PNIPAM could deliver fluids to a reaction zone in a specific sequence. This device had three staggered circular sample addition zones all with a diameter of 7.3 mm leading into a single channel that emptied into a waste zone. All three devices were laminated, and the lamination over the sample addition and waste zones was cut out and removed using a razor blade to allow for sample introduction and for fluids to evaporate from the waste zone. For the second and third devices, PNIPAM gels, loaded with red dye, blue dye and water respectively, were placed in the three sample addition zones at the same time, and the device was heated to 40 °C to observe the delivery of fluid from the gels to the devices.
2.8. Delivery of Reagents from PNIPAM to MicroPADs
PNIPAM was used to deliver four distinct, clinically-relevant classes of analytes to microPADs: small molecules (glucose), enzymes (HRP), antibodies (fluorescein-labeled IgG) and DNA (fluorescein-labeled 20 base pair oligonucleotide).
A paper-based 96-well plate with 5-mm-diameter circular reaction zones was used to demonstrate the delivery of glucose from a PNIPAM gel to a microPAD [
27]. The PNIPAM gels used for these experiments had a diameter of 7.24 mm and length of approximately 4 cm. The concentration of glucose delivered to the reaction zones was quantified using a colorimetric glucose assay based on a coupled enzymatic reaction using GOX, HRP, and ABTS, which produced a blue-green color in the reaction zones.
Glucose solutions of the following concentrations were prepared in duplicate: 0 mM (DI H2O), 1 mM, 2 mM, 2.5 mM, 3 mM, 3.5 mM, 4 mM, and 5 mM. One set of the glucose solutions was loaded into PNIPAM gels. The other set was used as external solutions for comparison. Ultimately, three different types of glucose samples were prepared: glucose loaded into PNIPAM (PNIPAM), the excess glucose solutions remaining in the vials after loading the PNIPAM gels (PNIPAM solution), and the external glucose solutions (solution). A reaction mixture was prepared consisting of GOX (75 kU·L−1), HRP (250 kU·L−1) and ABTS (6.25 mM) in 1XPBS. A volume of 1 μL of reaction mixture was spotted in the reactions zones of a paper-based 96-well plate and dried for 30 min under ambient conditions. The PNIPAM samples were placed in the wells for 60 s and then removed. For the PNIPAM loading solution and the external glucose solutions, 3 µL of each solution was added to the reaction zone using a micropipette. Three replicates were performed for each concentration. The colorimetric assays were allowed to develop for 30 min before being imaged and analyzed. Two calibration standard glucose solutions with concentrations of 2.5 mM and 3.5 mM, respectively, were also tested using the same colorimetric assay to evaluate the accuracy and precision of the assay.
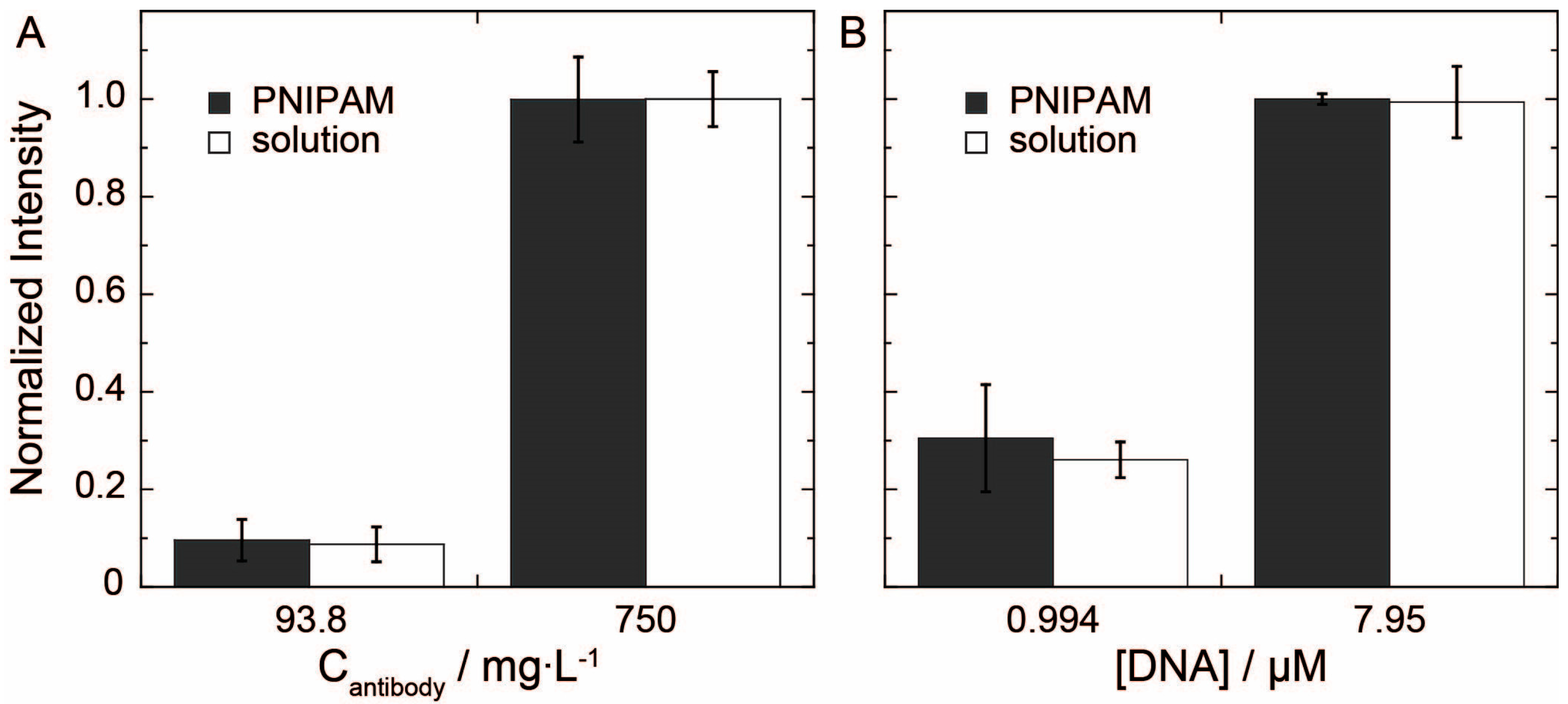
