A Naphthalimide-Benzothiazole Conjugate as Colorimetric and Fluorescent Sensor for Selective Trinitrophenol Detection
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Compound 1
2.3. Optical Detection
2.4. UV-vis Absorption Spectroscopy
2.5. Fluorescence Spectroscopy
2.6. Time Resolved Fluorescence Spectroscopy
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Optical Properties
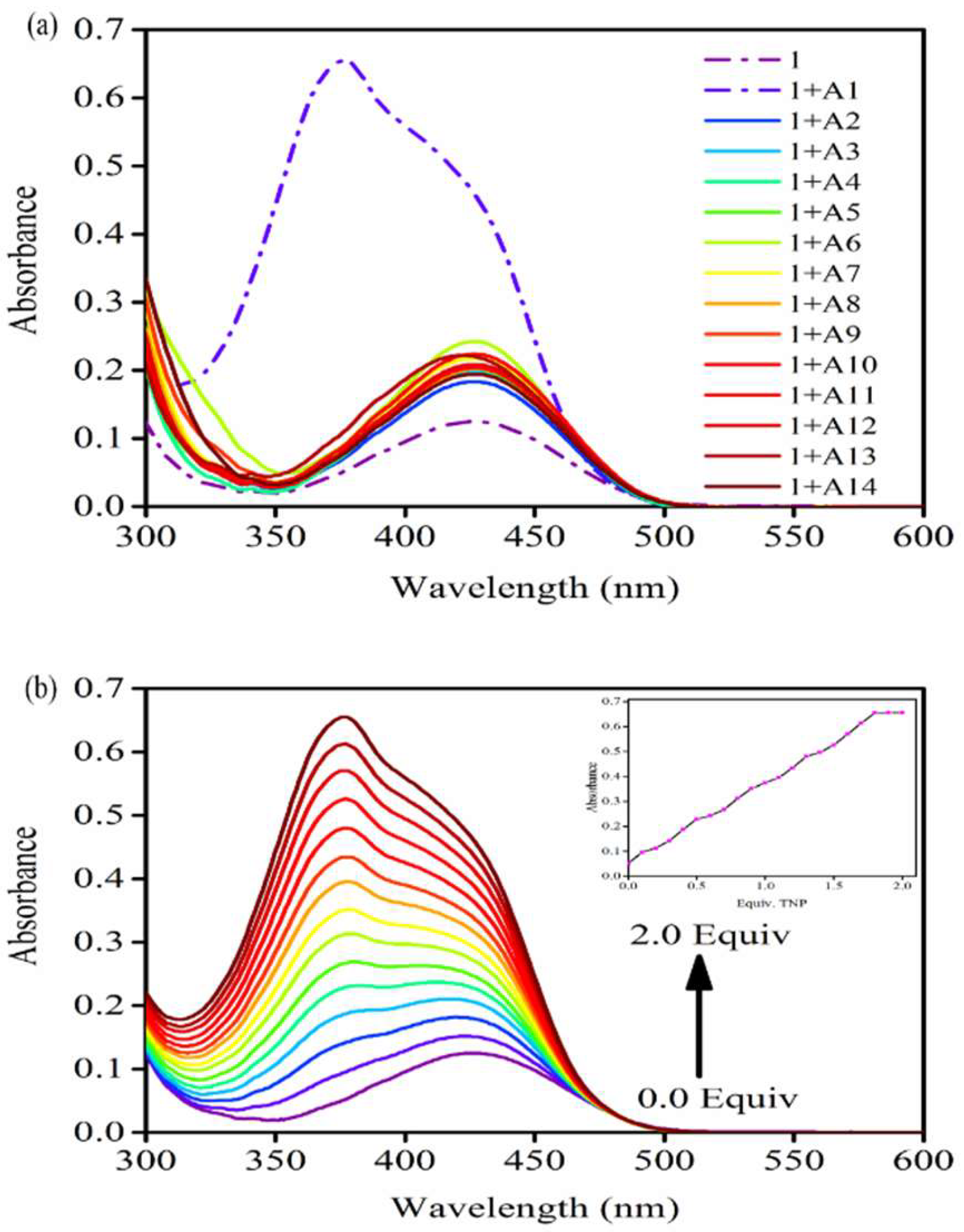
3.3. UV-vis Absorption
3.4. Florescence Properties
3.5. Time Correlated Single Photon Counting (TCSPC) Studies
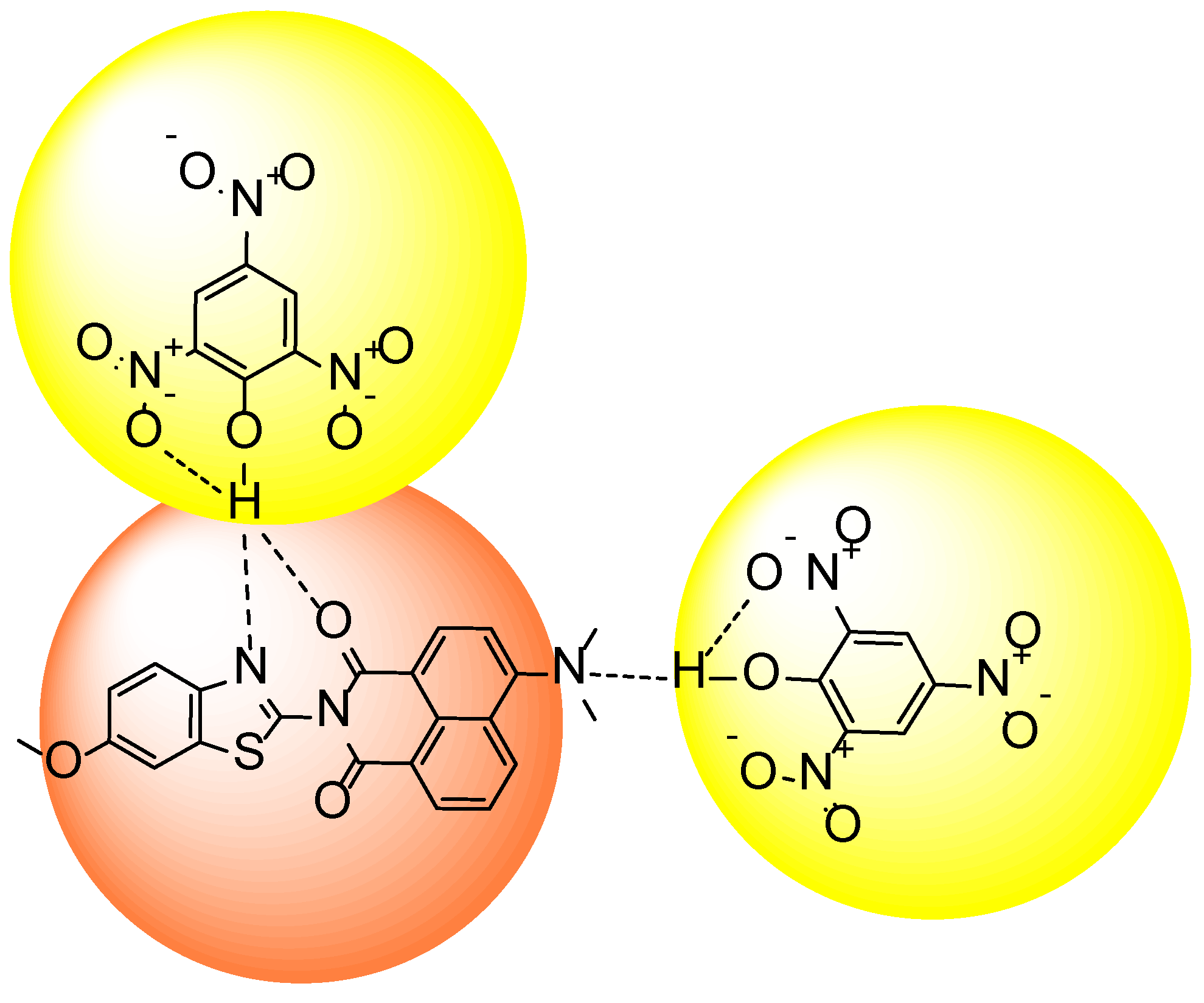
3.6. Fluorescence Quenching Mechanism
3.7. Stern–Volmer Constant
3.8. Binding Constant
3.9. Limit of Detection
3.10. 1H NMR Spectroscopy
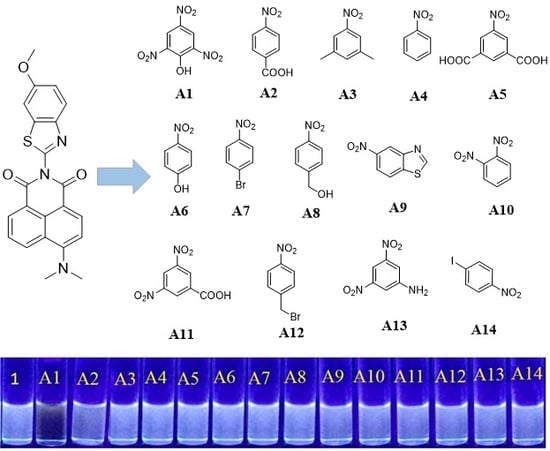
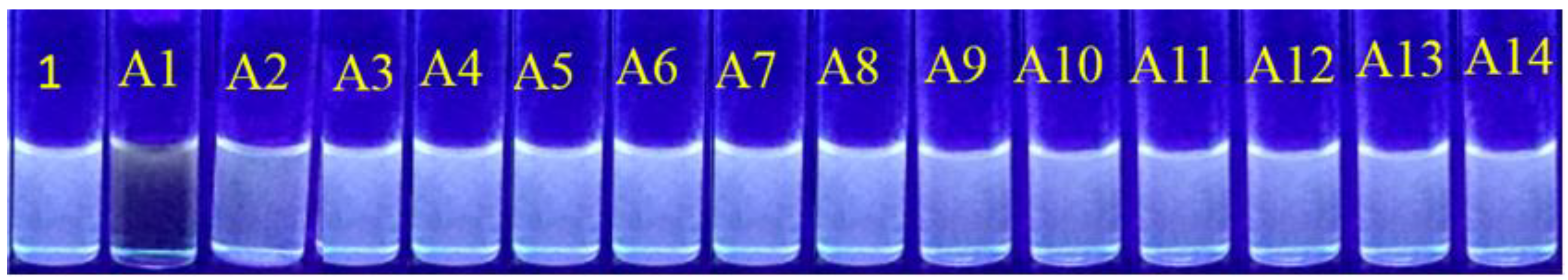
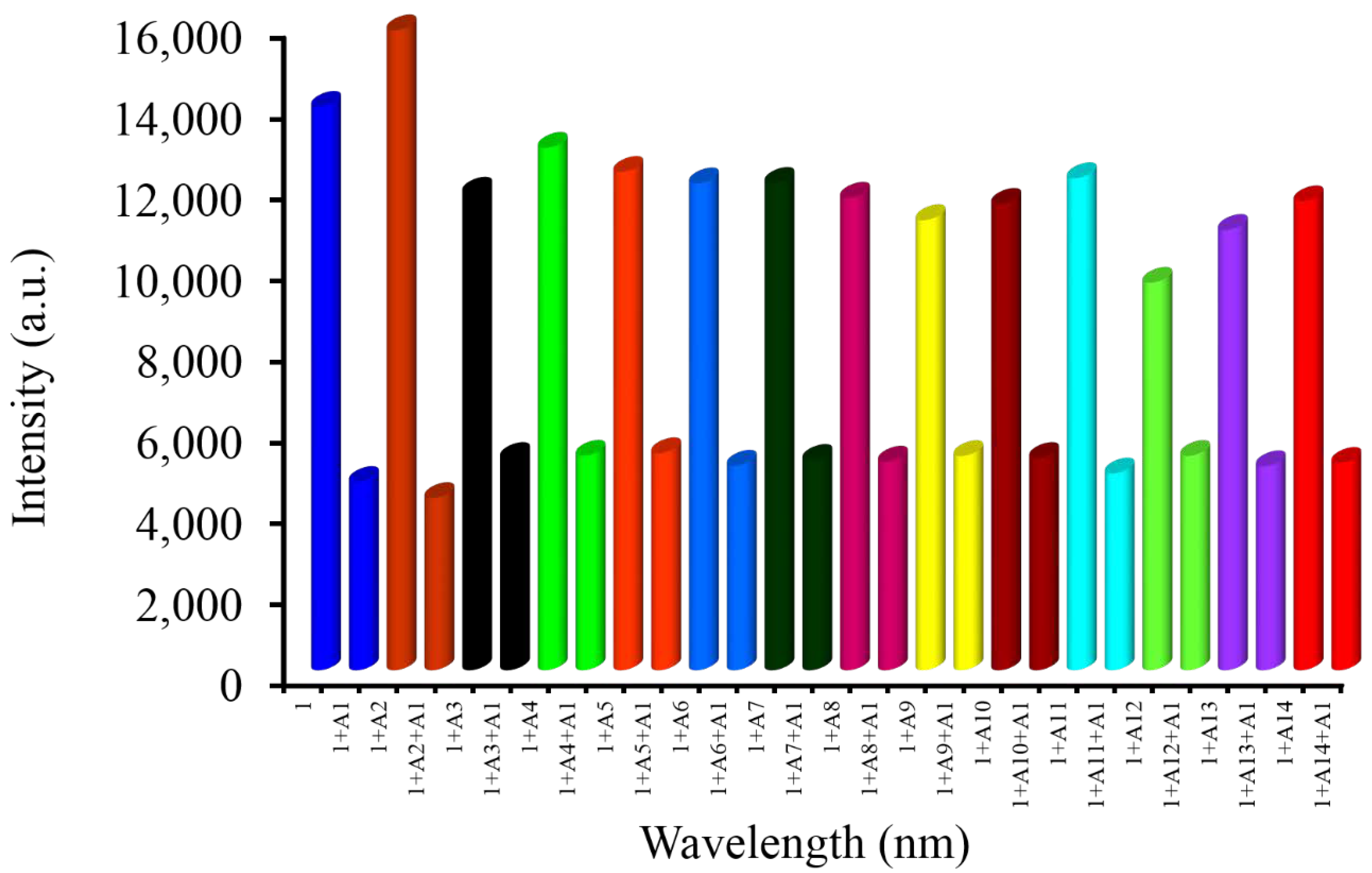
3.11. Competitive Sensing Properties
3.12. Detection of DNP
3.13. Detection of Hydroxyl Aromatics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akhavan, J. Chemistry of Explosives, 2nd ed.; Royal Society of Chemistry: London, UK, 2004. [Google Scholar]
- Wollin, K.-M.; Dieter, H.H. Toxicological guidelines for monocyclic nitro-, amino- and amino nitroaromatics, nitramines, and nitrate esters in drinking water. Arch. Environ. Contam. Toxicol. 2005, 49, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed]
- Salinas, Y.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Costero, A.M.; Parra, M.; Gil, S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Xiaomei, H.; Di, W. Förster resonance-energy-transfer detection of 2, 4, 6-trinitrophenol using copper nanoclusters. Anal. Bioanal. Chem. 2015, 407, 4607–4613. [Google Scholar]
