Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review
Abstract
:1. Introduction
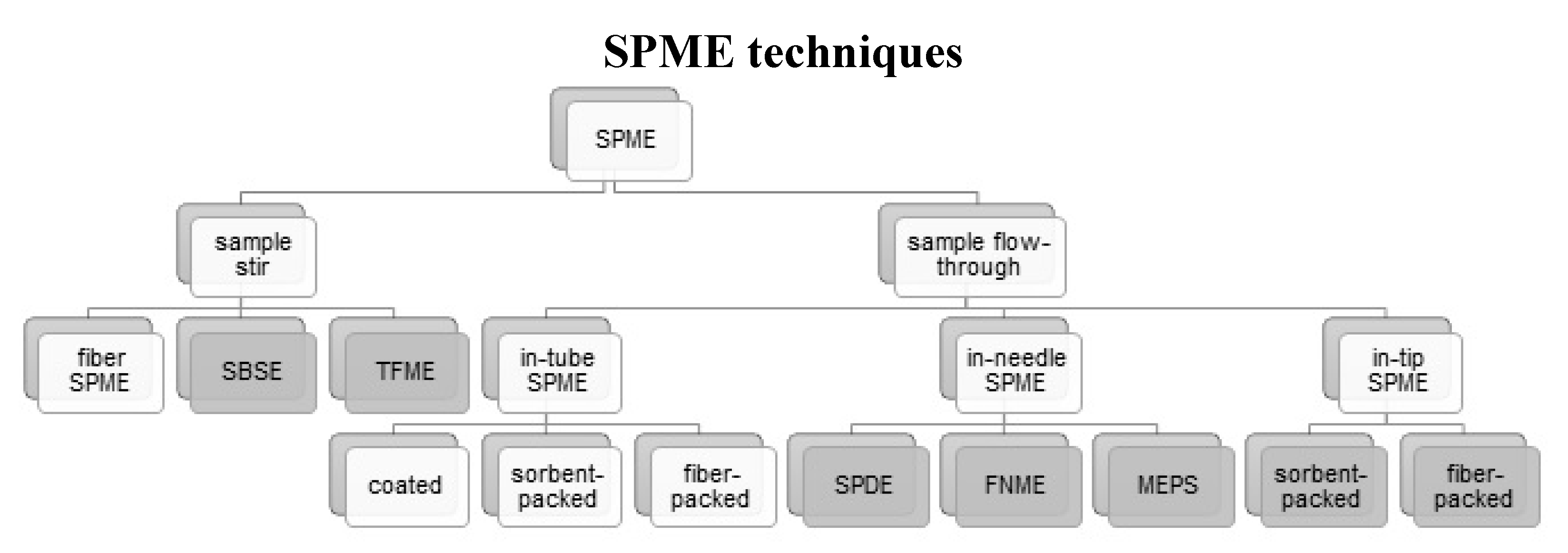
2. SPME Techniques
2.1. Fiber Solid-Phase Microextraction
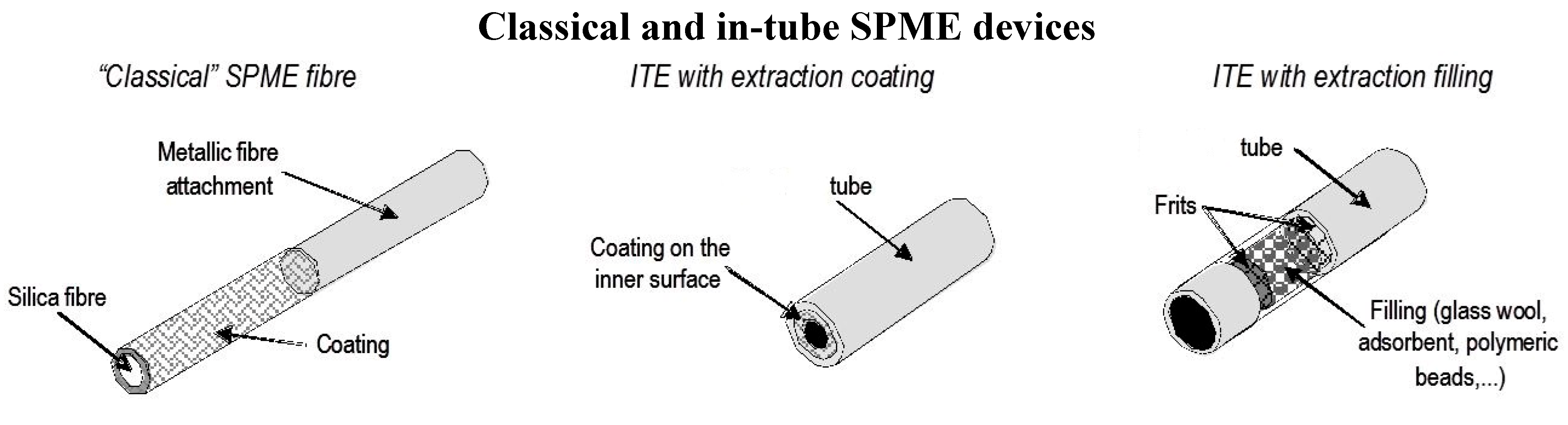
2.2. In-tube Solid-Phase Microextraction
2.3. Cooled Coated Fiber Device

2.4. Non-fiber SPME Techniques
3. SPME Process

3.1. Extraction and Desorption Techniques


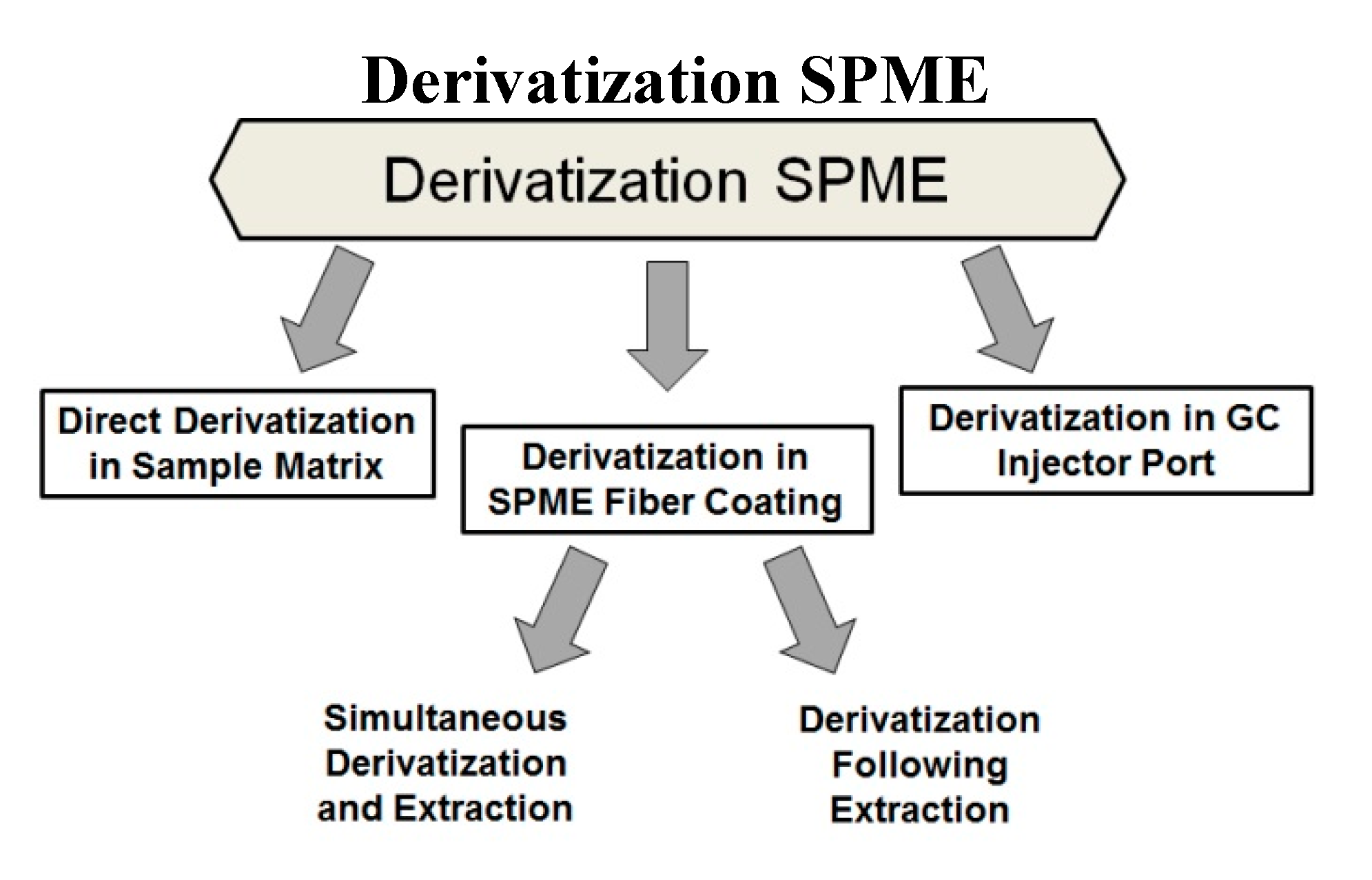
3.2. Derivatization
3.3. Salt-addition
3.4. Fiber Coatings
| Dipping and physical agglutinating methods | Sol-gel technology | Chemical grafting | Electrochemical methods | Electro-spinning | Liquid-phase deposition | Hydro-thermal methods |
|---|---|---|---|---|---|---|
| Carbon nanomaterials [26,120] Ordered mesoporous materials [121,122] Ionic liquids and polymeric ionic liquids [123,124] | Functionalized or polymer-functionalized carbon nanomaterials [125,126,127] Ionic liquid-mediated SPME coating [128,129] Sol-gel derived polymeric ionic liquid-based SPME coatings [130,131] Sol-gel ordered mesoporous silica SPME coating [132,133] Sol-gel coating on metal wires [134,135] Sol-gel molecularly imprinted polymer coatings [113,136] Aptamer Sol-gel SPME [137] | Nanomaterials [138,139] Immunoaffinity SPME [140] Molecularly imprinted polymers [141,142] Substrate-bonded ionic liquid coatings [143] Substrate-bonded polymeric ionic liquid coatings [144] | Electrodeposition Electropolymerized conductive polymers [88,145,146,147] Electropolymerized conductive polymers nanocomposite [148,149] Conductive polymer-ionic liquid composites [150,151] Metal oxides [152,153] Anodized metal wires Metal oxides [154,155] Electrophoretic deposition Carbon nanotubes (CNTs) [112,113,114,115] | Electrospun epoxide polymer Carbon nanofiber-based SPME [116,156,157] | SiO2, TiO2, SnO2, ZrO2 [117] Three dimensional transition metal oxides (V, Cr, Mn, Fe, Co, Ni, Cu, Zn, In, individually or combined) [158] | ZnO nanoparticles Organic frameworks (MOFs) [118,119] |
3.5. Quantitation
3.5.1. Traditional Calibration Methods
| Calibration method | Advantages | Disadvantages | Applications | |
|---|---|---|---|---|
| Traditional | External standard Standard addition Internal standard | No extensive sample preparation Correction of sample matrix effects Compensation of matrix effects and losses of analytes during sample preparation and irreproducibility in parameters (injection in GC/LC) | Need for availability of blank sample matrices Need for stable sampling procedure and chromatographic conditions Extensive sample preparation and analysis Limited availability of suitable internal standards High cost and limited availability of isotope-labelled standards | [176,177,178] [169,170] [165,166,167,168,171,172] |
| Equilibrium extraction | Possibility to calculate concentration of analytes by amount of extracted analytes Independence of amount of extracted analytes of sample volume | Need for knowledge about distribution coefficients | [181,182] | |
| Exhaustive extraction | Possibility to calculate concentration of analytes by amound of extracted analytes and sample volume | Suitable only for small sample volumes and large distribution coefficients or need for special devices | [56,183] | |
| Diffusion-based | Fick’s first law of diffusion Interface model and cross-flow mode Kinetic calibration with standard Standard-free kinetic calibration | Suitability for TWA sampling Independency of sampling rate of face velocity Minimizing of competitive effects for solid coating through high sampling rate and short time Suitability for on-site sampling Suitability for TWA sampling No need for standard loading Possibility to calculate concentrations of all extracted analytes in sample | Sorbent should be zero sink for target analytes Very low sample rate for water sampling Need for controlled or determined flow velocity of sampling matrix Application limited to linear sampling regime Need for determination of standard loading Need for stable sampling conditions Unsuitability for long-term monitoring | [184,185] [186,187] [188,189] [190] |
3.5.2. Equilibrium Extraction
3.5.3. Exhaustive Extraction
3.5.4. Diffusion-based Calibration
4. Applications in Food Analysis

| Extraction conditions | Desorption conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Food Sample | Analyte | Technique | Fiber | Time [min] | Temp. [°C] | Salt addition | Deriv. | Time [min] | Temp. [°C] | Detection | LOD | Recovery | Ref. |
| Aroma | |||||||||||||
| Dairy products | |||||||||||||
| Butter | diacetyl | - | PDMS-DVB | 5 | 37 | - | - | 1 | 250 | GC-MS-MS | 0.0078 ppm | - | [200] |
| European PDO hard cheeses | VVC | HS-SPME | DVB-CAR-PDMS | 60 | 45 | - | - | 10 | 260 | GC-MS / GC-FID | - | - | [201] |
| PDO Cheese, Oscypek | VVC | HS-SPME | CAR-PDMS | 15 | 50 | - | - | 5 | 260 | GC-MS | - | - | [202] |
| Van Herby Cheeses | esters, ketones, alde-hydes, acids, alcohols, hydrocarbons, terpenes | HS-SPME | DVB-CAR-PDMS | 30 | 40 | - | - | 5 | 250 | GC-MS / GC-FID | - | - | [203] |
| Milk, cheese and whey powder | VVC | HS-SPME | DVB-CAR-PDMS | 30 | 40 | 4 g NaCl | - | 5 | 250 | GC-MS | - | - | [204] |
| Meat and meat products | |||||||||||||
| Roasted pork of mini-pig | VVC | DI-SPME | CAR-PDMS | 60 | 80 | 0.22 g salt | - | 4 | 280 | GC-MS / GO-O / GC-FID | - | - | [205] |
| Traditional smoke-cured bacon (CSCB) | alkane, aldehydes, ketones, alcohols, thioethers, thiols, furans, phenols | - | CAR-PDMS / DVB-CAR-PDMS | 30 | 60 | - | - | 5 | 280 | GC-MS | - | - | [206] |
| Minced beef | VVC | HS-SPME | DVB-CAR-PDMS | 30 | 40 | 25% NaCl | - | 1 | 250 | GC-MS | - | - | [207] |
| Cooked, cured pork ham | VVC | HS-SPME / SBSE | CAR-PDB-DVB / 0.5mm PDMS phase thickness stir bars | 30 / 90 | 40 / RT | - | - | 10 | 250 / 30 | GC-MS | - | - | [208] |
| Slow fermented sausages | dimethyl trisulfide, 3-methyl thiophene, 2,3-butanedione, 2-nonanoneacetic acid | HS-SPME | CAR-PDMS | 180 | 37 | - | - | 5 | 240 | GC-MS / GC-O | - | - | [209] |
| Cooked, fermented sausage | VVC | SPME | CAR-PDMS | 40 | 47 | - | - | 10 | 250 | GC-MS | - | - | [210] |
| Cooked beef | VVC | HS-SPME | DVB–CAR–PDMS | 25 | 40 | 6% NaCl | - | 3 | 250 | GC-MS | - | - | [211] |
| Juice and alcoholic beverages | |||||||||||||
| Whisky | fatty acid ethyl esters, higher alcohols, fatty acids, carbonyl com-pounds, monoterpenols, C13 norisoprenoids, volatile phenols | HS-SPME | CAR-PDMS | 60 | 40 | 30% NaCl | - | 6 | 220 | GC-MS | - | - | [212] |
| Banana Terra spirit | 3-methylbutan-1-ol, 3-methylbutan-1-ol acetate, 2-phenylethylacetate, phenylethyl alcohol | HS-SPME | PDMS-CAR-DVB | 25 | 60 | - | - | 2 | 240 | GC-MS / GC-O | - | - | [213] |
| Chinese Laobaigan liquor | VVC | HS-SPME | DVB-CAR-PDMS / CAR-PDMS | 40 | 60 | 3 g NaCl | - | 5 | 250 | GC-MS | - | - | [214] |
| Ice wine | aroma compounds | HS-SPME | DVB-CAR-PDMS | 5 | 45 | 1 g NaCl | - | 2 | 260 | GC-TOF-MS | trans-OL: 0.015 g/mL; cis-OL: 0.01 g/mL | - | [215,216] |
| Wine | 2-methyl-3-furanthiol, 4-mercapto-4-methyl-2-pentanone, 3-mercaptohexanol, 2-furanmethane-thiol, 3-mercaptohexyl acetate | HS-SPME | PDMS–DVB | 10 | 55 | - | on-fiber deri-vatization | 2 | 250 | GC-NCI-MS | 0.03–0.8 fg | - | [85] |
| Spanish white wines | VVC | HS-SPME | CAR-PDMS | 60 | 46 | - | - | 2 | 280 | GC-MS / GC-FID | 0.1–900 ng/mL | 97–110% | [217] |
| Cherry wines | VVC | HS-SPME | DVB-CAR-PDMS | 45 | 50 | 2 g NaCl | - | 5 | 230 | GC-MS | 0.03–7.27 µg/L | 60.7–125.6% | [218] |
| China gingko wine | VVC | HS-SPME | CAR-PDMS | 45 | 50 | saturated NaCl solution | - | 4 | 250 | GC-MS | - | - | [219] |
| Black raspberry wines | VVC | HS-SPME | PDMS-DVB | 30 | 60 | - | - | 5 | 230 | GC-MS | - | - | [220] |
| Beer | alcohols, esters, organic acids, aldehydes, ketones, terpenes, sulfur com-pounds, amines, phenols | HS-SPME | TMSPMA-OH-TSO prepared by sol-gel technology | 30 | 40 | 2 g NaCl | - | 5 | 300 | GC | 0.01–35.2 µg/L | 92.8–105.8% | [221] |
| Yellow passion fruit juice | VVC | HS-SPME | PDMS-DVB | 20 | 50 | 17% NaCl | - | 6 | 250 | GC-MS | - | - | [222] |
| Grape juice | aroma compounds | HS-SBSE | PDMS | 120 | RT | - | - | 5 | -50 | GC-MS | - | 28.4% | [223] |
| Orange beverage emulsion | VVC | HS-SPME | CAR-PDMS | 15 | 45 | 15% NaCl | - | 8 | 250 | GC-MS | 0.06–2.27 mg/L | 88.3–121.7% | [224] |
| Coffee | aroma compounds | HS-SPME | PDMS-DVB | 20 | 60 | - | - | 1 | 270 | GC-MS | - | - | [225] |
| Coffee | VVC | HS-SPME | PDMS | 5 | 30 | - | - | - | 220 | SAW | - | - | [226] |
| Coffee | VVC | HS-SPME | DVB-CAR-PDMS | 30 | 60 | - | - | 5 | 230 | GC | - | - | [227] |
| Coffee | furans, methoxyphenols, pyrazines, and ketones | HS-SPME | poly [VC16Im][NTf2] with 50% [VBIm2C12]2[Ntf2] | 30 | RT | - | - | 5 | 175 | GC-MS / GC-FID | - | - | [123] |
| Fruits and vegetables | |||||||||||||
| Various apricot varieties | ethyl acetate, hexyl acetate, limonene, b-cyclocitral, c-decalactone, 6-methyl-5-hepten-2-one, linalool, b-ionone, menthone and (E)-hexen-2-al | HS-SPME | CAR-PDMS | 20 | 40 | - | - | 4 | 250 | GC-MS / GC-O | - | - | [228] |
| Apricot varieties | linalool, a-terpineol, b-ionone and c-decalactone | HS-SPME | CAR-PDMS | 20 | 40 | saturated NaCl solution | - | 4 | 250 | GC-MS | - | - | [229] |
| Apricot varieties | aldehydes, alcohols, acetates, esters, terpenes and acids | HS-SPME | CAR-PDMS | 30 | 40 | - | - | 2 | 250 | GC-MS | - | - | [230] |
| Jackfruit | ethyl isovalerate, 3-methylbutyl acetate, 1-butanol, propyl isovalerate, isobutyl isovalerate, 2-methyl-butanol, butyl isovalerate | HS-SPME | DVB-CAR-PDMS | 30 | 10 | - | - | 5 | 250 | GC-TOF-MS | - | - | [231] |
| Cooked peaches | VVC | HS-SPME | DVB-CAR-PDMS | 20 | 40 | - | - | 10 | 270 | GC-MS | - | - | [232] |
| Monstera deliciosa fruit | VVC | HS-SPME | PDMS-DVB | 60 | 40 | 15% NaCl | - | 6 | 250 | GC–qMS | - | - | [233] |
| Pineapple fruit | VVC | HS-SPME | PDMS-DVB | 30 | 40 | - | - | 2 | 240 | GC×GC-qMS | - | - | [234] |
| Sweet cherry cultivars | VVC | HS-SPME | CAR-PDMS | 20 | 45 | 0.2 g NaCl | - | 10 | 260 | GC-MS | - | - | [235] |
| Air-dried raisins | free and glycosidically bound volatile compounds | HS-SPME | CAR-PDMS-DVB | 40 | 60 | 1.3 g NaCl | - | 8 | - | GC-MS | - | - | [236] |
| Table grapes | alcohols, carbonyls, C6 compounds, terpenoids, esters | HS-SPME | PDMS-DVB | 30 | 40 | 2 g CaCl2, 20 g NaCl | - | 3 | 220 | GC-MS | - | - | [237] |
| Tomato | VVC | HS-SPME | DVB-CAR-PDMS | 15 | 50 | saturated CaCl2 solution | - | 10 | 250 | GC-MS | - | - | [238] |
| Miscellaneous | |||||||||||||
| Thistle honey | VVC | HS-SPME | DVB-CAR-PDMS | 40 | 60 | 30% NaCl | - | 2 | 250 | GC-MS | - | - | [239] |
| Croatian lime tree, fir honey-dew, sage honey | VVC | HS-SPME | DVB-CAR-PDMS | 20 | 40 | 0.5 g an-hydrous Na2SO4 | - | 3 | 250 | GC-MS | - | - | [240] |
| Honey | VVC | HS-SPME | PDMS-DVB | 40 | 50 | - | - | 2 | 250 | GC-QTOF-MS | - | - | [241] |
| Extra virgin olive oils | VVC | HS-SPME | DVB-CAR-PDMS | 10 | 40 | - | - | 5 | 260 | GC-FID | - | - | [242] |
| Extra virgin olive oils | VVC | HS-SPME | DVB-CAR-PDMS | 360 | 30 | - | - | 4 | 270 | GC-MS | - | - | [243] |
| Virgin olive oil | VVC | HS-SPME | DVB-CAR-PDMS | 40 | 40 | - | - | 5 | 300 | GC-MS | 0.1–2.54 mg/kg | - | [244] |
| Black and white rice bran | terpenoid flavor odorants | HS-SPME | PDMS | 30 | 100 | - | - | 0.2 | 250 | GCxGC-MS | - | - | [245] |
| Italian rice cultivars | VVC | HS-SPME | DVB-CAR-PDMS | 60 | 60 | - | - | 5 | 250 | GC-MS | - | - | [246] |
| Palm sugar | N-heterocyclic and O-heterocyclic compounds | HS-SPME | DVB-CAR-PDMS | 10 | 50 | - | - | 5 | 240 | GC-MS | - | - | [247] |
| Almond cultivars | VVC | HS-SPME | DVB-CAR-PDMS | 60 | 60 | - | - | 10 | 270 | GC-MS | - | - | [248] |
| Saffron | VVC | HS-SPME | PDMS | 20 | 36 | - | - | 0.4 | 250 | GC-MS | - | - | [249] |
| Garlic | VVC | HS-SPME | CAR-PDMS | 30 | 30 | - | - | 3 | 220 | GC-MS | - | - | [250] |
| Atlantic shellfish species | VVC | HS-SPME | CAR-PDMS | 30 | 80 | 10 mL saturated NaCl solution | - | 10 | 260 | GC-MS | 0.12–1.19 ppb | 59.3–119.6% | [251] |
| Off-Flavor | |||||||||||||
| Food products | |||||||||||||
| Beer and beverage | sulfur compounds | HS-SDME / DI-SPME / HS-SDME | PDMS | 5 | 25 | 2.0 g NaCl / 20% NaCl solution | - | 5 | 250 | GC-FPD | 0.5 ng/mL for DPrDS -208.1 ng/mL | beer: 85.5–106.9%; bevera-ges: 95.2–110.8% | [252] |
