Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
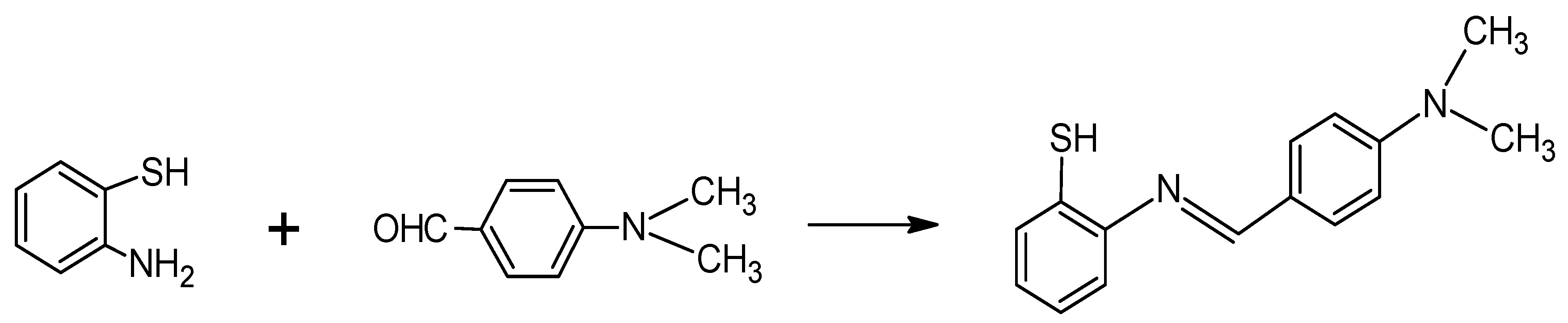
2.2. Preparation of 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene
2.3. UV–Visible Absorption Spectra for SL1
2.4. Detection of Metal Ions with SL1—Competition Experiments
2.4.1. UV–Visible Titration Experiment
2.4.2. Job Plot Quantification
3. Results and Discussion
3.1. Preparation of the Schiff Base Sensor Compound
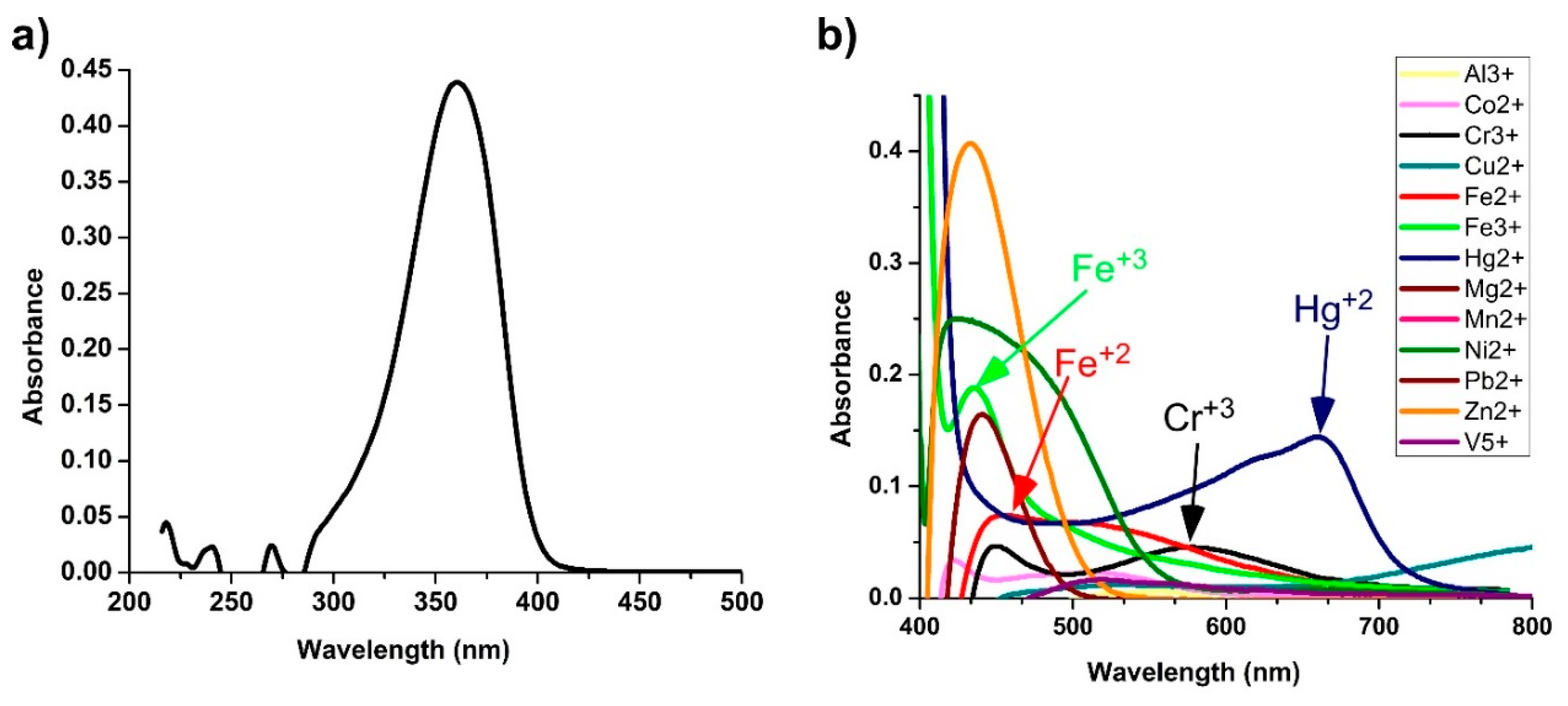
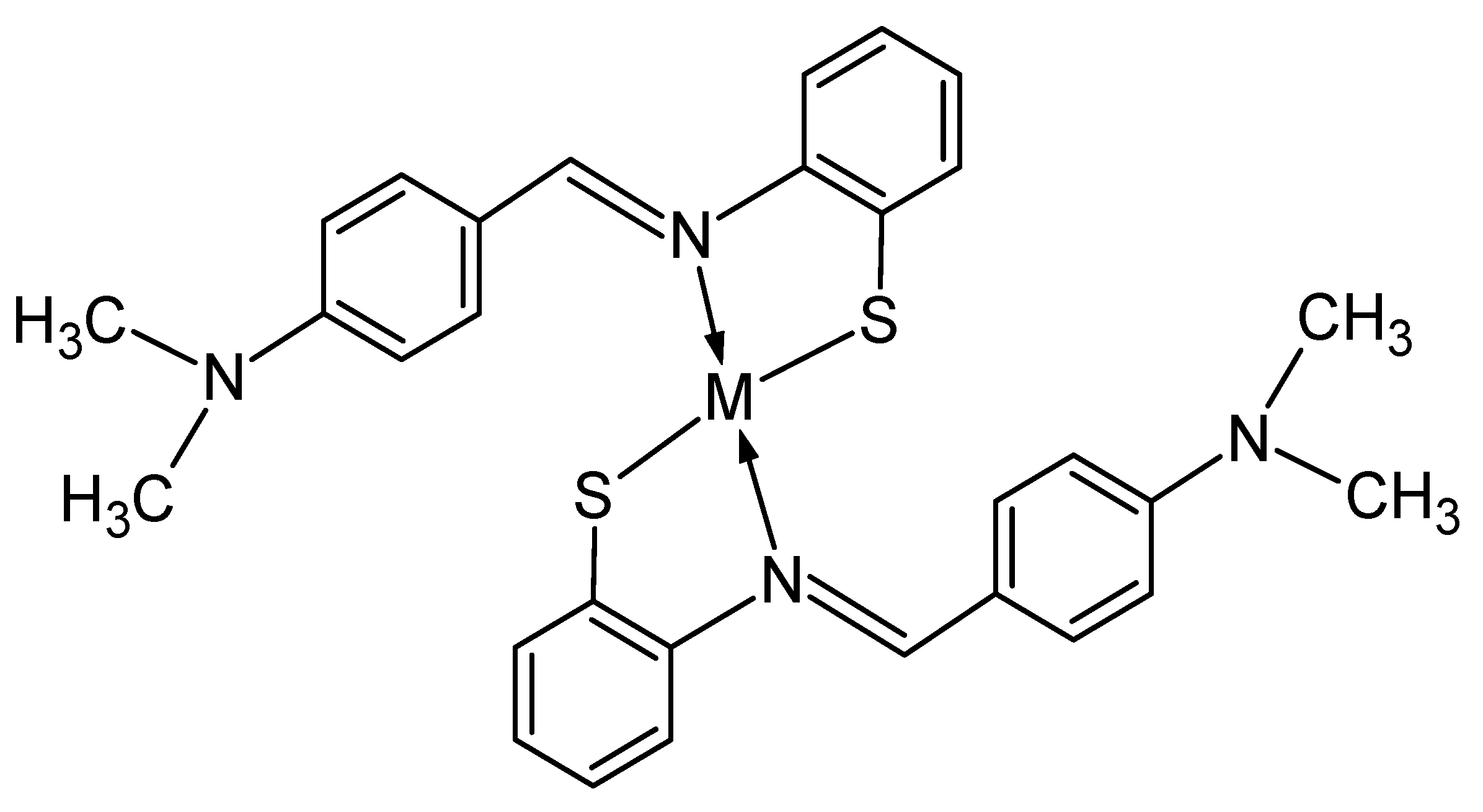
3.2. Transition Metal Ions Sensing with Colorimetry Analysis
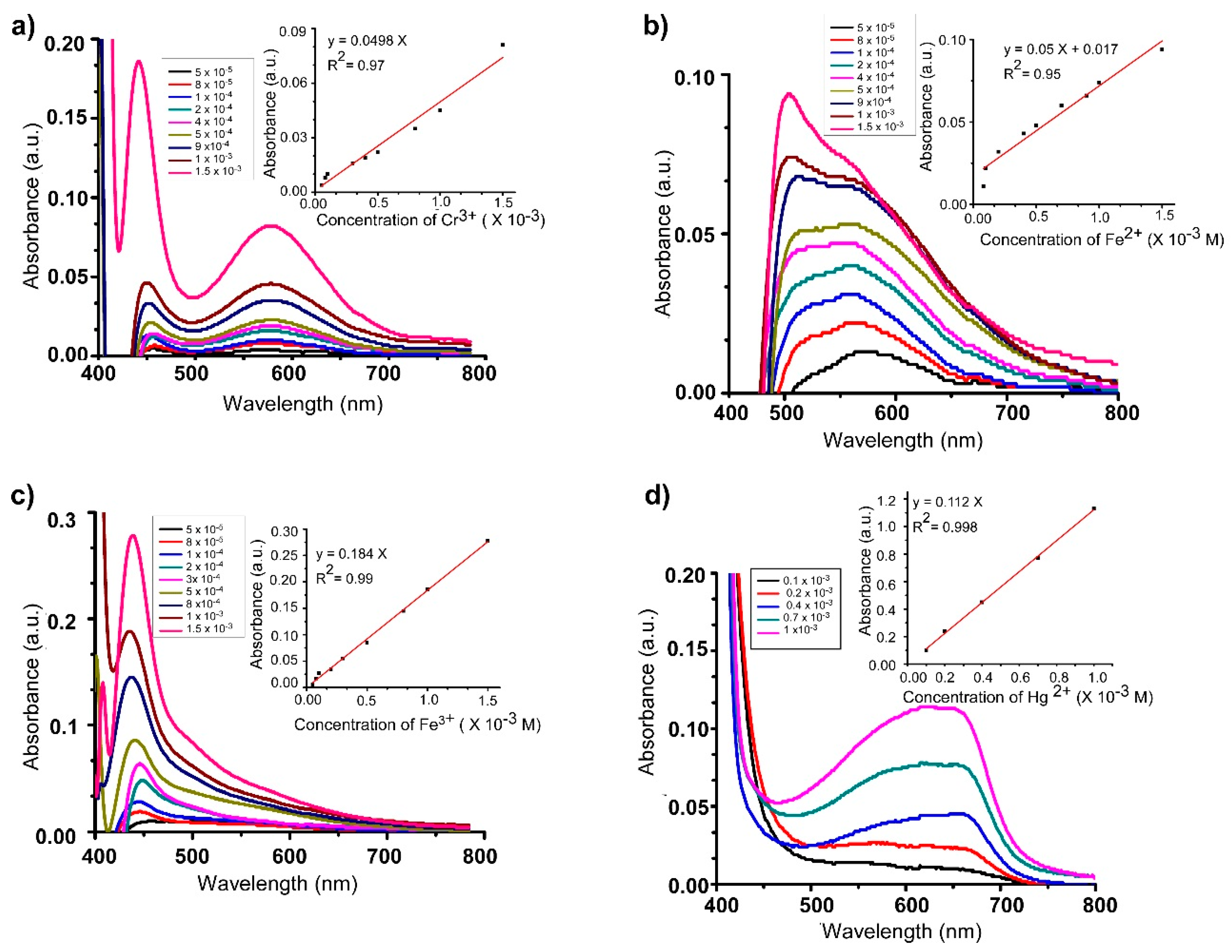
3.3. UV–Visible Monitoring
3.4. Job’s Plot and Detection Limit
3.5. Comparison with Other Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Hasan, M.M. Colorimetric detection and removal of copper (II) ions from wastewater samples using tailor-made composite adsorbent. Sens. Actuators B Chem. 2015, 206, 692–700. [Google Scholar] [CrossRef]
- Peralta-Domínguez, D.; Rodriguez, M.; Ramos-Ortiz, G.; Maldonado, J.; Luna-Moreno, D.; Ortiz-Gutierrez, M.; Barba, V. A Schiff base derivative used as sensor of copper through colorimetric and surface plasmon resonance techniques. Sens. Actuators B Chem. 2016, 225, 221–227. [Google Scholar] [CrossRef]
- Kundu, A.; Hariharan, P.; Prabakaran, K.; Anthony, S.P. Developing new Schiff base molecules for selective colorimetric sensing of Fe3+ and Cu2+ metal ions: Substituent dependent selectivity and colour change. Sens. Actuators B Chem. 2015, 206, 524–530. [Google Scholar] [CrossRef]