- McQueen, C.A. Comprehensive Toxicology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Shen, J.; Zhang, J.; Zuo, Y.; Wang, L.; Sun, X.; Li, J.; Han, W.; He, R. Biodegradation of 2, 4, 6-trinitrophenol by Rhodococcus sp. Isolated from a picric acid contaminated soil. J. Hazard. Mater. 2009, 163, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Haknsson, K.; Coorey, R.V.; Zubarev, R.; Talrose, V.L.; Hakansson, P. Low-mass ions observed in plasma desorption mass spectrometry of high explosives. J. Mass Spectr. 2000, 35, 337–346. [Google Scholar] [CrossRef]
- Sylvia, J.M.; Janni, J.A.; Klein, J.D.; Spencer, K.M. Surface enhanced Raman detection of 2, 4-dinitrotoluene impurity vapor as a marker to locate landmines. Anal. Chem. 2000, 72, 5834–5840. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.H.; Simpson, G. Capabilities and limitations of ion mobility spectrometry for field screening applications field. Anal. Chem. Technol. 1997, 1, 119–134. [Google Scholar] [CrossRef]
- Krausa, M.; Schorb, K. Trace detection of 2, 4, 6-trinitrotoluene in the gaseous phase by cyclic voltammetry. J. Electroanal. Chem. 1999, 461, 10–13. [Google Scholar] [CrossRef]
- Walsh, M.E. Determination of nitroaromatic, nitramine, and nitrate ester explosives in soil by gas chromatography and an electron capture detector. Talanta 2001, 54, 427–438. [Google Scholar] [CrossRef]