| Beer | esters and vicinal diketones | HS-SPME | DVB-CAR-PDMS | 30 | 60 | 3.0 g NaCl | - | - | 260 | GC-MS | - | - | [253] |
| Wine | volatile sulfur compouds | HS-SPME | CAR-PDMS | 20 | 35 | - | - | 7 | 300 | GC-pFPD | 0.5 µg/L | 0–100% | [254] |
| Chardonnay and Pinot gris wines | 2-aminoacetophenone | DI-SPME | DVB-CAR-PDMS | 30 | 30 | - | - | - | 250 | GC-MS | - | 70–80% | [255] |
| Orange juice | guaiacol and halogenated phenol | HS-SPME | DVB-CAR-PDMS | 30 | 40 | - | - | 5 | 220 | GC-MS / GC-O | - | - | [256] |
| Water and apple juice | geosmin | HS-SPME | PDMS synthesized as coated fiber by sol-gel technology | 25 | 40 | 37% NaCl | - | 4 | 250 | GC-MS | 1–1.000 ng/L | 95–102% | [257] |
| Coffee beverage | volatile compounds produced by fungi | HS-SPME | DVB-CAR | 30 | 65 | - | - | 0.7 | 270 | GC-MS | - | - | [258] |
| Rapeseed oil | hexanal, 2,4-heptadienal, 2-heptenal and 1-pentene-3-ol | HS-SPME | CAR-DVB-PDMS | 35 | 50 | - | - | - | 150 | MS | - | - | [259] |
| Conventional and high-oleic sunflower oil | hexanal, (E)-2-heptenal, (E)-2-decenal, (E,E)-2,4-nonadienal | HS-SPME | CAR-DVB-PDMS | 60 / 90 / 120 | 40 / 60 / 80 | - | - | 5 | 270 | MS | 0.4–4.3 mg/L | - | [260] |
| Conventional and high-oleic rapeseed oil | octanal, 3-octanone, propanal, (E,E)-2,4-hexa-dienal, (E)-2-heptenal | HS-SPME | CAR-DVB-PDMS | 90 | 40 | - | - | 5 | 270 | MS | 3.7–816.5 µg/L | - | [261] |
| Various frying oils | (E,E)-2,4-decadienal, heptanal, (E,E)-2,4-heptadienal, (E)-2-decenal | HS-SPME | CAR-DVB-PDMS | 90 | 40 | - | - | 5 | 270 | MS | 0.03–47.2 µg/L | - | [262] |
| Almond oils | hexanal, (E)-2-heptenal, (E)-2-octenal, nonanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal | HS-SPME | DVB-CAR-PDMS | 60 | 60 | - | - | 10 | 270 | GC-MS | - | - | [263] |
| Butter | hexanal | HS-SPME | CAR-PDMS | 180 | 4 | - | - | 5 | 250 | GC-MS | - | 97.37% | [264] |
| Soymilk | aldehydes, alcohols, ketones, aromatic compounds, esters, furans | HS-SPME | CAR-PDMS | 20 | 40 | - | - | 3 | 300 | GC-MS | - | - | [265] |
| Fresh chilled pasteurised milk | microbially induced changes in volatile constituents | HS-SPME | CAR-PDMS | 30 | 40 | - | - | 2 | 240 | GC-MS / PTR-MS | - | - | [266] |
| Full fat bovine milk | volatile compounds (pentanal, pentanol, hexanal) produced by photooxidation | HS-SPME | CAR-PDMS | 30 | 50 | - | - | 0.02 | 250 | GC-MS | - | - | [267] |
| Chicken breast | sulfides methanethiol, dimethyl disulfide, di-methyl trisulfide, ethanol, 1- and 2-butanol, 1-butanol isomers, free fatty acids | HS-SPME | PDMS | 15 | 50 | - | - | 3 | 200 | GC-MS-FASST | - | - | [268] |
| Whiting | trimethylamine, 3-methyl-butanal, 2-methyl-butanal, 3-hydroxy-2-butanone, 3-methyl-1-butanol, 2-methyl-1-butanol | HS-SPME | CAR-PDMS | 40 | 50 | saturated NaCl solution | - | 0.16 | 250 | GC-MS | - | - | [269] |
| Rainbow trout | geosmin | HS-SPME | DVB-PDMS | 20 | 65 | 3.0 g NaCl | - | 3 | 270 | GC-MS | - | - | [270] |
| Potato crisps | VVC | HS-SPME | CAR-PDMS | 20 | 50 | - | - | 3 | 300 | MS e-nose / GS e-nose | - | - | [271] |
| Food packaging migrants | |||||||||||||
| Cork | chloroanisoles | CF-HS-SPME | PDMS | 10 | 130 / 10 | - | - | 3 | 260 | GC-TOF-MS | - | >90% | [56] |
| Wine | chlorophenols and chloroanisoles | MHS-SPME | DVB-CAR-PDMS | 60 | 70 | - | KHCO3 and acetic acid anhy-dride | 5 | 280 | GC-MS-MS | 0.004–0.077 ng | - | [80] |
| Wine | 4-ethylphenol, 4-ethylguaiacol | SPME | PDMS-CAR | 30 | 60 | - | - | 15 | 220 | GC-MS | - | - | [272] |
| Wine | 2,4,6-trichloroanisole, 2,3,4,6-tetrachloroani-sole, pentachloroanisole, 2,4,6-tribromoanisole, 4-ethylphenol (4-EP), 4-ethylguaiacol, 4-vinyl-phenol, 4-vinylguaiacol | MHS-SPME | DVB-CAR-PDMS | 60 | 70 | - | - | 5 | 270 | GC-MS-MS | 4-EP: 1800 g/L; others: 1000 g/L | 93.85–101.27% | [273] |
| Wine | haloanisoles | MHS-SPME | DVB-CAR-PDMS | 35 | 60 | 99.8% NaCl | - | 4 | 250 | GC-ion-trap MS | 120.70–150 pg | 88.8% | [274] |
| Water and honey | chlorophenols | DMSPE-HS-SPME | PVC/MWCNTs nanocomposite | 15 | 60 | 5 mol/L NaCl solution | - | 4 | 215 | GC-ECD | 0.08–0.6 ng/mL | 91–109% | [120] |
| Volatlile toxic compounds | |||||||||||||
| Contaminants | |||||||||||||
| Milk | diethylstilbestrol | DI-SPME | CNT reinforced hollow fiber | 30 | 60 | - | - | 10 | - | HPLC | 5.1 mg/L | 57.50%–120.42% | [125] |
| Milk | enzyme-generated volatile organic com-pounds associated with Listeria monocytogenes | HS-SPME | PA | 10 | 37 | - | - | 2 | 230 | GC-MS | - | - | [275] |
| Milk | PAHs | DI-SPME | diethoxydiphenyl-silane prepared by sol-gel technology | 60 | 60 | - | - | 2 | 330 | GC-MS | 0.01–0.08 µg/L | - | [276] |
| Milk and honey | benzimidazole | DI-SPME | MEMF | 70 | - | - | - | 20 | - | HPLC-DAD | 0.11–0.30 μg/L | 72.3–121%; 83.1–119% | [277] |
| Chicken muscle and milk | tetracyclines (antibiotic) | SPME | molecularly imprinted polymer | 30 | - | - | - | 10 | - | HPLC | 1.0 - 2.3 µg/L | - | [141] |
| Baby formula | furfural and hydroxymethylfurfural | HS-SPME | dodecylbenzene-sulfonate-doped polypyrrole | 30 | 50 | 2 mol/L NaCl | - | - | 200 | IMS | 6 ng/g; 5 ng/g | 95% / 92% | [278] |
| Baby food and fruit juice | furan | HS-SPME | PEG and PEG/CNTs fibers prepared by sol-gel technology | 25 / 30 | 3 g NaCl | - | 0.25 | 230 | GC-FID | 0.001 ng/mL; 0.00025 ng/mL | 92–98.5% | [126] | |
| Fruit juices | carbamate and phenylurea pesticide residues | DI-SPME | PDMS-DVB and CW-TPR | 90 | 20 | 0.3 g 30% NaCl | - | 15 | 250 | LC/QIT-MS | 0.001–0.01 mg/kg | 0–82% | [279] |
| Soft drinks | 4-methylimidazole | HS-SPME | PDMS-DVB | 50 | 110 | saturated NaCl solution | - | - | 270 | GC-MS / LC-MS/MS | 1.9 µg/L | - | [280] |
| Brazilian sugarcane juice | pesticideand benzo[a]pyrene | SBSE andMASE | - | 180 / 30 | 280 / 45 | saturated NaCl solution | - | 11 | 250 | TD-GC-MS / LVI-GC-MS | 0.002–0.4 µg/L; 0.004 - 0.56 µg/L | 0.2–55.3%; 13.6–103.1% | [281] |
| Carbonated drink, juice drink, sauce, jam, succade | benzoic and sorbic acids | in-tube SPME | diethylamine-modified poly(GMA-co-EDMA) monolithic capillary | 5 | - | - | - | 7 | - | HPLC-UV | 1.2; 0.9 ng/mL | 84.4–106% | [282] |
| Water and juice | benzoylurea insecticides | MMF-SPME | MMF/MAED | 70 | - | - | - | - | - | HPLC-DAD | water: 0.026–0.075 mg/L; juice: 0.053–0.29 mg/L | 65.1–118% | [283] |
| Drinking water | organic micro-pollutants | DI-SPME | PDMS-DVB | 30 | 60 | - | - | - | 280 | GC-MS | 0.5–10 µg/L | - | [284] |
| Apple, apple juice, tomato | organophosphorus pesticides | HS-SPME | PDMS-DVB including B15C5 prepared by sol-gel technology | 45 | 70 | 5 g NaCl | - | 5 | 270 | GC-FPD | 0.003–0.09 ng/g | apple juice: 71.5 –01.6% apple: 83.3–97.7% tomato: 55.3–105.3% (spiked 5 ng/g) | [285] |
| Apples | polycyclic aromatic hydrocarbons, benzene, toluene, ethylbenzene, xylene | HS-SPME | CAR-PDMS | 45 | 60 | - | - | 5 | 250 | GC-MS | 0.02 mg < LOD < 0.26 mg | 0.012–0.140 µg | [286] |
| Fruit and vegetables | pesticide residues | HS-SPME | PDMS | 34 | 62 | 10% NaCl | - | 7 | 270 | GC-MS | 0.35–8.33 µg/kg | 73–118% | [287] |
| Packaged fresh vegetables | volatiles derived from Salmonella typhimurium | HS-SPME | CAR-PDMS | 15 | 20 | - | - | 3 | - | GC-MS | - | - | [288] |
| Vegetables | PAHs | HS-SPME | benzoxy-C[6]/OH-TSO prepared by sol-gel technology | 40 | 60 | NaCl | - | 10 | 280 | GC-FID | 0.04–2.32 ng/g | 81.07–107.5% | [289] |
| Vegetables | organophosphorus pesticide residues | DI-SPME | PA | 30 | RT | 10% NaCl | - | 11 | 260 | GC-FPD | 0.01–0.14 µg/L | - | [290] |
| Radish | organochlorine pesticides | HS-SPME | calix[4]arene/hy-droxy-terminated silicone oil prepared by sol-gel technology | 30 | 70 | 1.0 g K2SO4 | - | 2 | 270 | GC-ECD | 1.27–174 ng/kg | 83.05–119.3% | [291] |
| Roasted coffee | furan | HS-SPME | CAR-PDMS | 30 | 35 | - | - | - | - | GC-MS | 3–10 μg/kg | 76–101% | [292] |
| Wine | 2,4,6-trichloroanisole, dibutyl phthalate | SR-SPME | graphene and graphene oxide prepared by sol-gel technology | 20 | 45 | 20% NaCl | - | 5 | 250 | GC-MS | 0.3 ng/L | 96.96% / 98.20% | [293] |
| Still and fortified wines | fungicides captan, chlorthalonil, folpet, iprodione, procymidone and vinclozolin, acaricide dicofol | DI-SPME | PDMS | 60 | 35 | - | - | 3 | 250 | GC-MS/MS | - | 70–120% | [294] |
| Breaded fish products | furanic compounds | HS-SPME | CAR-PDMS | 40 | 37 ± 1 | 3 g NaCl | - | 10 | 280 | GC-MS | - | - | [295] |
| Fresh, deep frozen, canned, boiled, roasted fish | formaldehyde | HS-SPME | CAR-PDMS | 30 | 80 | - | PFBHA | 3 | 310 | GC-MS | 17 µg/kg | 94.8 ± 1.7% | [81] |
| Cooked, peeled tropical shrimps | 3-methyl-1-butanal, 2,3-butanedione, 2-methyl-1-butanal, 2,3-heptanedione and trimethylamine induced by isolated bacteria | HS-SPME | CAR-PDMS | 25 | 40 | - | - | 5 | 280 | GC-MS | - | - | [296] |
| Packaged, aged, fresh beef | VOCs associated with Salmonella | HS-SPME | CAR-PDMS | 30 | 23 ± 2 °C | - | - | 10 | 270 | GC-MS | - | - | [297] |
| Smoked meat products | PAHs | DI-SPME | PDMS | 60 | 25 | - | - | 30 | 250 | GC-MS | 0.008–0.138 ng/mL | - | [298] |
| Meat roasted in plastic bags | plasticisers (phthalates) | DI-CF-SPME | PA | 30 | 45 | - | - | 0.02 | 250 | GC-MS | 0.01–0.18 µg/kg | - | [134] |
| Fruit leathers | carbonyl compounds generated from ozone-based food colorants decomposition | HS-SPME | PDMS-DVB | 15 | 60 | - | PFBHA methanol | - | 250 | GC-MS | 0.016–0.030 μg/L | - | [86] |
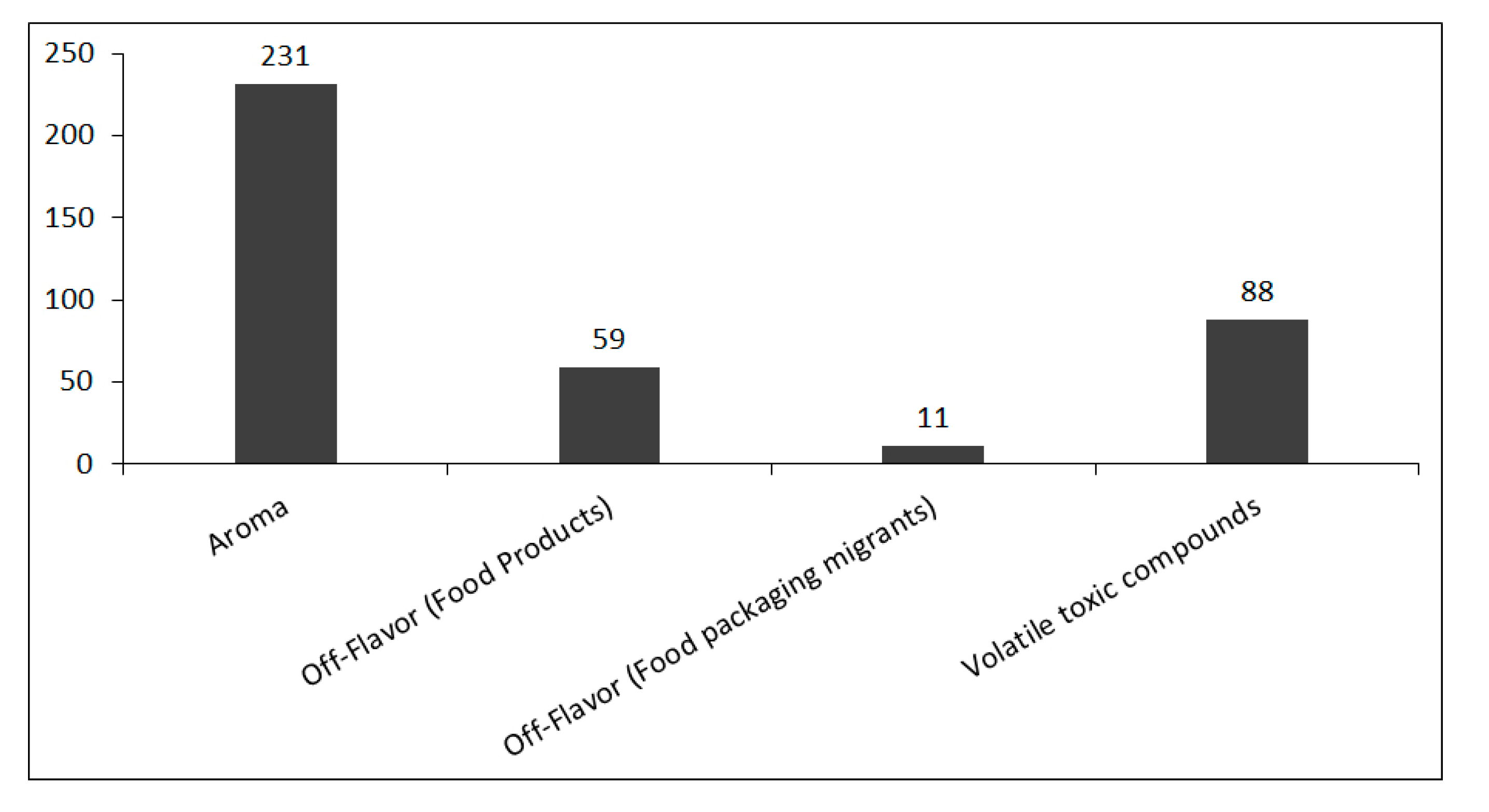
4.1. Aroma
4.2. Off-Flavors
4.3. Volatile Toxic Compounds
5. Environmental Applications
5.1. Air Samples
| Extraction conditions | Desorption conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Environmental Sample | Technique | Fiber | Time [min] | Temp. [°C] | (Salt) Addition | Deriv. | Time [min] | Temp. [°C] | Detection | LOD | Recovery [%] | Ref. |
| Air | |||||||||||||
| Pollutants | |||||||||||||
| Volatile organohalogen compounds | workplace air | SPME | sol-gel SWCNT / silica | 15 | RT | - | - | 3 | 280 | GC-MS | 0.09–0.2 ng/mL | - | [127] |
| Formaldehyde and other carbonyl compounds | indoor air | SPME | PDMS-DVB | 15 | RT | - | PFBHA | 4 | 250 | GC-MS | 0.002–0.032 µg/m³ | - | [82] |
| Acetaldehyde, acetone, BTX, pinene, trichloroethylene, alkanes | indoor air (classrooms) | SPME | PDMS-CAR | 240 | RT | - | - | 2.5 | 320 | GC-MS | 0.05–5.9 µg/m³ | 79–120 | [181] |
| BTEX | indoor and outdoor air | SPME | PDMS-CAR | 30 | 14–24 | - | - | 2 | 260 | GC-MS | 0.05–0.1 µg/m³ (benzene) | - | [319] |
| PAH | ambient air particulate matter (PM10, TPS), sampling on quarz filter disks and extraction with water/methylene chloride/acetone | DI-CF-SPME | PDMS | 60 | 70 | - | - | 1 | 270 | GC-MS | 0.02–1.16 ng | 88–98 | [330] |
| Alkyl- and methoxy-phenolic compounds | biomass smoke, absorption in aqueous NaOH prior to SPME | HS-SPME | CW-DVB | 90 | - | 35% NaCl, pH 2 | - | 3 | 250 | GC-MS | 1.13–4.60 ng/mL | - | [324] |
| Organophosphate triesters | indoor air (lecture room, office) | dynamic SPME | PDMS | 40–90 min / >18 h; air flow rate 10–35 cm/s | 22 | - | - | 2 | 280 | GC-NPD | - | - | [327] |
| Volatile organic compounds | |||||||||||||
| VOC | indoor air | SPME | PDMS-CAR | 10 | RT | - | - | 10 | 300 | GC-MS / GC-FID | - | 122 ± 24 | [331] |
| VOC | indoor air | SPME | PDMS-CAR | 1–45 | - | - | - | 2.5 | 320 | GC-MS | - | - | [332] |
| VOC | air from volcanic and geothermal areas, landfill gas | SPME | PDMS-DVB-CAR | 30 | 20 | - | - | 2 | 230 | GC-MS | - | 78–84 | [323] |
| Odorous compounds | |||||||||||||
| VOC and odorous compounds | air from swine barn, cattle feedlots | SPME | PDMS-CAR | 60 | RT | - | - | 5 | 260 | GC-MS-O | - | - | [320] |
| Volatile carbon, sulfur and nitrogen compounds | air from livestock operations | dynamic SPME | PDMS-DVB-CAR | 5 min / air flow rate 16 cm/s | 20 | - | - | - | 300 | GC-MS | - | - | [328] |
| VOC and odorous compounds | swine barn particulate matter (TPS, PM10, PM2.5, PM1), adsorption on TEOM filters prior to SPME | HS-SPME | PDMS-CAR | 180 | 25 | - | - | 40 | 260 | GC-MS-O | - | - | [333] |
| Odorous compounds | waste gas from fat refinery | SPME | PDMS-CAR | 30 | RT | - | - | 5 | 290 | GC-MS / GC-FID-O | - | - | [102] |