- Özdemir, Ö. Novel symmetric diimine-Schiff bases and asymmetric triimine-Schiff bases as chemosensors for the detection of various metal ions. J. Mol. Struct. 2016, 1125, 260–271. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Patil, A.; Salunke-Gawali, S. Overview of the chemosensor ligands used for selective detection of anions and metal ions (Zn2+, Cu2+, Ni2+, Co2+, Fe2+, Hg2+). Inorg. Chim. Acta 2018, 482, 99–112. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Small-molecule fluorescent sensors for investigating zinc metalloneurochemistry. Acc. Chem. Res. 2008, 42, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-H.; Xue, L.; Qian, Y.-Y.; Jiang, H. Novel ratiometric fluorescent sensor for silver ions. Org. Lett. 2009, 12, 292–295. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef]
- Azadbakht, R.; Keypour, H. A new Schiff base system bearing two naphthalene groups as fluorescent chemodosimeter for Zn2+ ion and its logic gate behavior. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 85, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, J.; Feng, H.; Zhao, Z.; Xu, L.; Qiu, H.; Tang, B. Zn2+-selective fluorescent turn-on chemosensor based on terpyridine-substituted siloles. Dyes Pigment. 2012, 95, 174–179. [Google Scholar] [CrossRef]
- Li, M.; Lu, H.-Y.; Liu, R.-L.; Chen, J.-D.; Chen, C.-F. Turn-on fluorescent sensor for selective detection of Zn2+, Cd2+, and Hg2+ in water. J. Org. Chem. 2012, 77, 3670–3673. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Thirupathi, P.; Lee, K.-H. Selective and sensitive ratiometric detection of Hg (II) ions using a simple amino acid based sensor. Org. Lett. 2011, 13, 5028–5031. [Google Scholar] [CrossRef]