- Ostafin, M.; Nogaj, B. 14N-NQR based Device for Detection of Explosives in Landmines. Measurement 2007, 40, 43–54. [Google Scholar] [CrossRef]
- Pan, J.; Tang, F.; Ding, A.; Kong, L.; Yang, L.; Tao, X.; Tian, Y.; Yang, J. A small-molecule chemosensor for the selective detection of 2, 4, 6-trinitrophenol (TNP). RSC Adv. 2015, 5, 191–195. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrogenerated thin films of microporous polymer networks with remarkably increased electrochemical response to nitroaromatic analytes. ACS Appl. Mater. Interfaces 2015, 7, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Räupke, A.; Palma-Cando, A.; Shkura, E.; Teckhausen, P.; Polywka, A.; Görrn, P.; Scherf, U.; Riedl, T. Highly sensitive gas-phase explosive detection by luminescent microporous polymer networks. Sci. Rep. 2016, 6, 29118. [Google Scholar]
- Qin, Z.-S.; Dong, W.-W.; Zhao, J.; Wu, Y.-P.; Zhang, Q.; Li, D.-S. A water-stable Tb(III)-based metal–organic gel (MOG) for detection of antibiotics and explosives. Inorg. Chem. Front. 2018, 5, 120–126. [Google Scholar] [CrossRef]
- Kartha, K.K.; Sandeep, A.; Praveen, V.K.; Ajayaghosh, A. Detection of Nitroaromatic Explosives with Fluorescent Molecular Assemblies and π-Gels. Chem. Rec. 2015, 15, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, Z.; Xu, S.; Li, D.; Zhang, Q.; Ma, W.; Zhou, H.; Wu, J.; Tian, Y. Fluorescent metal–organic frameworks based on mixed organic ligands: New candidates for highly sensitive detection of TNP. Dalton Trans. 2019, 48, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J. A luminescent porous metal–organic framework with Lewis basic pyridyl sites as a fluorescent chemosensor for TNP detection. Inorg. Chem. Commun. 2018, 89, 51–54. [Google Scholar] [CrossRef]