| Odorous compounds | gaseous effluents from production of poultry feather and viscera meal, condensed prior to SPME | HS-SPME | PDMS-DVB-CAR | 20 | 50 | - | - | 4 | 250 | GC-MS | - | - | [325] |
| Volatile organic sulfur compounds | air from different areas of sewage treatment plant | SPME | PDMS-CAR | 45 | 22 /RT | - | - | 2 | 200 | GC-MS | 0.01–0.08 µg/m³ | 75–96 | [322] |
| Synthetic musks | indoor air, adsorption on Tenax TA prior to SPME | HS-SPME | DVB-CAR-PDMS | 20 | 100 | 100 µL acetone | - | 5 | 270 | GC-MS | 0.029–0.380 ng/m³ | 85–103 | [326] |
| Trimethylamine | ambient air | SPME | PDMS-DVB | 10 | 22 / RT | - | - | 3 | 210 | GC-FID | [321] | ||
| Diacetyl | air | SPME | PDMS | 2 | RT | - | - | 1 | 250 | GC-MS | 0.05 ppm | - | [200] |
| Monoterpenes | plant emissions, ambient air | SPME | PDMS-DVB | 20 | 25 / RT | - | - | 5 | 250 | GC-MS | 4–20 ppt | - | [329] |
| Water | |||||||||||||
| Odorous compounds | |||||||||||||
| Earthy-musty odorants | source, product, and tap water from different waterworks | HS-SPME | PDMS-DVB-CAR | 30 | 60 | 25% NaCl | - | 5 | 230 | GC-MS | 0.1–1.3 ng/L | 83–112 | [334] |
| Earthy-musty odorants | tap water, river water, lake water | HS-SPME | PDMS-DVB-CAR | 30 | 90 | - | - | - | - | GC-MS | 0.25–0.61 ng/L | 65–92 | [335] |
| Earthy-musty odorants | tap water, lake water | HS-SPME | PDMS-DVB-CAR | 30 | 50 | 30% NaCl | - | 3 | 265 | GC-MS | 0.32–0.66 ng/L | 86–113 | [336] |
| Odorous trichlorobromo- phenols | tap water, river water | HS-SPME | PDMS-DVB-CAR | 90 | 60 | NaCl 6.5 g/30 mL | di-methyl sulfate / NaOH | 1 | 270 | GC-MS | 0.22–0.95 ng/L | - | [337] |
| Algal taste and odor compounds | lake water | HS-SPME | PDMS-DVB-CAR | 30 | 65 | NaCl | - | 3 | 250 | GC-MS-O | sub to low ppt range | 80–115 | [87] |
| Volatile sulfur compounds | odorous freshwater lakes | HS-SPME | PDMS-CAR | 30 | 45 | - | - | 3 | 250 | GC-FPD | 1.6–93.5 ng/L | 87–112 | [338] |
| Nitro musk fragrances | tap water, wastewater | HS-SPME | PDMS-CAR, PDMS-DVB | 25 | 100 | - | - | 2 | 300 / 270 | GC-μECD | 0.25–3.6 ng/L | 96–108 | [339] |
| Volatile and semivolatile organic compounds | |||||||||||||
| VOC | surface water, wastewater from wastewater treatment plant and from municipal solid-waste treatment plant | HS-SPME | PDMS-CAR | 30 | 50 | 10% NaCl | - | 5 | 280 | GC-MS-MS | 0.005–2 µg/L | 70–120 | [340] |
| Volatile and semivolatile organic compounds | landfill leachate | SBSE | PDMS | 60–120 | RT | - | none, acetate or BSTFA | 30 °C to 280 °C at 60 °C/min, 5 min hold at 280 °C | GC-MS | - | - | [341] | |
| Volatile and semivolatile organic compounds | snow | Di-SPME/HS-SPME | PDMS-DVB | 40 / 180 | RT | - | - | 5 | 250 | GC-MS | 0.11–1.93 µg/L | - | [342] |
| Pesticides | |||||||||||||
| Pyrethroid pesticides | tap water, waste water from sewage treatment plant, run-off water, water from container used for washing oranges | DI-SPME | PDMS | 20 | 50 | 0.02% Na2S2O3, 2% acetone | - | 3 | 280 | GC-MS | 0.9–35 pg/mL | - | [343] |
| Pyrethroid pesticides | groundwater | DI-SPME | PDMS/DVB | 30 | 65 | - | - | 5 | - | LC-PIF-FD | 0.003–0.009 µg/L | 92–109 | [344] |
| Benzimidazole fungicides | seawater, ground water, sewage | DI-SPME | PDMS-CAR | 40 | 60 | 15% NaCl | - | 10 (in metha-nol) | - | HPLC-FD | 0.03–1.30 ng/mL | 81–120 | [345] |
| Organochlorine pesticides | lake water | HS-SPME | PMPVS | 30 | 80 | - | - | 2 | 270 | GC-ECD | 0.84–13.0 ng/L | 72–116 | [346] |
| Organochlorine pesticides | groundwater | DI-SPME | PDMS-DVB-CAR | 45 | RT | - | - | 2 | 260 | GC-ECD | 0.0013–0.45 ng/L | 92–105 | [347] |
| Organochlorine pesticides | seawater | HS-SPME | poly(3,4-ethylene dioxythio-phene) | 15 | 70 | - | - | 2 | 270 | GC-µECD | 0.16–0.84 ng/L | 63–127 | [146] |
| Organochlorine pesticides and metabolites | seawater, sewage, groundwater | DI-SPME | PDMS-DVB, CW/TPR | 40 | 50 /45 | - | - | 8 / 10 | - | HPLC-UV-DAD | 0.3–3.6 ng/mL | 90–104 | [348] |
| Pesticides tebuthiuron and diuron | water | DI-SPME | PA | 50 | - | - | - | 15 | - | LC-DAD | 10–50 µg/L | - | [349] |
| Pesticides | surface and groundwater | DI-SPME | PA | 30 | 50 | - | - | 5 | 280 | GC-MS | 0.02–0.3 ng/mL | 78–109 | [350] |
| Organo-phosphorus pesticides | river water | DI-SPME | sol-gel based amino fiber | 40 | 30 | 20% NaCl | - | 4 | 250 | GC-MS | 0.05–1.0 ng/L | 80–115 | [351] |
| Organochlorine (OCP), organo-phosphorus (OPP), triazine, pyrethroid, and miscellaneous pesticides | groundwater | HS-SPME | PDMS-DVB | 60 | 60 | - | - | - | - | GC-ECD- TSD / GC-MS / GC-MS-MS | <0.1 µg/L | - | [352] |
| PAH and related compounds | |||||||||||||
| PAH | tap water, surface water, underground water, rainwater | HS-SPME | PDMS | 30 | 60 | 10% NaCl | - | 2 | 280 | GC-FID | 0.06–0.5 μg/L | 71–109 | [353] |
| PAH | rainwater, strom water | DI-SPME | PDMS | 60 | 65 | 0.5 M sodium mono-chloro-acetate | - | - | - | GC-MS | 0.001–0.041 µg/L | 72–111 | [354] |
| PAH | wastewater from scrubber of pilot-scale fluidized bed incinerator system | MA-HS-SPME | PDMS-DVB | 30 | 20 | - | - | 5 | 290 | GC-FID | 0.3–1.0 µg/L | 88–103 | [355] |
| PAH | surface waters, leaching waters of contaminated soils | HS-SPME | PA | 60 | 50 | - | - | 2 | 250 | GC-FID | 0.08–0.20 µg/L | - | [356] |
| Hydroxy metabolites of PAH | mini pore water, minimal salts medium, soil extract culture medium | DI-SPME | PA | 40 | 40 | 8% NaCl | BSTFA | 3 | 280 | GC-MS | 0.002–0.134 µg/L | - | [357] |
| Phenols | |||||||||||||
| Volatile phenols | well water, drinking water | HS-SPME | PANI | 60 | 80 | 30% NaCl, pH 2 | - | 5 | 275 | GC-FID | 1.3–12.8 ng/mL | 88–103 | [88] |
| Phenols | real-life water samples | HS-SPME | PANI | 50 | 50 | NaCl, pH 2 | - | 5 | 200 | GC-FID | 0.69–3.7 ng/mL | 69–112 | [147] |
| Phenols | river water, waste water | DI-SPME | oxidized MWCNTs | 30 | 20 | 36% NaCl | - | 3 (in ACN / water 70:30) | RT | HPLC-DAD | 0.25–3.67 ng/L | 86–119 | [138] |
| Phenols and nitrophenols | rainwater | SPME | PA | 40 | RT | NaCl, pH 3.0 | MDBSTFA | 5 | 280 | GC-MS | 0.2–99 μg/L | - | [358] |
| Chlorophenols | landfill leachate | IL-HS-SPME | 1-butyl-3-methylimida-zolium hexafluoro-phosphate | 4 | 25 | pH 2 | - | 4 | 240 | GC-MS | 0.008–0.026 µg/L | 87–99 | [124] |
| Chlorophenols | landfill leachate | purge-assisted HS-SPME | PA | 30 | 75 | - | - | 3 | 130 °C (0.05 min) to 300 °C at 8 °C/s, 4 min hold | GC-MS | 0.1–0.4 pg/mL | 83–114 | [359] |
| Miscellaneous | |||||||||||||
| Organometallic compounds (mercury, lead, tin) | river water, seawater | HS-SPME | PDMS-DVB-CAR | 15 | 40 | - | sodium tetra-ethyl-borate | 2 | 260 | GC-MS-MS | 4–33 ng/L | 50–109 | [360] |
| Organo-phosphorus fire retardants and plasticizers | wastewater, MAE prior to SPME | DI-SPME | PDMS | 30 | 65 | 10% NaCl | - | 0.5 | 250 | GC-ICP-MS / GC-TOF-MS | 29–50 ng/L | 38–43 | [83] |
| Non-halogenated solvents | textile wastewater | HS-SPME | PDMS | 10 | 35 | - | - | 5 | 240 | GC-MS | 0.1–300 µg/L | - | [361] |
| Acetone | seawater | SPME | PDMS-DVB | 30 | RT | NaCl, pH 3.7 | PFBHA | 5 | 250 | GC-MS | 3.0 nM | - | [362] |
| BTEX | waste water | HS-SPME | PDMS-DVB | 1 | RT | 35% NaCl | - | 0.16 | 200 | portable GC-µFID | 0.4–1.4 µg/L | 98–111 | [89] |
| BTEX | groundwater | HS-SPME | PDMS-CAR | 15 | 25 | 267 g/L NaCl | - | 2 | 290 | cryo-trap-GC-MS | 0.01–0.05 ng/L | - | [363] |
| Acrolein | surface water, drinking water | HS-SPME | PDMS-CAR | 50 | 60 | saturated NaCl solution | 2,2,2-trifluo-roethyl-hydra-zine | - | 220 | GC-MS | 0.06 µg/L | 91–104 | [364] |
| Pharmaceutical compounds | wastewater | dSPME | CW-TPR | 30 | 75 | 300 g/L NaCl, pH 3/11 | - | 10 | - | LC-MS-MS | LOQ: 0.005–0.05 µg/L | 89–110 | [90] |
| Endocrine disrupting chemicals and steroid hormones | river water | DI-SPME | PA | 90 | 45 | 10 g/L NaCl 10, pH 5 | BSTFA | 5 | 290 | GC-MS | 0.002–0.378 µg/L | - | [365] |
| Bisphenol A | landfill leachate | HS-SPME | PA | 60 | 25 | 100 g/L NaCl, pH 2 | BSTFA | 5 | 280 | GC-MS | 0.03 µg/L | - | [91] |
| Estrogens | surface water, wastewater | IT-SPME | DVB | 20 cycles à 40 µL (100 µL/min) | - | - | - | - | - | LC-MS-MS | 2.7–11.7 pg/mL | 86–107 | [92] |
| Organic pollutants (pesticides, octyl/nonyl phenols, pentachloro-benzene, PAHs) | wastewater, landfill leachate | DI-SPME | CW-DVB | 45 | RT | NaCl 10% | - | 5 | 250 | GC-TOF-MS | - | - | [366] |
| Organochlorine pesticides and polychlorinated bisphenyls | river water | HS-SPME | PDMS | 60 | 80 | - | - | 5 | 250 | GC-MS-MS | 0.4–26 pg/L | 75–105 | [367] |
| Volatile halogenated hydrocarbons, benzene | groundwater from waste-oil recycling facility | HS-SPDE | PDMS-AC | 15 cycles à 1 mL (50 µL/s) | 60 | 5% NaCl | - | 1 mL (10 µL/s) | 300 | GC-MS | 12–870 ng/L | - | [368] |
| BTEX and halocarbons | contaminated water | HS-SPME | PAC | 20 | 40–45 | 25% NaCl | - | 1 | 260 | GC-FID-ECD | LOQ: 0.01–0.94 µg/L | 88–113 | [369] |
| Chlorobenzene | tap water, river water | HS-SPME | nanofiber coated by electrospun PU | 5 | 30 | 25% NaCl | - | 2 | 200 | GC-MS | 10 ng/L | 94–102 | [370] |
| 1,2-cis-dichloroethylene, trichloroethylene | effluent from soil column experiments | HS-SPME | PDMS | 5 | 25 | - | - | 1 | 250 | GC-FID | 2.4–4.2 µg/L | - | [371] |
| 1,3-dichloro-2-propanol | tap water, river water, paper mill sewage | HS-SPME | PDMS-DVB-CAR | 30 | 25 | NaCl | BSTFA | 5 | 260 | GC-MS-MS | 0.4 ng/mL | 93–103 | [318] |
| Epichlorohydrin | water and sewage | HS-SPME | PDMS-CAR | 15 | 50 | - | - | 1 | 240 | GC-MS, GC-ECD | water: 1.0 ng/L | - | [93] |
| Polybrominated diphenyl ethers | river water, waste water | DI-SPME | MWCNT | 30 | RT | - | - | 2 | 295 | GC-ECD | 3.6–8.6 ng/L | 90–119 | [372] |
| Soil / Sediment and other Solid Matrices | |||||||||||||
| Odorous compounds | |||||||||||||
| VOC and odorous compounds | dairy manure | HS-SPME | PDMS-DVB-CAR | 30 | 30 | - | - | - | 230 | MD-GC-MS-O | - | - | [373] |
| Odorous volatile compounds | compost | HS-SPME | PDMS-CAR | 60 | 20 | - | - | 40 | 250 | GC-MS | 0.06–2.38 ppb | - | [374] |
| Volatile and semivolatile organic compounds | |||||||||||||
| VOC | soil, manure, compost, biochar | HS-SPME | PDMS-DVB-CAR | 20 | 20 | - | - | 5 | 230 | GC-MS | 0.01–310 ng/g | - | [375] |
| VOC | cow slurry | HS-SPME | PDMS-DVB-CAR / PDMS-CAR | 15 | 35 | NaCl | - | 3 | 300 | GC-MS | 0.02–1441 µg/L | - | [376] |
| Volatile and semivolatile organic compounds | urban landfill soil | HS-SPME | PDMS-DVB | 30 | 50 | NaCl | - | - | 250 | GC-MS | - | - | [377] |
| Pesticides | |||||||||||||
| Pyrethroids, organochlorine pesticides | agricultural soils | HS-SPME | PA | 30 | 100 | - | - | 5 | 290 | GC-µECD | 0.004–1.2 ng/g | 71–147 | [378] |
| Pyrethroids | sediment pore water | SPME | PDMS | 20 | - | - | - | 3 | 260 | GC-ECD | - | - | [379] |
| PAH and related compounds | |||||||||||||
| PAH | sediment pore water, sediments | SPME | PDMS | 500 h | 18 | 1 mL 10 mM NaN3 | - | 15 | 50 °C to 250 °C at 200 °C/min | - | - | [380] | |
| PAH | coastal sediments, MAE with acetone prior to SPME | DI-SPME | PDMS | 60 | 60 | - | - | - | 270 | GC-MS | 0.07–0.76 µg/kg | 70–110 | [381] |
| PAH | sand, sediment | CF-HS-SPME | PDMS | 40 | 150 (fiber: 5) | - | - | 2 | 300 | GC-FID | 0.3–3 pg/g | - | [54] |
| Parent and alkyl PAH | sediment pore water | HS-SPME | PDMS | 30 | - | - | - | 5 | 320 | GC-MS | 0.002–0.6 ng/mL | - | [382] |
| Miscellaneous | |||||||||||||
| 4-t-octylphenol, nonylphenol, bisphenol A | activated sludge | nd-SPME | PA | - | 20 | - | - | 5 | 2800 | GC-MS | - | - | [383] |
| Phenols and indoles | cow slurry | HS-SPME | PDMS-DVB-CAR | 15 | 35 | NaCl | MTBSTFA | 9 | 300 | GC-MS | 0.004–707 µg/L | >64 | [94] |
| Polybrominated diphenyl ethers | soil | HS-SPME | sol-gel M-β-CD-OH-TSO | 60 | 95 | methanol | - | 12 | 300 | GC-MS | 13.0–78.3 pg/g | 78–99 | [384] |
| Perfluoro-carboxylic acids | harbour sediments, PFE prior to SPME | HS-SPME | PDMS | 30 | 30 | saturated NaCl solution | boron trifluo-ride | 3 | 300 | GC-MS | 0.5–0.8 ng/g | 99–103 | [95] |
| Organotin compounds | sediment | HS-SPME | PDMS | 30 | 80 | ethanol, pH 5.3 | sodium tetra-ethyl-borate | 1 | 250 | GC-MS | 1.0–6.3 µg/kg | 98–117 | [84] |
| Mono-, di- and tri-butyltin | sediment, extraction with hydrochloric acid/ethanol prior to SPME | HS-SPME | PDMS | 30 | 40 | pH 4 | sodium tetra-ethyl-borate | 2 | 250 | GC-MS-MS | 0.03–1.0 pg/g | - | [96] |