- Bhalla, V.; Kumar, M.; Sharma, P.R.; Kaur, T. New fluorogenic sensors for Hg2+ ions: Through-bond energy transfer from pentaquinone to rhodamine. Inorg. Chem. 2012, 51, 2150–2156. [Google Scholar] [CrossRef]
- Alizadeh, K.; Parooi, R.; Hashemi, P.; Rezaei, B.; Ganjali, M.R. A new Schiff’s base ligand immobilized agarose membrane optical sensor for selective monitoring of mercury ion. J. Hazard. Mater. 2011, 186, 1794–1800. [Google Scholar] [CrossRef]
- Mergu, N.; Gupta, V.K. A novel colorimetric detection probe for copper (II) ions based on a Schiff base. Sens. Actuators B Chem. 2015, 210, 408–417. [Google Scholar] [CrossRef]
- Chang, I.J.; Choi, M.G.; Jeong, Y.A.; Lee, S.H.; Chang, S.-K. Colorimetric determination of Cu2+ in simulated wastewater using naphthalimide-based Schiff base. Tetrahedron Lett. 2017, 58, 474–477. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Velmathi, S. Colorimetric chemosensor for multi-signaling detection of metal ions using pyrrole based Schiff bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 428–435. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Govindaraju, T. Aldazine-based colorimetric sensors for Cu2+ and Fe3+. Sens. Actuators B Chem. 2012, 161, 304–310. [Google Scholar] [CrossRef]
- Sivaraman, G.; Sathiyaraja, V.; Chellappa, D. Turn-on fluorogenic and chromogenic detection of Fe (III) and its application in living cell imaging. J. Lumin. 2014, 145, 480–485. [Google Scholar] [CrossRef]