- Sharma, A.; Kim, D.; Park, J.-H.; Rakshit, S.; Seong, J.; Jeong, G.H.; Kwon, O.-H.; Lah, M.S. Mechanistic insight into the sensing of nitroaromatic compounds by metal-organic frameworks. Commun. Chem. 2019, 2, 39. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.K. A dual-functionalized, luminescent and highly crystalline covalent organic framework: Molecular decoding strategies for VOCs and ultrafast TNP sensing. J. Mater. Chem. A 2018, 6, 16246–16256. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yan, Y.; Xia, F.; Huang, A.; Xian, Y. Highly fluorescent polyimide covalent organic nanosheets as sensing probes for the detection of 2, 4, 6-trinitrophenol. ACS Appl. Mater. Interfaces 2017, 9, 13415–13421. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hu, W.L.; Xia, H.Y.; Zou, G.; Zhang, Q.J. Highly selective and reproducible detection of picric acid in aqueous media, based on a polydiacetylene microtube optical waveguide. J. Mater. Chem. A 2014, 2, 15560–15565. [Google Scholar] [CrossRef]
- Mukherjee, S.; Desai, A.V.; Inamdar, A.I.; Manna, B.; Ghosh, S.K. Selective detection of 2, 4, 6-trinitrophenol (TNP) by a π-stacked organic crystalline solid in water. Cryst. Growth Des. 2015, 15, 3493–3497. [Google Scholar] [CrossRef]