| Cyclopentadienyl-manganese tricarbonyl, (methylcyclo-penta-di-enyl)manganese tricarbonyl | seawater, soil | HS-SPME | PDMS-DVB | 20 | 60 | 5% NaCl | - | 0,25 | 250 | GC-MIP-AED | 0.62–0.65 pg Mn/g | 76–113 | [385] |
| Nitrous oxide | estuarine soils and sediments | HS-SPME | PDMS-CAR | 2 | 50 | - | - | 1 | 200 | GC-MS | 18 ppb | - | 386] |
5.2. Aqueous Samples
5.3. Solid Samples
6. Prospects and Trends
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Nerín, C. Focus on sample handling. Anal. Bioanal. Chem. 2007, 388, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Salafranca, J.; Aznar, M.; Batlle, R. Critical review on recent developments in solventless techniques for extraction of analytes. Anal. Bioanal. Chem. 2009, 393, 809–833. [Google Scholar] [CrossRef] [PubMed]
- Mondello, L.; Costa, R.; Tranchida, P.Q.; Dugo, P.; Presti, M.L.; Festa, S.; Fazio, A.; Dugo, G. Reliable characterization of coffee bean aroma profiles by automated headspace solid phase microextraction-gas chromatography-mass spectrometry with the support of a dual-filter mass spectra library. J. Sep. Sci. 2005, 28, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; Wiley, VCH: New York, NY, USA, 1997. [Google Scholar]
- Lord, H.; Pawliszyn, J. Evolution of solid-phase microextraction technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Batenburg, A.M.; Rosing, E.A.E.; Brinkman, U.A.T. Application of Solid-Phase Micro-Extraction and Comprehensive Two-Dimensional Gas Chromatography (GC x GC) for Flavour Analysis. Chromatographia 2002, 55, 361–367. [Google Scholar] [CrossRef]
- Chai, M.; Pawliszyn, J. Analysis of Environmental Air Samples by Solid-Phase Microextraction and Gas Chromatography/lon Trap Mass Spectrometry. Environ. Sci. Technol. 1995, 29, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kleeberg, K.K.; Dobberstein, D.; Hinrichsen, N.; Müller, A.; Weber, P.; Steinhart, H. Sampling Procedures with Special Focus on Automatization. In Advances in Food Diagnostics, 1st ed.; Nollet, L.M.L., Toldrá, F., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 253–293. [Google Scholar]
- Pawliszyn, J. Method and Device for Solid Phase Microextraction and Desorption. US Patent 1997. [Google Scholar]
- Dietz, C.; Sanz, J.; Cámara, C. Recent developments in solid-phase microextraction coatings and related techniques. J. Chromatogr. A 2006, 1103, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Pillonel, L.; Bosset, J.O.; Tabacchi, R. Rapid Preconcentration and Enrichment Techniques for the Analysis of Food Volatile. A Review. Lebensm.-Wiss. Technol. 2002, 35, 1–14. [Google Scholar] [CrossRef]
- Wardencki, W.; Michulec, M.; Curylo, J. A review of theoretical and practical aspects of solid-phase microextraction in food analysis. Int. J. Food Sci. Technol. 2004, 39, 703–717. [Google Scholar] [CrossRef]
- Alpendurada, M.F. Solid-phase microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [PubMed]
- Buchberger, W.; Zaborsky, P. Sorptive Extraction Techniques for Trace Analysis of Organic Pollutants in the Aquatic Environment. Acta Chim. Slov. 2007, 54, 1–13. [Google Scholar] [CrossRef]
- Ouyang, G.; Pawliszyn, J. Recent developments in SPME for on-site analysis and monitoring. TrAC Trends Anal. Chem. 2006, 25, 692–703. [Google Scholar] [CrossRef]
- Ribeiro, C.; Ribeiro, A.R.; Maia, A.S.; Gonçalves, V.M.; Tiritan, M.E. New Trends in Sample Preparation Techniques for Environmental Analysis. Crit. Rev. Anal. Chem. 2014, 44, 142–185. [Google Scholar] [CrossRef] [PubMed]
- Wardencki, W.; Curyło, J.; Namieśnik, J. Trends in solventless sample preparation techniques for environmental analysis. J. Biochem. Biophys. Methods 2007, 70, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Padrón, M.E.; Afonso-Olivares, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Microextraction Techniques Coupled to Liquid Chromatography with Mass Spectrometry for the Determination of Organic Micropollutants in Environmental Water Samples. Molecules 2014, 19, 10320–10349. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Pawliszyn, J. A critical review in calibration methods for solid-phase microextraction. Anal. Chim. Acta 2008, 627, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Zanjani, M.O.; Mehdinia, A. Electrochemically prepared solid-phase microextraction coatings—A review. Anal. Chim. Acta 2013, 781, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Zanjani, M.O.; Mehdinia, A. A review on procedures for the preparation of coatings for solid phase microextraction. Microchim. Acta 2014, 181, 1169–1190. [Google Scholar] [CrossRef]
- Kumar, A.; Gaurav; Malik, A.K.; Tewary, D.K.; Singh, B. A review on development of solid phase Microextraction fibers by sol–gel methods and their applications. Anal. Chim. Acta 2008, 610, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, Y.; Lin, S. Review: Recent developments of carbon nanotubes hybrid assemblies for sensing. Am. J. Nano Res. Appl. 2015, 3, 23–28. [Google Scholar]
- Moein, M.M.; Said, R.; Bassyouni, F.; Abdel-Rehim, M. Solid Phase Microextraction and Related Techniques for Drugs in Biological Samples. J. Anal. Methods Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, J.S.; Malik, A.K.; Kaur, V.; Schmitt-Kopplin, P. A Review on Solid Phase Micro Extraction—High Performance Liquid Chromatography (SPME-HPLC) Analysis of Pesticides. Crit. Rev. Anal. Chem. 2005, 35, 71–85. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Automated sample preparation using in-tube solid-phase microextraction and its application—A review. Anal. Bioanal. Chem. 2002, 373, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M. Before the injection—Modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef]
- Nerín, C.; Philo, M.R.; Salafranca, J.; Castle, L. Determination of bisphenol-type contaminants from food packaging materials in aqueous foods by solid-phase microextraction–high-performance liquid chromatography. J. Chromatogr. A 2002, 963, 375–380. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Headspace solid-phase microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Tuduri, L.; Desauziers, V.; Fanlo, J.L. A simple calibration procedure for volatile organic compounds sampling in air with adsorptive solid-phase microextraction fibers. Analyst 2003, 128, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namieśnik, J. Current trends in solid-phase microextraction (SPME) fibre coatings. Chem. Soc. Rev. 2010, 39, 4524. [Google Scholar] [CrossRef] [PubMed]
- Namieśnik, J.; Zygmunt, B.; Jastrzebska, A. Application of solid-phase microextraction for determination of organic vapours in gaseous matrices. J. Chromatogr. A 2000, 885, 405–418. [Google Scholar] [CrossRef]
- Souza-Silva, E.A.; Pawliszyn, J. Direct Immersion Solid-Phase Microextraction with Matrix-Compatible Fiber Coating for Multiresidue Pesticide Analysis of Grapes by Gas Chromatography-Time-of-Flight Mass Spectrometry (DI-SPME-GC-ToFMS). J. Agric. Food Chem. 2015, 63, 4464–4477. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, J.S.; Malik, A.K.; Kaur, V.; Schmitt-Kopplin, P. A Review on Solid Phase Micro Extraction—High Performance Liquid Chromatography (SPME-HPLC) Analysis of Pesticides. Crit. Rev. Anal. Chem. 2005, 35, 71–85. [Google Scholar] [CrossRef]
- Spietelun, A.; Marcinkowski, Ł.; Kloskowski, A.; Namieśnik, J. Application of green sample preparation techniques for the isolation, preconcentration and gas chromatographic determination of organic environmental pollutants. In Proceeedings of the 6th Shanghai International Symposium on Analytical Chemistry, Shanghai, China, 16–18 October 2012.
- Gan, J.; Bondarenko, S. Determination of Pesticides in Water. In Analysis of Pesticides in Food and Enviromental Samples; Tadeo, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; Volume 3, pp. 231–256. [Google Scholar]
- Vas, G.; Vékey, K. Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 2004, 39, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Zupančič-Kralj, L. Solid-phase microextraction. TrAC Trends Anal. Chem. 1999, 18, 272–282. [Google Scholar] [CrossRef]
- Ai, J. Solid Phase Microextraction for Quantitative Analysis in Nonequilibrium Situations. Anal. Chem. 1997, 69, 1230–1236. [Google Scholar] [CrossRef]
- Ai, J. Headspace Solid Phase Microextraction. Dynamics and Quantitative Analysis before Reaching a Partition Equilibrium. Anal. Chem. 1997, 69, 3260–3266. [Google Scholar] [CrossRef]
- Eisert, P.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. Anal. Chem. 1997, 69, 3140–3147. [Google Scholar] [CrossRef]
- Globig, D.; Weickhardt, C. Fully automated in-tube solid-phase microextraction for liquid samples coupled to gas chromatography. Anal. Bioanal. Chem. 2005, 381, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Cavaco, C.; Perestrelo, R.; Pereira, J.; Câmara, J.S. Microextraction by Packed Sorbent (MEPS) and Solid-Phase Microextraction (SPME) as Sample Preparation Procedures for the Metabolomic Profiling of Urine. Metabolites 2014, 4, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Kubinec, R.; Berezkin, V.G.; Górová, R.; Addová, G.; Mracnová, H.; Soják, L. Needle concentrator for gas chromatographic determination of BTEX in aqueous samples. J. Chromatogr. B 2004, 800, 295–301. [Google Scholar] [CrossRef]
- Jochmann, M.A. Solventless extraction and enrichment methods for compound-specific isotope analysis. Ph.D. Thesis, University of Tubingen, Germany, 19 December 2006. [Google Scholar]
- Kataoka, H. Recent developments and applications of microextraction techniques in drug analysis. Anal. Bioanal. Chem. 2010, 396, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Jinno, K. Miniaturized sample preparation combined with liquid phase Separations. J. Chromatogr. A 2003, 1000, 53–67. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Quantitative Extraction Using an Internally Cooled Solid Phase Microextraction Device. Anal. Chem. 1995, 67, 34–43. [Google Scholar] [CrossRef]
- Chen, Y.; Pawliszyn, J. Miniaturization and Automation of an Internally Cooled Coated Fiber Device. Anal. Chem. 2006, 78, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.R.; Hosseinzadeh, S.; Pawliszyn, J. New cold-fiber headspace solid-phase microextraction device for quantitative extraction of polycyclic aromatic hydrocarbons in sediment. J. Chromatogr. A 2006, 1124, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Carasek, E.; Pawliszyn, J. Screening of Tropical Fruit Volatile Compounds Using Solid-Phase Microextraction (SPME) Fibers and Internally Cooled SPME Fiber. J. Agric. Food Chem. 2006, 54, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Carasek, E.; Cudjoe, E.; Pawliszyn, J. Fast and sensitive method to determine chloroanisoles in cork using an internally cooled solid-phase microextraction fiber. J. Chromatogr. A 2007, 1138, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mirnaghi, F.S.; Hein, D.; Pawliszyn, J. Thin-Film Microextraction Coupled with Mass Spectrometry and Liquid Chromatography–Mass Spectrometry. Chromatographia 2013, 76, 1215–1223. [Google Scholar] [CrossRef]
- Jiang, R.; Pawliszyn, J. Thin-film microextraction offers another geometry for solid-phase microextraction. TrAC Trends Anal. Chem. 2012, 39, 245–253. [Google Scholar] [CrossRef]
- Koziel, J.A.; Odziemkowski, M.; Pawliszyn, J. Sampling and Analysis of Airborne Particulate Matter and Aerosols Using In-Needle Trap and SPME Fiber Devices. Anal. Chem. 2001, 73, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Fang, F.; Pawliszyn, J. Sampling and determination of volatile organic compounds with needle trap devices. J. Chromatogr. A 2005, 1072, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ueta, I.; Kotera, K.; Ogawa, M.; Wada, H.; Jinno, K. In-needle extraction device designed for gas chromatographic analysis of volatile organic compounds. J. Chromatogr. A 2006, 1106, 190–195. [Google Scholar] [CrossRef] [PubMed]
- SPDETM: The Magic Needle. flyer; CHROMTECH GmbH: Idstein, Germany, 2006. Available online: http://www.chromtech.de/download/20130130160105/CT-ProduktblattSPDE-231205.pdf (accessed on 31 March 2015).