- Saluja, P.; Sharma, H.; Kaur, N.; Singh, N.; Jang, D.O. Benzimidazole-based imine-linked chemosensor: Chromogenic sensor for Mg2+ and fluorescent sensor for Cr3+. Tetrahedron 2012, 68, 2289–2293. [Google Scholar] [CrossRef]
- Zamfir, G.; Stanica, N.; Draghici, C.; Kriza, C.A.A. Some Transitional Metal Complexes of 4-dimethylaminobenzilidene-2 mercaptoaniline. Rev. Chim. 2012, 63, 1176–1180. [Google Scholar]
- Jeong, U.; Kim, Y. Colorimetric detection of heavy metal ions using aminosilane. J. Ind. Eng. Chem. 2015, 31, 393–396. [Google Scholar] [CrossRef]
- Arunachalam, S.; Priya, N.P.; Jayabalakrishnan, C.; Chinnusamy, V. Ruthenium (II) Schiff base: complexes, physico-chemical, spectrometric, microbial and DNA binding and cleaving studies. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 110–122. [Google Scholar]
- Santos-Figueroa, L.E.; Moragues, M.E.; Climent, E.; Agostini, A.; Martínez-Máñez, R.; Sancenon, F. Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem. Soc. Rev. 2013, 42, 3489–3613. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Singh, A.; Ganjali, M.; Norouzi, P.; Faridbod, F.; Mergu, N. Comparative study of colorimetric sensors based on newly synthesized Schiff bases. Sens. Actuators B Chem. 2013, 182, 642–651. [Google Scholar] [CrossRef]
- Bourson, J.; Pouget, J.; Valeur, B. Ion-responsive fluorescent compounds. 4. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza-and diaza-crown ethers. J. Phys. Chem. 1993, 97, 4552–4557. [Google Scholar] [CrossRef]
- Heo, G.; Lee, D.; Kim, C.G.; Do, J.Y. Rhodamine spirolactam sensors operated by sulfur-cooperated metal complexation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 285–290. [Google Scholar] [CrossRef]