- Acharyya, K.; Mukherjee, P.S. A fluorescent organic cage for picric acid detection. Chem. Commun. 2014, 50, 15788–15791. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-Y.; Zhu, Y.-X.; Pan, M.; Wei, Z.-W.; Wang, H.-P.; Fan, Y.-N.; Su, C.-Y. Nanosized NIR-luminescent Ln metal–organic cage for picric acid sensing. Eur. J. Inorg. Chem. 2017, 3, 646–650. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, A.-J.; Dong, M.; Wang, Y.-W. A colorimetric and fluorescent chemosensor for the detection of an explosive-2, 4, 6-trinitrophenol (TNP). Chem. Commun. 2011, 47, 4505–4507. [Google Scholar] [CrossRef]
- Bandela, A.K.; Bandaru, S.; Rao, C.P. A fluorescent 1, 3-diaminonaphthalimide conjugate of calix[4]arene for sensitive and selective detection of trinitrophenol: Spectroscopy, microscopy, and computational studies, and its applicability using cellulose strips. Chem. Eur. J. 2015, 21, 13364–13374. [Google Scholar] [CrossRef]
- Roy, B.; Bar, A.K.; Gole, B.; Mukherjee, P.S. Fluorescent tris-imidazolium sensors for picric acid Explosive. J. Org. Chem. 2013, 78, 1306–1310. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly selective detection of nitro explosives by a luminescent metal–organic framework. Angew. Chem. Int. Ed. 2013, 52, 2881–2885. [Google Scholar] [CrossRef]
- Kumar, A.; Pandith, A.; Kim, H.-S. Pyrene-appended imidazolium probes as 3, 5-dinitrosalicylic acid sensors in 10% aqueous media. Dyes Pigm. 2015, 122, 351–358. [Google Scholar] [CrossRef]