- Ampuero, S.; Bogdanov, S.; Bosset, J.O. Classification of unifloral honeys with an MS-based electronic nose using different sampling modes: SHS, SPME and INDEX. Eur. Food Res. Technol. 2004, 218, 198–207. [Google Scholar] [CrossRef]
- Lipinski, J. Automated solid phase dynamic extraction—Extraction of organics using a wall coated syringe needle. Fresenius. J. Anal. Chem. 2001, 369, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioprocess Technol. 2011, 4, 1–26. [Google Scholar] [CrossRef]
- Nilsson, T.; Ferrari, R.; Facchetti, S. Inter-laboratory studies for the validation of solid-phase microextraction for the quantitative analysis of volatile organic compounds in aqueous samples. Anal. Chim. Acta 1997, 356, 113–123. [Google Scholar] [CrossRef]
- Bene, A.; Luisier, J.L.; Fornage, A. Applicability of a SPME method for the rapid determination of VOCs. Chimia 2002, 56, 289–291. [Google Scholar] [CrossRef]
- Basheer, C.; Lee, H.K. Hollow fiber membrane-protected solid phase microextraction of triazine herbicides in bovine milk and sewage sludge samples. J. Chromatogr. A 2004, 1047, 189–194. [Google Scholar] [CrossRef]
- Razote, E.; Jeon, I.; Maghirang, R.; Chobpattana, W. Dynamic air sampling of volatile organic compounds using solid phase microextraction. J. Environ. Sci. Health Part B 2002, 37, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Razote, E.B.; Maghirang, R.G.; Seitz, L.M.; Jeon, I.J. Characterization of volatile organic compounds on airborne dust in a swine finishing barn. Transac. ASAE 2004, 47, 1231–1238. [Google Scholar] [CrossRef]
- Parreira, F.V.; de Carvalho, C.R.; Cardeal, Z.L. Evaluation of indoor exposition to benzene, toluene, ethylbenzene, xylene and styrene by passive sampling with a solid-phase microextraction device. J. Chromatogr. Sci. 2002, 40, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.M.; Ebeler, S.E. Headspace solid-phase microextraction method for the study of the volatility of selected flavor compounds. J. Agric. Food Chem. 2003, 51, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Solid phase Microextraction. In A century of Separation Science; Issaq, H.J., Ed.; Marcel Dekker, Inc: New York, NY, USA, 2002; pp. 399–419. [Google Scholar]
- Costas-Rodriguez, M.; Pena-Pereira, F. Method Development with Miniaturized Sample Preparation Techniques. In Miniaturization in Sample Preparation; Pena-Pereira, F., Ed.; De Gruyter Open: Warsaw, Poland, 2014; pp. 276–307. [Google Scholar]
- Pawliszyn, J. Handbook of solid phase Microextraction, 1st ed.; Elsevier Inc: Waltham, MA, USA, 2012. [Google Scholar]
- Lord, H.L. Strategies for interfacing solid-phase microextraction with liquid chromatography. J. Chromatogr. A 2008, 1152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Sze, N.; Thomson, B.; Nacson, S.; Pawliszyn, J. Solid phase microextraction with matrix assisted laser desorption/ionization introduction to mass spectrometry and ion mobility spectrometry. Analyst 2002, 127, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D.; Fiamegos, Y.C. Microextraction combined with derivatization. TrAC Trends Anal. Chem. 2008, 27, 533–542. [Google Scholar] [CrossRef]
- Pan, L.; Pawliszyn, J. Derivatization/Solid-Phase Microextraction: New Approach to Polar Analytes. Anal. Chem. 1997, 69, 196–205. [Google Scholar] [CrossRef]
- Martínez-Uruñuela, A.; González-Sáiz, J.M.; Pizarro, C. Multiple solid-phase microextraction in a non-equilibrium situation Application in quantitative analysis of chlorophenols and chloroanisoles related to cork taint in wine. J. Chromatogr. A 2005, 1089, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Careri, M.; Musci, M.; Mangia, A. Fish and food safety: Determination of formaldehyde in 12 fish species by SPME extraction and GC–MS analysis. Food Chem. 2007, 100, 1049–1053. [Google Scholar] [CrossRef]
- Bourdin, D.; Desauziers, V. Development of SPME on-fiber derivatization for the sampling of formaldehyde and other carbonyl compounds in indoor air. Anal. Bioanal. Chem. 2014, 406, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Shah, M.; Kubachka, K.M.; Caruso, J.A. Determination of organophosphorus fire retardants and plasticizers in wastewater samples using MAE-SPME with GC-ICPMS and GC-TOFMS detection. J. Environ. Monit. 2007, 9, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Devos, C.; Vliegen, M.; Willaert, B.; David, F.; Moens, L.; Sandra, P. Automated headspace-solid-phase micro extraction–retention time locked-isotope dilution gas chromatography–mass spectrometry for the analysis of organotin compounds in water and sediment samples. J. Chromatogr. A 2005, 1079, 408–414. [Google Scholar] [CrossRef]
- Mateo-Vivaracho, L.; Ferreira, V.; Cacho, J. Automated analysis of 2-methyl-3-furanthiol and 3-mercaptohexyl acetate at ng L−1 level by headspace solid-phase microextracion with on-fibre derivatisation and gas chromatography–negative chemical ionization mass spectrometric determination. J. Chromatogr. A 2006, 1121, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Koziel, J.A.; Cai, L.; Özsoy, D.H.; Leeuwen, J.H. Quantification of Carbonyl Compounds Generated from Ozone-Based Food Colorants Decomposition Using On-Fiber Derivatization-SPME-GC-MS. Chromatography 2015, 2, 1–18. [Google Scholar] [CrossRef]
- Peter, A.; Köster, O.; Schildknecht, A.; Gunten, U. Occurrence of dissolved and particle-bound taste and odor compounds in Swiss lake waters. Water Res. 2009, 43, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Noroozian, E.; Jalali-Heravi, M.; Mollahosseini, A. Optimization of solid-phase microextraction of volatile phenols in water by a polyaniline-coated Pt-fiber using experimental design. Anal. Chim. Acta 2007, 581, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Deng, C.; Shen, W.; Zhang, X. Field analysis of benzene, toluene, ethylbenzene and xylene in water by portable gas chromatography–microflame ionization detector combined with headspace solid-phase microextraction. Talanta 2006, 69, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Unceta, N.; Sampedro, M.C.; Bakar, N.K.A.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Multi-residue analysis of pharmaceutical compounds in wastewaters by dual solid-phase microextraction coupled to liquid chromatography electrospray ionization ion trap mass spectrometry. J. Chromatogr. A 2010, 1217, 3392–3399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, L.; Zou, S.; Lan, C.; Luan, T. Determination of bisphenol A in landfill leachate by solid phase microextraction with headspace derivatization and gas chromatography-mass spectrophotometry. Chin. J. Anal. Chem. 2006, 34, 325–328. [Google Scholar] [CrossRef]
- Mitani, K.; Fujioka, M.; Kataoka, H. Fully automated analysis of estrogens in environmental waters by in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2005, 1081, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Gaca, J.; Wejnerowska, G. Determination of epichlorohydrin in water and sewage samples. Talanta 2006, 70, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Larreta, J.; Usobiaga, A.; Etxebarria, N.; Arana, G.; Zuloaga, O. Optimisation of the on-fibre derivatisation of volatile fatty acids in the simultaneous determination together with phenols and indoles in cow slurries. Anal. Bioanal. Chem. 2007, 389, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Alzaga, R.; Salgado-Petinal, C.; Jover, E.; Bayona, J.M. Development of a procedure for the determination of perfluorocarboxylic acids in sediments by pressurised fluid extraction, headspace solid-phase microextraction followed by gas chromatographic–mass spectrometric determination. J. Chromatogr. A 2006, 1083, 1–6. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Pinto, L.F.; Clara, M.; Basto, P.; Teresa, M.; Vasconcelos, S.D. Headspace solid-phase micro-extraction and gas chromatography-ion trap tandem mass spectrometry method for butyltin analysis in sediments: Optimization and validation. Microchem. J. 2007, 87, 147–153. [Google Scholar] [CrossRef]
- Quintana, J.B.; Rodriguez, I. Strategies for the microextraction of polar organic contaminants in water samples. Anal. Bioanal. Chem. 2006, 384, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Wu, J.; Xie, W.; Pawliszyn, J. Automated in tube solid-phase microextraction coupled with HPLC–ES–MS for the determination of catechins and caffeine in tea. Analyst 2000, 125, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Górecki, T.; Yu, X.; Pawliszyn, J. Theory of analyte extraction by selected porous polymer SPME fibres. Analyst 1999, 124, 643–649. [Google Scholar] [CrossRef]
- Cai, J.; Liu, B.; Su, Q. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J. Chromatogr. A 2001, 930, 1–7. [Google Scholar] [CrossRef]
- Kleeberg, K.K.; Liu, Y.; Jans, M.; Schlegelmilch, M.; Streese, J.; Stegrnann, R. Development of a simple and sensitive method for the characterization of odorous waste gas emissions by means of solid-phase microextraction (SPME) and GC MS/olfactometry. Waste Manage. 2005, 25, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.P.; Wang, G.R.; Huang, B.Y.; Liu, C.Y. Preparation and application of ionic liquid-coated fused-silica capillary fibers for solid-phase microextraction. Anal. Chim. Acta 2009, 645, 42–47. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pohl, J.; Engel, R.; Rothman, L.; Thomas, M. Preparation of ionic liquid based solid-phase microextraction fiber and its application to forensic determination of methamphetamine and amphetamine in human urine. J. Chromatogr. A 2009, 1216, 4824–4830. [Google Scholar] [CrossRef] [PubMed]
- Mullett, W.M.; Martin, P.; Pawliszyn, J. In-Tube Molecularly Imprinted Polymer Solid-Phase Microextraction for the Selective Determination of Propranolol. Anal. Chem. 2001, 73, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Hofacker, S.; Mechtel, M.; Mager, M.; Kraus, H. Sol–gel: A new tool for coatings chemistry. Prog. Org. Coat. 2002, 45, 159–164. [Google Scholar] [CrossRef]
- Mullett, W.M.; Levsen, K.; Lubda, D.; Pawliszyn, J. Bio-compatible in-tube solid-phase microextraction capillary for the direct extraction and high-performance liquid chromatographic determination of drugs in human serum. J. Chromatogr. A 2002, 963, 325–334. [Google Scholar] [CrossRef]
- Chong, S.; Wang, D.; Hayes, J.; Wilhite, B.; Malik, A. Sol-gel coating technology for the preparation of solid-phase microextraction fibers of enhanced thermal stability. Anal. Chem. 1997, 69, 3889–3898. [Google Scholar] [CrossRef] [PubMed]
- Young, S.K. Sol-Gel Science for Ceramic Materials. Mater. Matt. 2006, 1, 8. [Google Scholar]
- Hu, B.; He, M.; Chen, B. Novel Materials in Solid-Phase Microextraction and Related Sample Preparation Approaches. In Miniaturization in Sample Preparation; Pereira, F.P., Ed.; De Gruyter Open Ltd: Warsaw, Poland; Berlin, Germany, 2014; pp. 88–190. [Google Scholar]
- Djozan, D.; Assadi, Y.; Haddadi, S.H. Anodized Aluminum Wire as a Solid-Phase Microextraction Fiber. Anal. Chem. 2001, 73, 4054–4058. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, Y.; Yuan, D. Electrosorption-enhanced solid-phase microextraction of trace anions using a platinum plate coated with single-walled carbon nanotubes. Talanta 2011, 85, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Lei, N.; Gong, C.; Yu, Y.; Lam, K.; Lam, M.H.; Yu, H.; Lam, P.K. An organically modified silicate molecularly imprinted solid-phase microextraction device for the determination of polybrominated diphenyl ethers. Anal. Chim. Acta 2009, 633, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Q.; Yuan, D. Determination of endocrine-disrupting compounds in water by carbon nanotubes solid-phase microextraction fiber coupled online with high performance liquid chromatography. Talanta 2011, 85, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, S.; Noroozian, E. HP-SPME of Volatile Polycyclic Aromatic Hydrocarbons from Water Using Multiwalled Carbon Nanotubes Coated on a Steel Fiber through Electrophoretic Deposition. Chromatographia 2012, 75, 913–921. [Google Scholar] [CrossRef]
- Zewe, J.W.; Steach, J.K.; Olesik, S.V. Electrospun fibers for solid-phase microextraction. Anal. Chem. 2010, 82, 5341–5348. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Li, T.; Zhao, Y.; Huang, F.-K.; Guo, L.; Feng, Y.-Q. Preparation of a TiO2 nanoparticle-deposited capillary column by liquid phase deposition and its application in phosphopeptide analysis. J. Chromatogr. A 2008, 1192, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Najafi, N.M.; Kharrazi, S. A new solid phase micro extraction for simultaneous head space extraction of ultra traces of polar and non-polar compounds. Anal. Chim. Acta 2011, 689, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Liu, H.; Chen, J.; Zeng, J.; Huang, J.; Gao, L.; Wang, Y.; Chen, X. ZnO nanorod coating for solid phase microextraction and its applications for the analysis of aldehydes in instant noodle samples. J. Chromatogr. A 2012, 1246, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, K.; Matin, A.A.; Amanzadeh, H.; Biparva, P.; Tajik, H.; Farshid, A.A.; Pirkharrati, H. A novel dispersive micro solid phase extraction using zein nanoparticles as the sorbent combined with headspace solid phase micro-extraction to determine chlorophenols in water and honey samples by GC–ECD. Talanta 2014, 128, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Anbia, M.; Khazaei, M. Ordered nanoporous carbon-based SPME and determination by GC. Chromatographia 2011, 73, 379–384. [Google Scholar] [CrossRef]
- Rahimi, A.; Hashemi, P.; Badiei, A.; Arab, P.; Ghiasvand, A.R. CMK-3 nanoporous carbon as a new fiber coating for solid-phase microextraction coupled to gas chromatography–mass spectrometry. Anal. Chim. Acta 2011, 695, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Toledo, B.R.; Hantao, L.W.; Ho, T.D.; Augusto, F.; Anderson, J.L. A chemometric approach toward the detection and quantification of coffee adulteration by solid-phase microextraction using polymericionic liquid sorbent coatings. J. Chomatogr. A 2014, 1346, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Chen, C.Y.; Li, Z.G.; Yang, T.C.C.; Lee, M.R. Determination of chlorophenols in landfill leachate using headspace sampling with ionic liquid-coated solid-phase microextraction fibers combined with gas chromatography–mass spectrometry. Anal. Chim. Acta 2012, 712, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Shi, J.P. Determination of diethylstilbestrol in milk using carbon nanotube-reinforced hollow fiber solid-phase microextraction combined with high-performance liquid chromatography. Talanta 2012, 97, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sarafraz-Yazdi, A.; Abbasian, M.; Amiri, A. Determination of furan in food samples using two solid phase microextraction fibers based on sol–gel technique with gas chromatography–flame ionization detector. Food Chem. 2012, 131, 698–704. [Google Scholar] [CrossRef]
- Attari, S.G.; Bahrami, A.; Shahna, F.G.; Heidari, M. Solid-phase microextraction fiber development for sampling and analysis of volatile organohalogen compounds in air. J. Environ. Health Sci. Eng. 2014, 12, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Shearrow, A.M.; Harris, G.A.; Fang, L.; Sekhar, P.K.; Nguyen, L.T.; Turner, E.B.; Bhansali, S.; Malik, A. Ionic liquid-mediated Sol-gel coatings for capillary microextraction. J. Chomatogr. A 2009, 1216, 5449–5458. [Google Scholar] [CrossRef] [PubMed]
- Shearrow, A.M.; Bhansali, S.; Malik, A. Ionic liquid-mediated bis(3-methyldimethoxysilyl)propyl. polypropylene oxide-based polar Sol-gel coatings for capillary microextraction. J. Chromatogr. A 2009, 1216, 6349–6355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shao, X.; Shu, J.J.; Liu, M.M.; Liu, H.L.; Feng, X.H.; Liu, F. Thermally stable ionic liquid-based Sol-gel coating for ultrasonic extraction–solid-phase microextraction–gas chromatography determination of phthalate esters in agricultural plastic films. Talanta 2012, 89, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, X.; Chen, Y.; Liu, H.; Feng, X.; Qiu, G.; Liu, F.; Zeng, Z. Innovative chemically bonded ionic liquids-based Sol-gel coatings as highly porous, stable and selective stationary phases for solid phase microextraction. Anal. Chim. Acta 2010, 683, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Feng, Y.Q.; Shi, Z.G.; Wang, J.B. Ordered mesoporous silica coated capillary for in-tube solid phase microextraction coupled to high performance liquid chromatography. Anal. Chim. Acta 2005, 543, 1–8. [Google Scholar] [CrossRef]
- Zhu, F.; Liang, Y.; Xia, L.; Rong, M.; Su, C.; Lai, R.; Li, R.; Ouyang, G. Preparation and characterization of vinyl-functionalized mesoporous organosilica-coated solid-phase microextraction fiber. J. Chromatogr. A 2012, 1247, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.; André, L.C.; Cardeal, Z.L. Analysis of plasticiser migration to meat roasted in plastic bags by SPME–GC/MS. Food Chem. 2015, 178, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Es-Haghi, A.; Hosseini, S.M.; Khoshhesab, Z.M. Development and application of a new solid-phase microextraction fiber by Solgel technology on titanium wire. Anal. Chim. Acta 2012, 742, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Gao, Y.L.; Wang, P.P.; Shang, H.; Pan, S.Y.; Li, X.J. Sol-gel molecularly imprinted polymer for selective solid phase microextraction of organophosphorous pesticides. Talanta 2013, 115, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Hu, X.; Wen, J.; Zhou, Q. Robust aptamer Sol-gel solid phase microextraction of very polar adenosine from human plasma. J. Chromatogr. A 2013, 1279, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, Y.; Zhang, Y.; Zhang, H.; Liu, M. Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J. Chromatogr. A 2007, 1165, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Ayazi, Z.; Sistani, H. Chemically bonded carbon nanotubes on modified gold substrate as novel unbreakable solid phase microextraction fiber. Microchim. Acta 2011, 174, 295–301. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; Oliveira, E.B.; Breton, F.; Pawliszyn, J. Immunoaffinity in-tube solid phase microextraction coupled with liquid chromatography–mass spectrometry for analysis of fluoxetine in serum samples. J. Chromatogr. A 2007, 1174, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Pan, J.; Hu, Y.; Huo, Y.; Li, G. Preparation and evaluation of solid-phase microextraction fiber based on molecularly imprinted polymers for trace analysis of tetracyclines in complicated samples. J. Chromatogr. A 2008, 1188, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Djozan, D.; Farajzadeh, M.A.; Sorouraddin, S.M.; Baheri, T. Determination of methamphetamine, amphetamine and ecstasy by inside-needle adsorption trap based on molecularly imprinted polymer followed by GC-FID determination. Microchim. Acta 2012, 179, 209–217. [Google Scholar] [CrossRef]
- Amini, R.; Rouhollahi, A.; Adibi, M.; Mehdinia, A. A novel reusable ionic liquid chemically bonded fused-silica fiber for headspace solid-phase microextraction/gas chromatography-flame ionization detection of methyl tert-butyl ether in a gasoline sample. J. Chromatogr. A 2011, 1218, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Liu, J.F. Development of a solid-phase microextraction fiber by chemical binding of polymeric ionic liquid on a silica coated stainless steel wire. J. Chromatogr. A 2012, 1230, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tamer, U.; Ertas, N.; Udum, Y.A.; Sahin, Y.; Pekmez, K.; Yıldız, A. Electrochemically controlled solid-phase microextraction (EC-SPME) based on overoxidized sulfonated polypyrrole. Talanta 2005, 67, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Banitaba, M.H.; Mohammadi, A.A.; Davarani, S.S.H.; Mehdinia, A. Preparation and evaluation of a novel solid-phase microextraction fiber based on poly(3,4-ethylenedioxythiophene) for the analysis of OCPs in water. Anal. Methods 2011, 3, 2061–2067. [Google Scholar] [CrossRef]
- Bagheri, H.; Babanezhad, A.M.E. An electropolymerized aniline-based fiber coating for solid phase microextraction of phenols from water. Anal. Chim. Acta 2005, 532, 89–95. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Ma, C.; Du, D.; Chen, X. Electropolymerized multiwalled carbon nanotubes/polypyrrole fiber for solid-phase microextraction and its applications in the determination of pyrethroids. Talanta 2011, 84, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Mehdinia, A.; Khani, H.; Mozaffari, S. Fibers coated with a graphene-polyaniline nanocomposite for the headspace solid-phase microextraction of organochlorine pesticides from seawater samples. Microchim. Acta 2014, 181, 89–95. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, M.; Ma, Y.; Zeng, B. Electrochemical preparation of polyaniline–ionic liquid based solid phase microextraction fiber and its application in the determination of benzene derivatives. J. Chromatogr. A 2011, 1218, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, W.; Liu, B.; Liang, F.; He, H.; Yang, S.; Sun, C. Nanostructured polyaniline-ionic liquid composite film coated steel wire for headspace solid-phase microextraction of organochlorine pesticides in water. J. Chromatogr. A 2011, 1218, 6285–6291. [Google Scholar] [CrossRef] [PubMed]
- Mehdinia, A.; Mousavi, M.F.; Shamsipur, M. Nano-structured lead dioxide as a novel stationary phase for solid-phase microextraction. J. Chromatogr. A 2006, 1134, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Budziak, D.; Martendal, E.; Carasek, E. Application of an NiTi alloy coated with ZrO2 solid-phase microextraction fiber for determination of haloanisoles in red wine samples. Microchim. Acta 2009, 164, 197–202. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Ji, L.; Li, J.; Liu, S.; Liu, X.; Jiang, S. A novel TiO2 nanotube array/Ti wire incorporated solid-phase microextraction fiber with high strength, efficiency and selectivity. J. Chromatogr. A 2010, 1217, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Lü, J.; Liu, J.; Jiang, G. In situ fabrication of nanostructured titania coating on the surface of titaniumwire: A new approach for preparation of solid-phase microextraction fiber. Anal. Chim. Acta 2008, 611, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Aghakhani, A.; Baghernejad, M.; Akbarinejad, A. Novel polyamide-based nanofibers prepared by electrospinning technique for headspace solid-phase microextraction of phenol and chlorophenols from environmental samples. Anal. Chim. Acta 2012, 716, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Newsome, T.E.; Zewe, J.W.; Olesik, S.V. Electrospun nanofibrous solid-phase microextraction coatings for preconcentration of pharmaceuticals prior to liquid chromatographic separations. J. Chromatogr. A 2012, 1262, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.W.; Ma, Q.; Feng, Y. Temperature-response polymer coating for in-tube solid-phase microextraction coupled to highperformance. Talanta 2011, 84, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Water Quality–Determination of Selected Plant Treatment Agents and Biocide Products–Method Using Solid-Phase Microextraction (SPME) Followed by Gas Chromatography-Mass Spectrometry (GC-MS); International Organization for Standardization: Genf, Switzerland, ISO 27108:2010.