To evaluate the delivery of fluorescein tagged antibody and DNA, devices with a circular sample addition zone and a circular test zone, both with a diameter of 7.25 mm, connected by a single channel (length 2 mm and width 2.2 mm) were used. Two sets of solutions with concentrations of 750 mg/L and 93.8 mg/L of fluorescein-labeled rabbit anti-sheep IgG were prepared in 1XPBS. One set of the solutions was loaded into 7.24-mm PNIPAM gels with an average mass of 0.1504 g, and the other set of solutions was used as external solutions for comparison. The PNIPAM gels were placed upon the sample addition zone of the microPADs at room temperature until the solution completely filled the test zone. The external solutions were tested by adding 10 µL of solution to the sample addition zone. All devices were dried for 20 min. Once the devices were dry, they were imaged using a fluorescent scanner (Typhoon TRIO+, Amersham Biosciences) and quantified in ImageJ.
Two sets of solutions of fluorescein tagged DNA (20 base-pair-long oligonucleotide) with concentrations of 7.95 µM and 0.994 µM were prepared in 1XPBS. One set of solutions was loaded into 7.24-mm PNIPAM gels with an average mass of 0.1389 g, and the other set of solutions was used as external solutions for comparison. Samples were tested by the same method as the fluorescein-labeled IgG samples.
2.9. Storage of Reagents in PNIPAM
PNIPAM was assessed for its ability to deliver an enzyme, HRP, by passive wicking to a microPAD using a paper-based 96-well plate. The effects of PNIPAM on the activity of HRP were also investigated. PNIPAM samples with a 7.24-mm diameter and length of approximately 4 cm were prepared for loading with HRP solution. A working HRP solution was prepared with a concentration of 550 kU·L−1 in 1XPBS. A portion of this solution was loaded into eight PNIPAM gels. PNIPAM gels loaded with 1XPBS were used as negative controls to account for any background signal from the assay. The remaining HRP solution was used as a control solution. After the PNIPAM gels were loaded with the HRP solution, they were divided into two groups: four gels were stored in dry, empty vials (dry storage), and the other four gels were stored in the HRP solution (solution storage). The dry storage gels, solution storage gels, and the HRP solution were all prepared on the same day (day 0) and then stored in a drawer at room temperature for the duration of the experiment. The activity of the HPR stored under the three conditions was monitored on select days up to day 35.
The activity of the HRP in the three samples was monitored using a colorimetric assay with ABTS. The PNIPAM samples (3-mm sections of the gels) were placed on reaction zones of a paper-based 96-well plate for 10 min to ensure delivery of HRP. For comparison, 1 µL of the HRP solution was added to separate reaction zones. Immediately after adding the HRP solutions, 3 µL of 1 Step ABTS was added to each reaction zone. The color from the assay was allowed to develop for 30 min under ambient conditions, and then the results were imaged and quantified.
As an additional experiment we assessed if a single PNIPAM gel could deliver reproducible amounts of HRP in sequential trials. The PNIPAM samples were placed in a reaction zone for 10 min for each trial. After 10 min, the samples were transferred directly to a new reaction zone. This process was repeated four times. The activity of the HRP delivered to the test zones was determined using the ABTS assay.