- Kumar, A.; Pandith, A.; Kim, H.-S. Pyrene appended imidazolium probe for 2, 4, 6-trinitrophenol in water. Sens. Actuator B Chem. 2016, 231, 293–301. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Pandith, A.; Kim, H.-S. Selective detection of 2, 4, 6-trinitrophenol based on in situ–generated fluorescent Zn2+-anthracene ensembles in 80% aqueous dimethyl sulfoxide. Bull. Korean Chem. Soc. 2018, 39, 14–23. [Google Scholar] [CrossRef]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishna, P.; Meher, N.; Iyer, P.K. Functional 1,8-naphthalimide AIE/AIEEgens: Recent advances and prospects. ACS Appl. Mater. Interfaces 2018, 10, 12081–12111. [Google Scholar] [CrossRef]
- Tomczyk, M.D.; Walczak, K.Z. l,8-Naphthalimide based DNA intercalators and anticancer agents. A systematic review from 2007 to 2017. Eur. J. Md. Chem. 2018, 159, 399–442. [Google Scholar] [CrossRef] [PubMed]
- Kalita, A.; Hussain, S.; Malik, A.H.; Barman, U.; Goswami, N.; Iyer, P.K. Anion-exchange induced strong π–π interactions in single crystalline naphthalene diimide for nitroexplosive sensing: An electronic prototype for visual on-site detection. ACS Appl. Mater. Interfaces 2016, 8, 25326–25336. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, N.; Lv, H.; Ding, Q.; Gao, A.; Jing, Q.; Yi, T. Strong blue emissive supramolecular self-assembly system based on naphthalimide derivatives and its ability of detection and removal of 2, 4, 6-trinitrophenol. Langmuir 2017, 33, 7788–7798. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Seth, S. A comprehensive review on recent advances in synthesis & pharmacotherapeutic potential of benzothiazoles. Antiinflamm Antiallergy Agents Med. Chem. 2015, 14, 98–112. [Google Scholar]