- Deutsches Institut für Normung. German Standard Methods for the Examination of Water, Waste Water and Sludge–Jointly Determinable Substances (group F)–Part 34: Determination of Selected Plant Treatment Agents, Biocides and Break-Down Products; Method Using Gas Chromatography (GC-MS) after Solid-Phase Micro Extraction (SPME) (F 34); Deutsches Institut für Normung: Berlin, Germany, DIN 38407-34:2006-05.
- Deutsches Institut für Normung. German Standard Methods for the Examination of Water, Waste Water and Sludge–Jointly Determinable Substances (group F)–Part 41: Determination of Selected Easily Volatile Organic Compounds in Water–Method Using Gas Chromatography (GC-MS) after Headspace Solid-Phase Micro Extraction (HS-SPME) (F 41); Deutsches Institut für Normung: Berlin, Germany, DIN 38407-41:2011-06.
- International Organization for Standardization. Water Quality–Determination of Volatile Organic Compounds in Water–Method Using Headspace Solid-Phase Micro-Extraction (HS-SPME) Followed by Gas Chromatography-Mass Spectrometry (GC-MS); International Organization for Standardization: Genf, Switzerland, ISO/DIS 17943:2014-07.
- Austrian Standards Institute. Determination of Volatile Compounds in Cellulose-based Materials by Solid Phase Micro Extraction (SPME); Austrian Standards Insitute: Vienna, Austria, OENORM A 1117: 2004-05-01.
- ASTM International. Standard Test Method for Determination of Parent and Alkyl Polycyclic Aromatics in Sediment Pore Water Using Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry in Selected Ion Monitoring Mode; ASTM International: West Conshohocken, PA, USA, ASTM D 7363a:2013.
- Bagheri, H.; Babanezhad, E.; Khalilian, F. A novel sol–gel-based amino-functionalized fiber for headspace solid-phase microextraction of phenol and chlorophenols from environmental samples. Anal. Chim. Acta 2008, 616, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yu, B.; Chen, W.; Lin, Z.; Zhang, L.; Lin, Z.; Chen, X.; Wang, X. Application of ceramic/carbon composite as a novel coating for solid-phase Microextraction. J. Chromatogr. A 2008, 1188, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Januszkiewicz, J.; Sabik, H.; Azarnia, S.; Lee, B. Optimization of headspace solid-phase microextraction for the analysis of specific flavors in enzyme modified and natural Cheddar cheese using factorial design and response surface methodology. J. Chromatogr. A 2008, 1195, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ravelo-Perez, L.M.; Hernandez-Borges, J.; Borges-Miquel, T.M.; Rodriguez Delgado, M.A. Multiple pesticide analysis in wine by MEKC combined with solid-phase microextraction and sample stacking. Electrophoresis 2007, 28, 4072–4081. [Google Scholar] [CrossRef] [PubMed]
- De Jager, L.S.; Perfetti, G.A.; Diachenko, G.W. Analysis of tetramethylene disulfotetramine in foods using solid-phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2008, 1192, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Optimisation of a complete method for the analysis of volatiles involved in the flavour stability of beer by solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1190, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Medina, I. Solid-phase microextraction method for the determination of volatile compounds associated to oxidation of fish muscle. J. Chromatogr. A 2008, 1192, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plutowska, B.; Wardencki, W. Determination of volatile fatty acid ethyl esters in raw spirits using solid phase microextraction and gas chromatography. Anal. Chim. Acta 2008, 613, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Peldszus, S. Organic Acids. In Chromatographic Analysis of the Environment, 3rd ed.; Nollet, L.M.L, Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 93, pp. 453–512. [Google Scholar]
- Chee, K.K.; Wong, M.K.; Lee, H.K. SPME for the Determination of Organochlorine Pesticides. In Applications of Solid Phase Microextraction; Pawliszyn, J., Ed.; The Royal Society of Chemistry: Cambridge, UK, 1999; pp. 212–226. [Google Scholar]
- Bocchini, P.; Pinelli, F.; Pozzi, R.; Ghetti, F.; Galletti, G.C. Quantitative determination of dimethyl fumarate in silica gel by solid-phase microextraction/gas chromatography/mass spectrometry and ultrasound-assisted extraction/gas chromatography/mass spectrometry. Environ. Monit. Assess. 2015, 187, 320. [Google Scholar] [CrossRef] [PubMed]
- Ezquerro, S.; Pons, B.; Tena, M.T. Direct quantitation of volatile organic compounds in packaging materials by headspace solid-phase microextraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 985, 247–257. [Google Scholar] [CrossRef]
- Wu, C.-M.; Wang, Z. Volatile Compounds in Fresh and Processed Shiitake Mushrooms (Lentinus edodes Sing.). Food Sci. Technol. Res. 2000, 6, 166–170. [Google Scholar] [CrossRef]
- Djozan, D.; Jouyban, A.; Norouzi, J. Ultrasonic assisted SPME coupled with GC and GC-MS using pencil lead as a fiber for monitoring the organic volatile impurities of ceftazidime. J. Chromatogr. Sci. 2008, 46, 680–685. [Google Scholar] [CrossRef] [PubMed]
- McGowin, A.E. Polycyclic Aromatic Hydrocarbons. In Chromatographic Analysis of the Environment, 3rd ed.; Nollet, L.M.L, Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 93, pp. 555–616. [Google Scholar]
- Luukkonen, V. Determination of chlorophenols from water by solid phase microextraction—Ion mobility spectrometry (SPME-IMS). Master’s thesis, Lappeenranta University of Technology, Lappeenranta, Finland, 29 November 2012. [Google Scholar]
- Larroque, V.; Desauziers, V.; Mocho, P. Development of a solid phase microextraction (SPME) method for the sampling of VOC traces in indoor air. J. Environ. Monit. 2006, 8, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Bragg, L.; Ouyang, G.; Pawliszyn, J. Comparison of thin-film microextraction and stir bar sorptive extraction for the analysis of polycyclic aromatic hydrocarbons in aqueous samples with controlled agitation conditions. J. Chromatogr. A 2008, 89, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Hernández, D. Optimization of Multiple Headspace Solid-Phase Microextraction for the Quantification of Volatile Compounds in Dry Fermented Sausages. J. Agric. Food Chem. 2007, 55, 8688–8695. [Google Scholar] [CrossRef] [PubMed]
- Paschke, A.; Vrana, B.; Popp, P.; Schürmann, G. Comparative application of solid-phase microextraction fibre assemblies and semi-permeable membrane devices as passive air samplers for semi-volatile chlorinated organic compounds. A case study on the landfill ‘‘Grube Antonie’’ in Bitterfeld, Germany. Environ. Pollut. 2006, 144, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Zhao, W.; Alaee, M.; Pawliszyn, J. Time-weighted average water sampling with a diffusion-based solid-phase microextraction device. J. Chromatogr. A 2007, 1138, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.; Jia, M.; Pawliszyn, J. Air Sampling with Porous Solid-Phase Microextraction Fibers. Anal. Chem. 2000, 72, 5178–5186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Koziel, Y.A.; Pawliszyn, J. Calibration for On-Site Analysis of Hydrocarbons in Aqueous and Gaseous Samples Using Solid-Phase Microextraction. Anal. Chem. 2003, 75, 6485–6493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ouyang, G.; Alaee, M.; Pawliszyn, J. On-rod standardization technique for time-weighted average water sampling with a polydimethylsiloxane rod. J. Chromatogr. A 2006, 1124, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Bragg, L.; Qin, Z.; Alaee, M.; Pawliszyn, J. Field Sampling with a Polydimethylsiloxane Thin-Film. J. Chromatogr. Sci. 2006, 44, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Cai, J.; Zhang, X.; Li, H.; Pawliszyn, J. Standard-free kinetic calibration for rapid on-site analysis by solid-phase microextraction. J. Sep. Sci. 2008, 31, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.; Pawliszyn, J. Time-weighted average sampling of volatile and semi-volatile airborne organic compounds by the solid-phase Microextraction device. J. Chromatogr. A 2000, 892, 455–467. [Google Scholar] [CrossRef]
- Chen, Y.; O’Reilly, J.; Wang, Y.; Pawliszyn, J. Standards in the extraction phase, a new approach to calibration of Microextraction processes. Analyst 2004, 129, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ouyang, G.; Pawliszyn, J. and application of in-fibre internal standardization solid-phase microextraction. Analyst 2007, 132, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Weurman, S.; van Lunteren, G. Application of gas chromatography to the study of food odours. In Aroma- and Flavour-Producing Substances in Foods; Solms, J., Neukom, S., Eds.; Forster: Zurich, Switzerland, 1967; pp. 21–34. [Google Scholar]
- Rijkens, F.; Boelens, M.H. The future of aroma research. In Aroma Research; Proceedings of the International Symposium on Aroma Research, Central Institute for Nutrition and Food Research TNO, Zeist, Netherlands, 26–29 May 1975; Maarse, H., Groenen, P.J., Eds.; Pudoc: Wageningen, The Netherlands, 1975; pp. 203–222. [Google Scholar]
- Pawliszyn, J. Sample Preparation: Quo Vadis? Anal. Chem. 2003, 75, 2543–2558. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid Phase MICROEXTRACTION; University of Waterloo: Waterloo, Canada, 2007. [Google Scholar]
- Ferreira, S.L.C.; Dos Santos, W.N.L.; Quintella, C.M.; Neto, B.B.; Bosque-Sendra, J.M. Doehlert matrix: A chemometric tool for analytical chemistry—Review. Talanta 2004, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace solid-phase microextraction–gas chromatographic–time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shirey, R.E.; Sidisky, L.M. Determination of Diacetyl in Butter and Air Samples by SPME Coupled with GC–MS. Chromatographia 2010, 72, 999–1004. [Google Scholar] [CrossRef]
- Mallia, S.; Fernández-Garcia, E.; Bosset, J.O. Comparison of purge and trap and solid phase microextraction techniques for studying the volatile aroma compounds of three European PDO hard cheeses. Int. Dairy J. 2005, 15, 741–758. [Google Scholar] [CrossRef]
- Majcher, M.A.; Kaczmarek, A.; Klensporf-Pawlik, D.; Pikul, J.; Jeleń, H.H. SPME-MS-Based Electronic Nose as a Tool for Determination of Authenticity of PDO Cheese, Oscypek. Food Anal. Methods 2015. [Google Scholar] [CrossRef]
- Ocak, E.; Javidipour, I.; Tuncturk, Y. Volatile compounds of Van Herby cheeses produced with raw and pasteurized milks from different species. J. Food Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Tunick, M.H.; Iandola, S.K.; Hekken, D.L. Comparison of SPME Methods for Determining Volatile Compounds in Milk, Cheese, and Whey Powder. Foods 2013, 2, 534–543. [Google Scholar] [CrossRef]
- Xie, J.; Sun, B.; Zheng, F.; Wang, S. Volatile flavor constituents in roasted pork of Mini-pig. Food Chem. 2008, 109, 506–514. [Google Scholar] [CrossRef]
- Yu, A.N.; Sun, B.G.; Tian, D.T.; Qu, W.Y. Analysis of volatile compounds in traditional smoke-cured bacon (CSCB) with different fiber coatings using SPME. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Mallouchos, A.; Panagou, E.Z.; Nychas, G.J.E. The dynamics of the HS/SPME–GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015, 193, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Benet, I.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J.; Roura, E. Analysis of SPME or SBSE extracted volatile compounds from cooked cured pork ham differing in intramuscular fat profiles. Food Sci. Technol. 2015, 60, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Corral, S.; Salvador, A.; Flores, M. Salt reduction in slow fermented sausages affects the generation of aroma active compounds. Meat Sci. 2013, 93, 776–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardini, F.; Tabanelli, G.; Lanciotti, R.; Montanari, C.; Luppi, M.; Coloretti, F.; Chiavari, C.; Grazia, L. Biogenic amine content and aromatic profile of Salama da sugo, a typical cooked fermented sausage produced in Emilia Romagna Region (Italy). Food Control 2013, 32, 638–643. [Google Scholar] [CrossRef]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Câmara, J.S.; Marques, J.C.; Perestrelo, R.M.; Rodrigues, F.; Oliveira, L.; Andrade, P.; Caldeira, M. Comparative study of the whisky aroma profile based on headspace solid phase microextraction using different fibre coatings. J. Chromatogr. A 2007, 1150, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Capobiango, M.; Mastello, R.B.; Chin, S.T.; Oliveira, E.S.; Cardeal, Z.L.; Marriott, P.J. Identification of aroma-active volatiles in banana Terra spirit usingmultidimensional gas chromatography with simultaneous massspectrometry and olfactometry detection. J. Chromatogr. A 2015, 1388, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, T.; Li, W.; Wang, R.; Xiao, D. Analysis of volatile compounds in Chinese Laobaigan liquor using headspace solid-phase microextraction coupled with GC-MS. Anal. Methods 2015, 7, 1906–1913. [Google Scholar] [CrossRef]
- Giraudel, J.L.; Setkova, L.; Pawliszyn, J.; Montury, M. Rapid headspace solid-phase microextraction–gas chromatographic–time-of-flight mass spectrometric method for qualitative profiling of ice wine volatile fraction III. Relative characterization of Canadian and Czech ice wines using self-organizing maps. J. Chromatogr. A 2007, 1147, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Setkova, L.; Risticevic, S.; Pawliszyn, J. Rapid headspace solid-phase microextraction-gas chromatographic–time-of-flight mass spectrometric method for qualitative profiling of ice wine volatile fraction II: Classification of Canadian and Czech ice wines using statistical evaluation of the data. J. Chromatogr. A 2007, 1147, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Jurado, J.M.; Ballesteros, O.; Alcázar, A.; Pablos, F.; Martín, M.J.; Vilchez, J.L.; Navalón, A. Differentiation of certified brands of origins of Spanish white wines by HS-SPME-GC and chemometrics. Anal. Bioanal. Chem. 2008, 390, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhou, X.; Niu, Y.; Yu, D.; Zhu, J.; Zhu, G. Optimization and application of headspace-solid-phasemicro-extraction coupled with gas chromatography–massspectrometry for the determination of volatile compounds in cherrywines. J. Chromatogr. B 2015, 978–979, 122–130. [Google Scholar]
- Wang, X.; Xie, K.; Zhuang, H.; Ye, R.; Fang, Z.; Feng, T. Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem. 2015, 182, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Park, S.K. Volatile aroma and sensory analysis of black raspberry wines fermented by different yeast strains. J. Inst. Brew. 2015, 121, 87–94. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Xiong, B. Preparation of novel solid-phase microextraction fibers by sol–gel technology for headspace solid-phase microextraction-gas chromatographic analysis of aroma compounds in beer. J. Chromatogr. A 2005, 1065, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Braga, C.G.; Prado, A.; Pinto, J.S.; Alencar, S.M. Volatile profile of yellow passion fruit juice by static headspace and solid phase microextraction techniques. Cienc. Rural 2015, 45, 356–363. [Google Scholar] [CrossRef]
- Caven-Quantrill, D.J.; Buglass, A.J. Comparison of micro-scale simultaneous distillation–extraction and stir bar sorptive extraction for the determination of volatile organic constituents of grape juice. J. Chromatogr. A 2006, 1117, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Salmah, Y.; Nazimah, S.A.H.; Tan, C.P. Solid-phase microextraction for headspace analysis of key volatile compounds in orange beverage emulsion. Food Chem. 2007, 105, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Várvölgyi, E.; Gere, A.; Szöllösi, D.; Sipos, L.; Kovács, Z.; Kókai, Z.; Csóka, M.; Mednyánszky, Z.; Fekete, A.; Korány, K. Application of Sensory Assessment, Electronic Tongue and GC–MS to Characterize Coffee Samples. Arab. J. Sci. Eng. 2015, 40, 125–133. [Google Scholar] [CrossRef]
- Barié, N.; Bücking, M.; Stahl, U.; Rapp, M. Detection of coffee flavour ageing by solid-phase microextraction/surface acoustic wave sensor array technique (SPME/SAW). Food Chem. 2015, 176, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S.R.; Pedrozo, M.G.; Miguel, C.P.; Cordeiro, C.S.; Silva, C.F.; Pinheiro, A.C.M.; Schwan, R.F. Inoculation of starter cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Guillot, S.; Peytavi, L.; Bureau, S.; Boulanger, R.; Lepoutre, J.P.; Crouzet, J.; Schorr-Galindo, S. Aroma characterization of various apricot varieties using headspacesolid phase microextraction combined with gas chromatography–mass spectrometry and gas chromatography–olfactometry. Food Chem. 2006, 96, 147–155. [Google Scholar] [CrossRef]
- Solis-Solis, H.M.; Calderón-Santoyo, M.; Schorr-Galindo, S.; Luna-Solano, G.; Ragazzo-Sánchez, J.A. Characterization of aroma potential of apricot varieties using different extraction techniques. Food Chem. 2007, 105, 829–837. [Google Scholar] [CrossRef]
- Gokbulut, I.; Karabulut, I. SPME–GC–MS detection of volatile compounds in apricot varieties. Food Chem. 2012, 132, 1098–1102. [Google Scholar] [CrossRef]
- Ong, B.T.; Nazimah, S.A.H.; Tan, C.P.; Mirhosseini, H.; Osman, A.; Hashim, D.M.; Rusul, G. Analysis of volatile compounds in five jackfruit (Artocarpus heterophyllus L.) cultivars using solid-phase microextraction (SPME) and gas chromatography-time-of-flight mass spectrometry (GC-TOFMS). J. Food Compos. Anal. 2008, 21, 416–422. [Google Scholar] [CrossRef]
- Kralj, M.B.; Jug, T.; Komel, E.; Fajt, N.; Jarni, K.; Živković, J.; Mujić, I. Aromatic compound in different peach cultivars and effect of preservatives on the final aroma of cooked fruits. Hem. Ind. 2014, 68, 767–779. [Google Scholar] [CrossRef]
- Spínola, V.; Perestrelo, R.; Câmara, J.S.; Castilho, P.C. Establishment of Monstera deliciosa fruit volatile metabolomic profile at different ripening stages using solid-phase microextraction combined with gas chromatography–mass spectrometry. Food Res. Int. 2015, 67, 409–417. [Google Scholar] [CrossRef]
- Steingass, C.B.; Carle, R.; Schmarr, H.G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: I. Characterization of pineapple aroma compounds by comprehensive two-dimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2591–2608. [Google Scholar] [CrossRef] [PubMed]
- Vavoura, M.V.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Characterization of Four Popular Sweet Cherry Cultivars Grown in Greece by Volatile Compound and Physicochemical Data Analysis and Sensory Evaluation. Molecules 2015, 20, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cai, J.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS–SPME with GC–MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Wu, B.; Fang, J.; Li, S. Volatile compounds evolution of three table grapes with different flavor during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Thissen, U.; Coulier, L.; Overkamp, K.M.; Jetten, J.; Werff, B.J.C.; Ven, T.; Werf, M.J. A proper metabolomics strategy supports efficient food quality improvement: A case study on tomato sensory properties. Food Qual. Pref. 2011, 22, 499–506. [Google Scholar] [CrossRef]
- Bianchi, F.; Mangia, A.; Mattarozzi, M.; Musci, M. Characterization of the volatile profile of thistle honey using headspace solid-phase microextraction and gas chromatography–mass spectrometry. Food Chem. 2011, 129, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Lušić, D.; Koprivnjak, O.; Ćurić, D.; Sabatini, A.G.; Conte, L.S. Volatile Profile of Croatian Lime Tree (Tilia sp.), Fir Honeydew (Abies alba) and Sage (Salvia officinalis) Honey. Food Technol. Biotechnol. 2007, 45, 156–165. [Google Scholar]