- Luthern, J.; Peredes, A. Determination of the stoichiometry of a thermochromic color complex via Job’s method. J. Mater. Sci. Lett. 2000, 19, 185–188. [Google Scholar] [CrossRef]
- Feng, G.; Geng, L.; Wang, T.; Li, J.; Yu, X.; Wang, Y.; Li, Y.; Xie, D. Fluorogenic and chromogenic detection of biologically important fluoride anion with schiff-bases containing 4-amino-1, 8-naphthalimide unit. J. Lumin. 2015, 167, 65–70. [Google Scholar] [CrossRef]
- Bosque-Sendra, J.M.; Almansa-López, E.; García-Campaña, M.; Cuadros-Rodríguez, L. Data analysis in the determination of stoichiometries and stability constants of complexes. Anal. Sci. 2003, 19, 1431–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shellaiah, M.; Rajan, Y.C.; Balu, P.; Murugan, A. A pyrene based Schiff base probe for selective fluorescence turn-on detection of Hg2+ ions with live cell application. New J. Chem. 2015, 39, 2523–2531. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alorabi, A.Q.; Abdelbaset, M.; Zabin, S.A. Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene. Chemosensors 2020, 8, 1. https://doi.org/10.3390/chemosensors8010001
Alorabi AQ, Abdelbaset M, Zabin SA. Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene. Chemosensors. 2020; 8(1):1. https://doi.org/10.3390/chemosensors8010001
Chicago/Turabian StyleAlorabi, Ali Q., Mohamed Abdelbaset, and Sami A. Zabin. 2020. "Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene" Chemosensors 8, no. 1: 1. https://doi.org/10.3390/chemosensors8010001