- Moniruzzaman, M.; Rodríguez, I.; Ramil, M.; Cela, R.; Sulaiman, S.A.; Gan, S.H. Assessment of gas chromatography time-of-flight accurate mass spectrometry for identification of volatile and semi-volatile compounds in honey. Talanta 2014, 129, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Akil, E.; Castelo-Branco, V.N.; Costa, A.M.M.; Vendramini, A.L.A.; Calado, V.; Torres, A.G. Oxidative Stability and Changes in Chemical Composition of Extra Virgin Olive Oils After Short-Term Deep-Frying of French Fries. J. Am. Oil Chem. Soc. 2015, 92, 409–421. [Google Scholar] [CrossRef]
- Cecchi, T.; Alfei, B. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME–GC–MS: Newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; García-González, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Validation of SPME–GCMS method for the analysis of virgin olive oil volatiles responsible for sensory defects. Talanta 2015, 134, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Chumpolsri, W.; Wijii, N.; Boontakham, P.; Nimmanpipug, P.; Sookwong, P.; Luangkamin, S.; Wongpornchai, S. Variation of Terpenoid Flavor Odorants in Bran of Some Black and White Rice Varieties Analyzed by GC×GC-MS. J. Food Nutr. Res. 2015, 3, 114–120. [Google Scholar] [CrossRef]
- Griglione, A.; Liberto, E.; Cordero, C.; Bressanello, D.; Cagliero, C.; Rubiolo, P.; Bicchi, C.; Sgorbini, B. High-quality Italian rice cultivars: Chemical indices of ageing and aroma quality. Food Chem. 2015, 172, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Aida, W.M.W.; Maskat, M.Y.; Osman, H. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC-MS) analysis of aroma compound in palm sugar (Arenga pinnata). J. Food Compos. Anal. 2006, 19, 822–830. [Google Scholar] [CrossRef]
- Sanahuja, A.B.; Santonja, M.R.; Teruel, N.G.; Carratalá, M.L.M.; Selva, M.C.G. Classification of Almond Cultivars Using Oil Volatile Compound Determination by HS-SPME–GC–MS. J. Am. Oil Chem. Soc. 2011, 88, 329–336. [Google Scholar] [CrossRef]
- D’Auria, M.; Mauriello, G.; Racioppi, R.; Rana, G.L. Use of SPME–GC–MS in the Study of Time Evolution of the Constituents of Saffron Aroma: Modifications of the Composition During Storage. J. Chromatogr. Sci. 2006, 44, 18–21. [Google Scholar] [CrossRef]
- Kim, N.Y.; Park, M.H.; Jang, E.Y.; Lee, J.H. Volatile Distribution in Garlic (Allium sativum L.) by Solid Phase Microextraction (SPME) with Different Processing Conditions. Food Sci. Biotechnol. 2011, 20, 775–782. [Google Scholar] [CrossRef]
- Fratini, G.; Lois, S.; Pazos, M.; Parisi, G.; Fratini, I.M.G.; Lois, S.; Pazos, M.; Parisi, G.; Medina, I. Volatile profile of Atlantic shellfish species by HS-SPME GC/MS. Food Res. Int. 2012, 48, 856–865. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, C.; Xing, J.; Hu, B. Comparison of headspace and direct single-drop microextraction and headspace solid-phase microextraction for the measurement of volatile sulfur compounds in beer and beverage by gas chromatography with flame photometric detection. J. Chromatogr. A 2006, 1125, 133–137. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.C.; da Silva, A.A.S.; da Silva, L.S.N.; Godoy, R.L.O.; Nogueira, L.C.; Quitério, S.L.; Raices, R.S.L. Method development by GC–ECD and HS-SPME–GC–MS for beer volatile Analysis. Food Chem. 2015, 167, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luesma, E.; Ferreira, V. Quantitative analysis of free and bonded forms of volatile sulfurcompouds in wine. Basic methodologies and evidences showing theexistence of reversible cation-complexed forms. J. Chromatogr. A 2014, 1359, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Tsai, I.M.; Qian, M.C. Analysis of 2-aminoacetophenone by direct-immersion solid-phase microextraction and gas chromatography–mass spectrometry and its sensory impact in Chardonnay and Pinot gris wines. Food Chem. 2007, 105, 1144–1150. [Google Scholar] [CrossRef]
- Gocmen, D.; Elston, A.; Williams, T.; Parish, M.; Rouseff, R.L. Identification of medicinal off-flavours generated by Alicyclobacillus species in orange juice using GC-olfactometry and GC-MS. Lett. Appl. Microbiol. 2005, 40, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Aghakhani, A.; Es-haghi, A. Sol-Gel-based SPME and GC–MS for Trace Determination of Geosmin in Water and Apple Juice Samples. Chromatographia 2007, 66, 779–783. [Google Scholar] [CrossRef]
- Iamanaka, B.T.; Teixeira, A.A.; Teixeira, A.R.R.; Vicente, E.; Frisvad, J.C.; Taniwaki, M.H.; Bragagnolo, N. Potential of volatile compounds produced by fungi to influence sensory quality of coffee beverage. Food Res. Int. 2014, 64, 166–170. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Mildner-Szkudlarz, S.; Jasińska, I.; Wąsowicz, E. A Headspace–SPME–MS Method for Monitoring Rapeseed Oil Autoxidation. J. Am. Oil Chem. Soc. 2007, 84, 509–517. [Google Scholar] [CrossRef]
- Petersen, K.D.; Kleeberg, K.K.; Jahreis, G.; Fritsche, J. Assessment of the oxidative stability of conventional and high-oleic sunflower oil by means of solid-phase microextraction-gas chromatography. Int. J. Food Sci. 2012, 63, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.D.; Kleeberg, K.K.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Comparison of analytical and sensory lipid oxidation parameters in conventional and high-oleic rapeseed oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1193–1203. [Google Scholar] [CrossRef]
- Petersen, K.D.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Chemical and sensory assessment of deep-frying media alternatives for the processing of French fries. Eur. J. Lipid Sci. Technol. 2013, 115, 935–945. [Google Scholar] [CrossRef]
- Beltrán, A.; Ramos, M.; Grané, N.; Martín, M.L.; Garrigós, M.C. Monitoring the oxidation of almond oils by HS-SPME–GC–MS and ATR-FTIR: Application of volatile compounds determination to cultivar authenticity. Food Chem. 2011, 126, 603–609. [Google Scholar] [CrossRef]
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME–GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chem. 2011, 127, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Achouri, A.; Boye, J.I.; Zamani, Y. Identification of volatile compounds in soymilk using solid-phase microextraction-gas chromatography. Food Chem. 2006, 99, 759–766. [Google Scholar] [CrossRef]
- Silcock, P.; Alothman, M.; Zardin, E.; Heenan, S.; Siefarth, C.; Bremer, P.J.; Beauchamp, J. Microbially induced changes in the volatile constituents of fresh chilled pasteurised milk during storage. Food Pack. Shelf Life 2014, 2, 81–90. [Google Scholar] [CrossRef]
- Wold, J.P.; Skaret, J.; Dalsgaard, T.K. Assessment of the action spectrum for photooxidation in full fat bovine milk. Food Chem. 2015, 179, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Mikš-Krajnik, M.; Yoon, Y.J.; Yuk, H.G. Detection of Volatile Organic Compounds as Markers of Chicken Breast Spoilage Using HS-SPME-GC/MS-FASST. Food Sci. Biotechnol. 2014, 24, 361–372. [Google Scholar] [CrossRef]
- Duflos, G.; Moine, F.; Coin, V.M.; Malle, P. Determination of Volatile Compounds in Whiting (Merlangius merlangus) Using Headspace–Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry. J. Chromatogr. Sci. 2005, 43, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.F.; Jauncey, K.; Beveridge, M.C.M.; Lawton, L.A. Depuration rates and the sensory threshold concentration of geosmin responsible for earthy-musty taint in rainbow trout, Onchorhynchus mykiss. Aquaculture 2005, 245, 89–99. [Google Scholar] [CrossRef]
- Vinaixa, M.; Vergara, A.; Duran, C.; Llobet, E.; Badia, C.; Brezmes, J.; Vilanova, J.; Correig, X. Fast detection of rancidity in potato crisps using e-noses based on mass spectrometry or gas sensors. Sens. Actuat. B 2005, 106, 67–75. [Google Scholar] [CrossRef]
- Gallardo-Chacón, J.J.; Karbowiak, T. Sorption of 4-ethylphenol and 4-ethylguaiacol by suberin from cork. Food Chem. 2015, 181, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Pérez-del-Notario, N.; González-Sáiz, J.M. Multiple headspace solid-phase microextraction for eliminating matrix effect in the simultaneous determination of haloanisoles and volatile phenols in wines. J. Chromatogr. A 2007, 1166, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ariza, J.L.; García-Barrera, T.; Lorenzo, F.; Beltrán, R. Use of multiple headspace solid-phase microextraction and pervaporation for the determination of off-flavours in wine. J. Chromatogr. A 2006, 1112, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tait, E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Bacteria detection based on the evolution of enzyme-generated volatile organic compounds: Determination of Listeria monocytogenes in milk samples. Anal. Chim. Acta 2014, 848, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Careri, M.; Mangia, A.; Mattarozzi, M.; Musci, M. Experimental design for the optimization of the extraction conditions of polycyclic aromatic hydrocarbons in milk with a novel diethoxydiphenylsilane solid-phase microextraction fiber. J. Chromatogr. A 2008, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, X.; Yuan, D. Determination of benzimidazole anthelmintics in milk and honey by monolithic fiber-based solid-phase microextraction combined with high-performance liquid chromatography–diode array detection. Anal. Bioanal. Chem. 2015, 407, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Kamalabadi, M.; Ghaemi, E.; Mohammadi, A.; Alizadeh, N. Determination of furfural and hydroxymethylfurfural from baby formula using headspace solid phase microextraction based on nanostructured polypyrrole fiber coupled with ion mobility spectrometry. Food Chem. 2015, 181, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Sagratini, G.; Mañes, J.; Giardiná, D.; Damiani, P.; Picó, Y. Analysis of carbamate and phenylurea pesticide residues in fruit juices by solid-phase microextraction and liquid chromatography–mass spectrometry. J. Chromatogr. A 2007, 1147, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Shin, H.S. Simple Determination of 4-Methylimidazole in Soft Drinks by Headspace SPME GC–MS. Chromatographia 2013, 76, 97–101. [Google Scholar] [CrossRef]
- Zuin, V.G.; Schellin, M.; Montero, L.; Yariwake, J.H.; Augusto, F.; Popp, P. Comparison of stir bar sorptive extraction and membrane-assisted solvent extraction as enrichment techniques for the determination of pesticide and benzoa.pyrene residues in Brazilian sugarcane juice. J. Chromatogr. A 2006, 1114, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Y.; Feng, Y.Q. A simple and rapid method for simultaneous determination of benzoic and sorbic acids in food using in-tube solid-phase microextraction coupled with high-performance liquid chromatography. Anal. Bioanal. Chem. 2007, 388, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Huang, X.; Liao, K.; Yuan, D. Sensitive monitoring of benzoylurea insecticides in water and juice samples treated with multiple monolithic fiber solid-phase microextraction and liquid chromatographic analysis. Anal. Chim. Acta 2015, 860, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Guillot, S.; Kelly, M.T.; Fenet, H.; Larroque, M. Evaluation of solid-phase microextraction as an alternative to the official method for the analysis of organic micro-pollutants in drinking water. J. Chromatogr. A 2006, 1101, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Gong, S.; Chen, M.; Wu, C. Vinyl crown ether as a novel radical crosslinked sol–gel SPME fiber for determination of organophosphorus pesticides in food samples. Anal. Chim. Acta 2006, 559, 89–96. [Google Scholar] [CrossRef]
- Poinot, P.; Qin, F.; Lemoine, M.; Yvon, V.; Ledauphin, J.; Gaillard, J.L. Study of current analytical strategies for the dual investigation of polycyclic aromatic hydrocarbons and benzene, toluene, ethylbenzene and xylene in apples. J. Food Compos. Anal. 2014, 35, 83–93. [Google Scholar] [CrossRef]
- Abdulra’uf, L.B.; Tan, G.H. Chemometric approach to the optimization of HS-SPME/GC–MS for the determination of multiclass pesticide residues in fruits and vegetables. Food Chem. 2015, 177, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Harte, B.R. Solid phase microextraction/gas chromatography/mass spectrometry integrated with chemometrics for detection of Salmonella typhimurium contamination in a packaged fresh vegetable. Anal. Chim. Acta 2007, 581, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.F.; Huang, J.Y.; Zhang, X.N.; Liu, X.J.; Li, X.J. Determination of Polycyclic Aromatic Hydrocarbons in Vegetables by Headspace SPME-GC. Chromatographia 2011, 74, 99–107. [Google Scholar] [CrossRef]
- Sapahin, H.A.; Makahleh, A.; Saad, B. Determination of organophosphorus pesticide residues in vegetables using solid phase micro-extraction coupled with gas chromatography–flame photometric detector. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Dong, C.; Zeng, Z.; Lia, X. Determination of organochlorine pesticides and their metabolites in radish after headspace solid-phase microextraction using calix4.arene fiber. Talanta 2005, 66, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Arisseto, A.P.; Vicente, E.; Ueno, M.S.; Toledo, M.C.F. Occurrence of furan in commercial samples of roasted coffee in Brazil. Food Addit. Contam. A 2012, 29, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Peng, Q.; Chen, Y.; Tang, Q.; Feng, Q. A rapid space-resolved solid-phase microextraction method as a powerful tool to determine contaminants in wine based on their volatility. Food Chem. 2015, 176, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Esteves, C.; Limpo-Faria, A.; Barros, P.; Ribeiro, N.; Simões, T.; Correia, M.; Delerue-Matos, C. Analysis of six fungicides and one acaricide in still and fortified wines using solid-phase microextraction-gas chromatography/tandem mass spectrometry. Food Chem. 2012, 132, 630–636. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Petisca, C.; Henriques, R.; Ferreira, I.M.P.L.V.O. Impact of cooking and handling conditions on furanic compounds in breaded fish products. Food Chem. Toxicol. 2013, 55, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Jaffrès, E.; Lalanne, V.; Macé, S.; Cornet, J.; Cardinal, M.; Sérot, T.; Dousset, X.; Joffraud, J.J. Sensory characteristics of spoilage and volatile compounds associated with bacteria isolated from cooked and peeled tropical shrimps using SPME–GC–MS analysis. Int. J. Food Microbiol. 2011, 147, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Panigrahi, S.; Lin, D.; Logue, C.M.; Sherwood, J.L.; Doetkott, C.; Marchello, M. A comparative qualitative study of the profile of volatile organic compounds associated with Salmonella contamination of packaged aged and fresh beef by HS-SPME/GC-MS. J. Food Sci. Technol. 2011, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Ruiz, J. Analysis of polycyclic aromatic hydrocarbons in solid matrixes by solid-phase microextraction coupled to a direct extraction device. Talanta 2007, 71, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Page, B.D.; Lacroix, G. Application of solid-phase microextraction to the headspace gas chromatographic analysis of halogenated volatiles in selected foods. J. Chromatogr. A 1993, 648, 199–211. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.; D’Souza, L.A. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. CRC Crit. Rev. Food Sci. Nutr. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Sostaric, T.; Boyce, M.C.; Spickett, E.E. Analysis of the volatile components in vanilla extracts and flavorings by solid-phase microextraction and gas chromatography. J. Agric. Food Chem. 2000, 48, 5802–5807. [Google Scholar] [CrossRef] [PubMed]
- Botondi, R.; DeSantis, D.; Bellincontro, A.; Vizovitis, K.; Mencarelli, F. Influence of ethylene inhibition by 1-methyl-cyclopropene on apricot quality, volatile production, and glycosidase activity of lowand high-aroma varieties of apricots. J. Agric. Food Chem. 2003, 51, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Greger, V.; Schieberle, P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J. Agric. Food Chem. 2007, 55, 5221–5228. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.H. Solid-Phase Microextraction in the Analysis of Food Taints and Off-Flavors. J. Chromatogr. Sci. 2006, 44, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Melchert, H.U.; Pabel, E. Reliable identification and quantification of trichothecenes and other mycotoxins by electron impact and chemical ionization-gas chromatography–mass spectrometry, using an ion-trap system in the multiple mass spectrometry mode: Candidate reference method for complex matrices. J. Chromatogr. A 2004, 1056, 195–199. [Google Scholar] [PubMed]
- Chan, D.; MacDonald, S.J.; Boughtflower, V.; Brereton, P. Simultaneous determination of aflatoxins and ochratoxin A in food using a fully automated immunoaffinity column clean-up and liquid chromatography–fluorescence detection. J. Chromatogr. A 2004, 1059, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, A.; Ferracane, R.; Ritieni, A. Ochratoxin A in Italian marketed cocoa products. Food Chem. 2004, 88, 487–494. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A. Esters of okadaic acid and dinophysistoxin-2 in Portuguese bivalves related to human poisonings. Toxicon 1999, 37, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.A.; Rudnick, D.; Williams, E. Mercury burdens in Chinese mitten crabs (Eriocheir sinensis) in three tributaries of southern San Francisco Bay, California, USA. Environ. Pollut. 2005, 133, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Vupputuri, S.; Longnecker, M.P.; Daniels, J.L.; Guo, X.; Sandler, D.P. Blood mercury level and blood pressure among US women: Results from the National Health and Nutrition Examination Survey 1999–2000. Environ. Res. 2005, 97, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Storelli, A.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Mercury speciation in the muscle of two commercially important fish, hake (Merluccius merluccius) and striped mullet (Mullus barbatus) from the Mediterranean sea: estimated weekly intake. Food Chem. 2005, 89, 295–300. [Google Scholar] [CrossRef]
- Meador, J.P.; Ernest, D.W.; Kagley, A.N. A comparison of the non-essential elements cadmium, mercury, and lead found in fish and sediment from Alaska and California. Sci. Total Environ. 2005, 339, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Cubadda, F.; Raggi, A. Determination of cadmium, lead, iron, nickel and chromium in selected food matrices by plasma spectrometric techniques. Microchem. J. 2005, 79, 91–96. [Google Scholar] [CrossRef]
- Zhao, F.J.; Adams, M.L.; Dumont, C.; McGrath, S.P.; Chaudri, A.M.; Nicholson, F.A.; Chambers, B.J.; Sinclair, A.H. Factors affecting the concentrations of lead in British wheat and barley grain. Environ. Pollut. 2004, 131, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C.G.; Pineiro, C.; Perez-Martin, R.I. Denaturation of fish proteins during frozen storage: role of formaldehyde. Lebensm. Unters. For. 1995, 200, 14–23. [Google Scholar] [CrossRef]
- Badii, F.; Howell, N.K. Changes in the texture and structure of cod and haddock fillets during frozen storage. Food Hydrocol. 2002, 16, 313–319. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Corradini, C.; Musci, M.; Mangia, A. Innovative Method for Ultratrace Determination of Formaldehyde in Frozen Fish: SPME Extraction and GC-ITMS/MS Analysis. Curr. Anal. Chem. 2005, 1, 129–134. [Google Scholar] [CrossRef]
- Carro, A.M.; González, P.; Fajar, N.M.; Lorenzo, R.A.; Cela, R. Solid-phase micro-extraction procedure for the determination of 1,3-dichloro-2-propanol in water by on-fibre derivatisation with bis(trimethylsilyl) trifluoroacetamide. Anal. Bioanal. Chem. 2009, 394, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Tumbiolo, S.; Gal, J.-F.; Maria, P.-C.; Zerbinati, O. SPME sampling of BTEX before GC/MS analysis: examples of outdoor and indoor air quality measurements in public and private sites. Ann. Chim. 2005, 95, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.A.; Cai, L.; Wright, D.W.; Hoff, S.J. Solid-Phase Microextraction as a Novel Air Sampling Technology for Improved, GC–Olfactometry-Based Assessment of Livestock Odors. J. Chromatogr. Sci. 2006, 44, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, D. A combination of Tedlar bag sampling and solid-phase microextraction for the analysis of trimethylamine in air: Relationship between concentration level and sample size. Microchem. J. 2009, 91, 16–20. [Google Scholar] [CrossRef]
- Ras, M.R.; Marcé, R.M.; Borrull, F. Solid-phase microextraction—Gas chromatography to determine volatile organic sulfur compounds in the air at sewage treatment plants. Talanta 2008, 77, 774–778. [Google Scholar] [CrossRef]
- Tassi, F.; Capecchiacci, F.; Buccianti, A.; Vaselli, O. Sampling and analytical procedures for the determination of VOCs released into air from natural and anthropogenic sources: A comparison between SPME (Solid Phase Micro Extraction) and ST (Solid Trap) methods. Appl. Chem. 2012, 27, 115–123. [Google Scholar] [CrossRef]
- Conde, F.J.; Afonso, A.M.; González, V.; Ayala, J.H. Optimization of an analytical methodology for the determination of alkyl- and methoxy-phenolic compounds by HS-SPME in biomass smoke. Anal. Bioanal. Chem. 2006, 385, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Krüger, R.L.; Dallago, R.M.; do Nascimento Filho, I.; Di Luccio, M. Study of odor compounds in gaseous effluents generated during production of poultry feather and viscera meal using headspace solid phase microextraction. Environ. Monit. Assess. 2009, 158, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Garcia-Jares, C.; Llompart, M.; Lamas, J.P.; Cela, R. Development of a method based on sorbent trapping followed by solid-phase microextraction for the determination of synthetic musks in indoor air. J. Chromatogr. A 2009, 1216, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Isetun, S.; Nilsson, U. Dynamic field sampling of airborne organophosphate triesters using solidphase microextraction under equilibrium and non-equilibrium conditions. Analyst 2005, 130, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.N.; Woodbury, B.L. A solid-phase microextraction chamber method for analysis of manure volatiles. J. Environ. Qual. 2006, 35, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Yassaa, N.; Custer, T.; Song, W.; Pech, F.; Kesselmeier, J.; Williams, J. Quantitative and enantioselective analysis of monoterpenes from plant chambers and in ambient air using SPME. Atmos. Meas. Tech. 2010, 3, 1615–1627. [Google Scholar] [CrossRef]
- Menezes, H.C.; Cardeal, Z.L. Determination of polycyclic aromatic hydrocarbons from ambient air particulate matter using a cold fiber solid phase microextraction gas chromatography–mass spectrometry method. J. Chromatogr. A 2011, 1218, 3300–3305. [Google Scholar] [CrossRef] [PubMed]
- Hippelein, M. Analysing selected VVOCs in indoor air with solid phase microextraction (SPME): A case study. Chemosphere 2006, 65, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Mocho, P.; Nicolle, J.; Desauziers, V. Modelling of adsorption kinetics and calibration curves of gaseous volatile organic compounds with adsorptive solid-phase microextraction fibre: Toluene and acetone for indoor air applications. Anal. Bioanal. Chem. 2008, 392, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Koziel, J.A.; Lo, Y.C.; Hoff, S.J. Characterization of volatile organic compounds and odorants associated with swine barn particulate matter using solid-phase microextraction and gas chromatography–mass spectrometry–olfactometry. J. Chromatogr. A 2006, 1102, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Peng, S.; Xia, W.; Zheng, W.; Chen, X.; Yin, L. Analysis of Five Earthy-Musty Odorants in Environmental Water by HS-SPME/GC-MS. Int. J. Anal. Chem. 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, J.N.; Zhao, M.; He, Y.J. Accurate analysis of trace earthy-musty odorants in water by headspace solid phase microextraction gas chromatography-mass spectrometry. J. Sep. Sci. 2012, 35, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.H.; Li, T.Y.; Huang, S.D. Analysis of earthy and musty odors in water samples by solid-phase microextraction coupled with gas chromatography/ion trap mass spectrometry. Talanta 2005, 65, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Ventura, F.; Galceran, M.T. Analysis of odorous trichlorobromophenols in water by in-sample derivatization/solid-phase microextraction GC/MS. Anal. Bioanal. Chem. 2006, 386, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Fan, C.; Shang, J.; Deng, J.; Yin, H. Headspace solid-phase microextraction for the determination of volatile sulfur compounds in odorous hyper-eutrophic freshwater lakes using gas chromatography with flame photometric detection. Microchem. J. 2012, 104, 26–32. [Google Scholar] [CrossRef]
- Polo, M.; Garcia-Jares, C.; Llompart, M.; Cela, R. Optimization of a sensitive method for the determination of nitro musk fragrances in waters by solid-phase microextraction and gas chromatography with micro electron capture detection using factorial experimental design. Anal. Bioanal. Chem. 2007, 388, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Cervera, M.I.; Beltran, J.; Lopez, F.J.; Hernandez, F. Determination of volatile organic compounds in water by head space-solid-phase microextraction gas chromatography coupled to tandem mass spectrometry with triple quadrupole analyzer. Anal. Chim. Acta 2011, 704, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Badoil, L.; Benanou, D. Characterization of volatile and semivolatile compounds in waste landfill leachates using stir bar sorptive extraction–GC/MS. Anal. Bioanal. Chem. 2009, 393, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Kos, G.; Ariya, P.A. Determination of a wide range of volatile and semivolatile organic compounds in snow by use of solid-phase micro-extraction (SPME). Anal. Bioanal. Chem. 2006, 385, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Casas, V.; Llompart, M.; Garcia-Jares, C.; Cela, R.; Dagnac, T. Effects of sample pretreatment and storrage conditions in the determination of pyrethroids in water samples by solid-phase microextraction and gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2007, 387, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, P.P.; Mughari, A.R.; Galera, M.M. Application of solid-phase microextraction for determination of pyrethroids in groundwater using liquid chromatography with post-column photochemically induced fluorimetry derivatization and fluorescence detection. J. Chromatogr. A 2008, 1188, 61–68. [Google Scholar] [CrossRef] [PubMed]
- López Monzón, A.; Vega Moreno, D.; Torres Padron, M.E.; Sosa Ferrera, Z.; Santana Rodriguez, J.J. Solid-phase microextraction of benzimidazole fungicides in environmental liquid samples and HPLC-fluorescence determination. Anal. Bioanal. Chem. 2007, 387, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zeng, Z.; Yang, M. Determination of organochlorine pesticides and their derivations in water after HS-SPME using polymethylphenylvinylsiloxane-coated fiber by GC-ECD. Water Res. 2005, 39, 4204–4210. [Google Scholar] [CrossRef] [PubMed]
- Raposo, J.L., Jr.; Ré-Poppi, N. Determination of organochlorine pesticides in ground water samples using solid-phase microextraction by gas chromatography-electron capture detection. Talanta 2007, 72, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Padrón, M.E.T.; Ferrera, Z.S.; Rodríguez, J.J.S. Optimisation of solid-phase microextraction coupled to HPLC-UV for the determination of organochlorine pesticides and their metabolites in environmental liquid samples. Anal. Bioanal. Chem. 2006, 386, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Brondi, S.H.G.; Rodrigues da Silva, J.C.; Lanças, F.M. Development and validation of a methodology for the determination of pesticides in water by SPME–LC/DAD. J. Liquid Chromatogr. Rel. Technol. 2005, 28, 2909–2919. [Google Scholar] [CrossRef]
- Filho, A.M.; Neves dos Santos, F.; Pereira, P.A.P. Development, validation and application of a method based on DI-SPME and GC–MS for determination of pesticides of different chemical groups in surface and groundwater samples. Microchem. J. 2010, 96, 139–145. [Google Scholar] [CrossRef]
- Bagheri, H.; Ayazi, Z.; Babanezhad, E. A sol-gel-based amino functionalized fiber for immersed solid-phase microextraction of organophosphorus pesticides from environmental samples. Microchem. J. 2010, 94, 1–6. [Google Scholar] [CrossRef]
- GonÇalves, C.M.; Esteves da Silva, J.C.G.; Alpendurada, M.F. Evaluation of the Pesticide Contamination of Groundwater Sampled over Two Years from a Vulnerable Zone in Portugal. J. Agric. Food Chem. 2007, 55, 6227–6235. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Ferreira, C.; Almeida, C.M.M. Analysis of Polynuclear Aromatic Hydrocarbons by SPME-GC-FID in Environmental and Tap Waters. J. Braz. Chem. Soc. 2008, 19, 1084–1097. [Google Scholar] [CrossRef]
- Rianawati, E.; Balasubramanian, R. Optimization and validation of solid phase micro-extraction (SPME) method for analysis of polycyclic aromatic hydrocarbons in rainwater and stormwater. Phys. Chem. Earth 2009, 34, 857–865. [Google Scholar] [CrossRef]
- Wei, M.C.; Jen, J.F. Determination of polycyclic aromatic hydrocarbons in aqueous samples by microwave assisted headspace solid-phase microextraction and gas chromatography/flame ionization detection. Talanta 2007, 72, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Zuazagoitia, D.; Millán, E.; Garcia, R. A Screening Method for Polycyclic Aromatic Hydrocarbons Determination in Water by Headspace SPME with GC-FID. Chromatographia 2007, 66, 773–777. [Google Scholar] [CrossRef]
- Luan, T.; Fang, S.; Zhong, Y.; Lin, L.; Chan, S.M.N.; Lan, C.; Tam, N.F.Y. Determination of hydroxy metabolites of polycyclic aromatic hydrocarbons by fully automated solid-phase microextraction derivatization and gas chromatography–mass spectrometry. J. Chromatogr. A 2007, 1173, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Jaber, F.; Schummer, C.; Chami, J.A.; Mirabel, P.; Millet, M. Solid-phase microextraction and gas chromatography–mass spectrometry for analysis of phenols and nitrophenols in rainwater, as their t-butyldimethylsilyl derivatives. Anal. Bioanal. Chem. 2007, 387, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.P.; Lee, R.J.; Lee, M.R. Purge-assisted headspace solid-phase microextraction combined with gas chromatography–mass spectrometry for determination of chlorophenols in aqueous samples. J. Chromatogr. A 2008, 1213, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Beceiro-González, E.; Guimaraes, A.; Alpendurada, M.F. Optimisation of a headspace-solid-phase micro-extraction method for simultaneous determination of organometallic compounds of mercury, lead and tin in water by gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 5563–5569. [Google Scholar] [CrossRef] [PubMed]
- López-Grimau, V.; Guadayol, J.M.; Griera, J.A.; Gutiérrez, M.C. Determination of non halogenated solvents in industrial wastewater using solid phase microextraction (SPME) and GC-MS. Lat. Am. Appl. Res. 2006, 36, 45–55. [Google Scholar]
- Hudson, E.D.; Okuda, K.; Ariya, P.A. Determination of acetone in seawater using derivatization solid-phase microextraction. Anal. Bioanal. Chem. 2007, 388, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Chang, C.M.; Jianpeng, D. Determination of benzene, toluene, ethylbenzene, xylenes in water at sub-ng l_1 levels by solid-phase microextraction coupled to cryo-trap gas chromatography–mass spectrometry. Chemosphere 2007, 69, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Shin, H.S. Simple Determination of Acrolein in Surface and Drinking Water by Headspace SPME GC–MS. Chromatographia 2012, 75, 943–948. [Google Scholar] [CrossRef]
- Yang, L.; Luan, T.; Lan, C. Solid-phase microextraction with on-fiber silylation for simultaneous determinations of endocrine disrupting chemicals and steroid hormones by gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1104, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Portolés, T.; Pitarch, E.; López, F.J. Target and Nontarget Screening of Organic Micropollutants in Water by Solid-Phase Microextraction Combined with Gas Chromatography/High-Resolution Time-of-Flight Mass Spectrometry. Anal. Chem. 2007, 79, 9494–9504. [Google Scholar] [CrossRef] [PubMed]
- Derouiche, A; Driss, M.R.; Morizur, J.P.; Taphanel, M.H. Simultaneous analysis of polychlorinated biphenyls and organochlorine pesticides in water by headspace solid-phase microextraction with gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1138, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Jochmann, M.A.; Yuan, Y.; Schmidt, T.C. Determination of volatile organic hydrocarbons in water samples by solid-phase dynamic extraction. Anal. Bioanal. Chem. 2007, 387, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Shutao, W.; Yan, W.; Hong, Y.; Jie, Y. Preparation of a Carbon-Coated SPME Fiber and Application to the Analysis of BTEX and Halocarbons in Water. Chromatographia 2006, 63, 365–371. [Google Scholar] [CrossRef]
- Bagheri, H.; Aghakhani, A. Novel nanofiber coatings prepared by electrospinning technique for headspace solid-phase microextraction of chlorobenzenes from environmental samples. Anal. Methods 2011, 3, 1284–1289. [Google Scholar] [CrossRef]
- Avila, M.A.S.; Breiter, R.; Mott, H. Development of a simple, accurate SPME-based method for assay of VOCs in column breakthrough experiments. Chemosphere 2007, 66, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Jiang, D.Q.; Gu, Z.Y.; Yan, Y.P. Multiwalled carbon nanotubes coated fibers for solid-phase microextraction of polybrominated diphenyl ethers in water and milk samples before gas chromatography with electron-capture detection. J. Chromatogr. A 2006, 1137, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Laor, Y.; Koziel, J.A.; Cai, L.; Ravid, U. Chemical-Sensory Characterization of Dairy Manure Odor Using Headspace Solid-Phase Microextraction and Multidimensional Gas Chromatography Mass Spectrometry-Olfactometry. J. Air Waste Manage. Assoc. 2008, 58, 1187–1197. [Google Scholar] [CrossRef]
- Kim, H.; McConnell, L.L.; Millner, P. Comparison of odorous volatile compounds from fourteen different commercial composts using solid-phase microextraction. Trans. ASAE 2005, 48, 315–320. [Google Scholar] [CrossRef]
- Higashikawa, F.S.; Cayuela, M.L.; Roig, A.; Silva, C.A.; Sanchez-Monedero, M.A. Matrix effect on the performance of headspace solid phase microextraction method for the analysis of target volatile organic compounds (VOCs) in environmental samples. Chemosphere 2013, 93, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Larreta, J.; Vallejo, A.; Bilbao, U.; Alonso, A.; Arana, G.; Zuloaga, O. Experimental design to optimise the analysis of organic volatile compounds in cow slurry by headspace solid-phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1136, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Banar, M.; Özkan, A.; Vardar, C. Characterization of an urban landfill soil by using physicochemical analysis and solid phase microextraction (SPME)—GC/MS. Environ. Monit. Assess. 2007, 127, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Cela, R.; Dagnac, T. Simultaneous determination of traces of pyrethroids, organochlorines and other main plant protection agents in agricultural soils by headspace solid-phase microextraction–gas chromatography. J. Chromatogr. Sci. 2008, 1188, 154–163. [Google Scholar] [CrossRef]
- Bondarenko, S.; Spurlock, F.; Gan, J. Analysis of Pyrethroids in sediment pore water by solid-phase microextraction. Environ. Toxicol. Chem. 2007, 26, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Witt, G.; Liehr, G.A.; Borck, D.; Mayer, P. Matrix solid-phase microextraction for measuring freely dissolved concentrations and chemical activities of PAHs in sediment cores from the western Baltic Sea. Chemosphere 2009, 74, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.J.; Ferreira, P.C.; Reis, P.A.; Cruzeiro, C.; Rocha, E. Determination of Polycyclic Aromatic Hydrocarbons in Coastal Sediments from the Porto Region (Portugal) by Microwave-Assisted Extraction, Followed by SPME and GC–MS. J. Chromatogr. Sci. 2011, 49, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, S.B.; Grabanski, C.B.; Miller, D.J.; Kreitinger, J.P. Solid-phase microextraction measurement of parent and alkyl polycyclic aromatic hydrocarbons in milliliter sediment pore water samples and determination of KDOC values. Environ. Sci. Technol. 2005, 39, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Huang, J.; Yu, G. Measurement of the free concentrations of alkyl phenols and bisphenol A to determine their biodegradation kinetics by activated sludge. Chin. Sci. Bull. 2007, 52, 2766–2770. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, F.; Cha, D.; Zeng, Z.; Xu, Y. Headspace solid-phase microextraction with novel sol–gel permethylated-ß-cyclodextrin/hydroxyl-termination silicone oil fiber for determination of polybrominated diphenyl ethers by gas chromatography–mass spectrometry in soil. Talanta 2007, 73, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Peñalver, R.; Hernández-Córdoba, M. Solid-phase microextraction combined with gas chromatography and atomic emission detection for the determination of cyclopentadienylmanganese tricarbonyl and (methylcyclopentadienyl)manganese tricarbonyl in soils and seawaters. J. Chromatogr. A 2007, 1173, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Drescher, S.R.; Brown, S.D. Solid phase microextraction-gas chromatographic-mass spectrometric determination of nitrous oxide evolution to measure denitrification in estuarine soils and sediments. J. Chromatogr. A 2006, 1133, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Bicchi, C.; Cordero, C.; Liberto, E.; Rubiolo, P.; Sgorbini, B. Automated headspace solid-phase dynamic extraction to analyse the volatile fraction of food matrices. J. Chromatogr. A 2004, 1024, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Grosch, W. Evaluation of the Key Odorants of Foods by Dilution Experiments, Aroma Models and Omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P. New Developments in Methods for Analysis of Volatile Flavor Compounds and their Precursors. In Characterization of Food: Emerging Methods; Goankar, A.G., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1995; pp. 403–433. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293-381. https://doi.org/10.3390/chromatography2030293
Merkle S, Kleeberg KK, Fritsche J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography. 2015; 2(3):293-381. https://doi.org/10.3390/chromatography2030293
Chicago/Turabian StyleMerkle, Sybille, Kim Karen Kleeberg, and Jan Fritsche. 2015. "Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review" Chromatography 2, no. 3: 293-381. https://doi.org/10.3390/chromatography2030293