- Chaudhary, S.; Sharma, H.; Milton, M.D. Novel 2-arylbenzothiazoles: Selective chromogenic and fluorescent probes for the detection of picric acid. Chem. Sel. 2018, 3, 4598–4608. [Google Scholar] [CrossRef]
- Dikundwar, A.G.; Dutta, G.K.; Guru Row, T.N.; Patil, S. Polymorphism in opto-electronic materials with a benzothiazole-fluorene core: A consequence of high conformational flexibility of π-conjugated backbone and alkyl side chains. Cryst. Growth Des. 2011, 11, 1615–1622. [Google Scholar] [CrossRef]
- Juollard, J. Dimethylformamide: Purification, tests for purity and physical properties. Pure Appl. Chem. 1977, 49, 885–892. [Google Scholar]
- Impurities of Dimethylacetamide. Available online: https://www.labhut.com//media/download/ dimethylacetamide_impurities-CGB-WAX.pdf (accessed on 22 May 2019).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool, Version 1.1.0. Available online: http://avogadro.openmolecules.net/ (accessed on 19 July 2019).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization and analysis platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Urano, Y.; Setsukinai, H.; Takakusa, H.; Kojima, H.; Kikuchi, K.; Ohkubo, S.; Fukuzumi, S.; Nagano, T. Rational principles for modulating fluorescence properties of fluorescein. J. Am. Chem. Soc. 2004, 126, 14079–14085. [Google Scholar] [CrossRef] [PubMed]
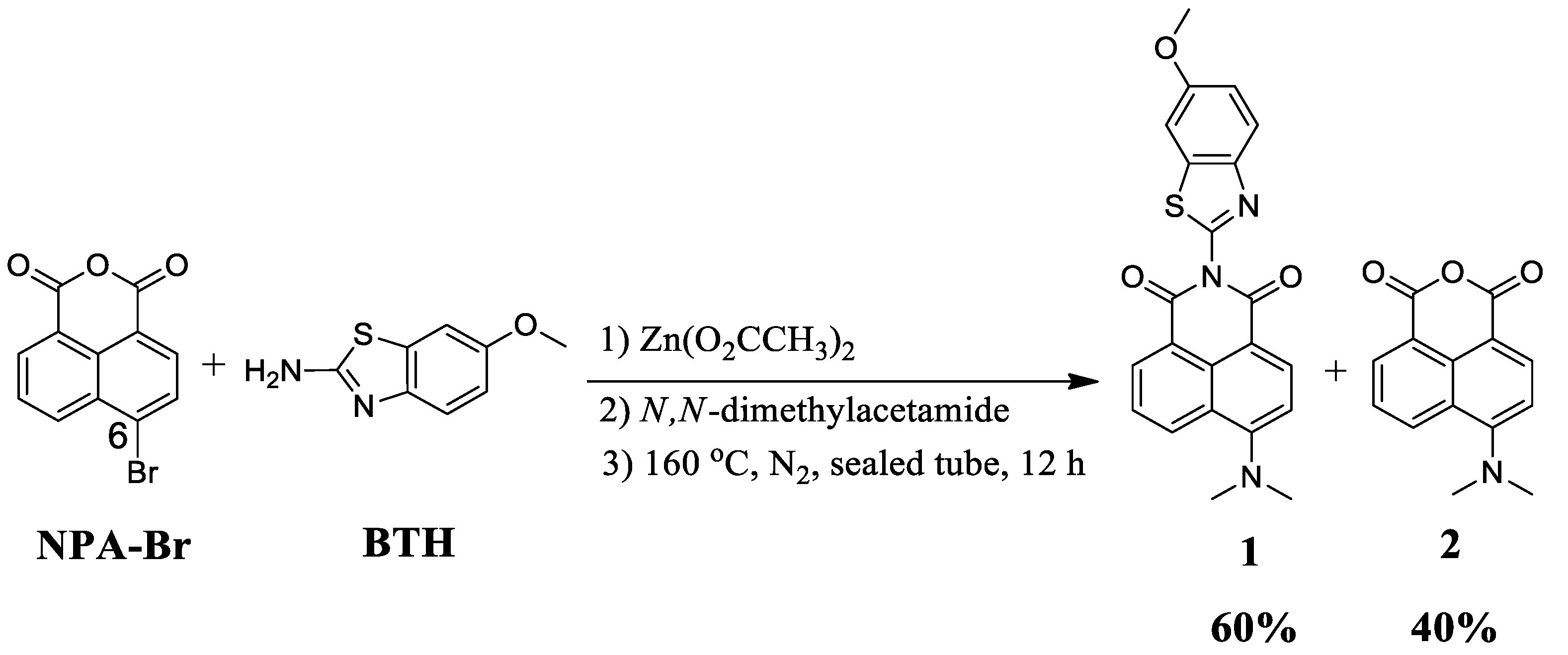





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D. Jawale Patil, P.; D. Ingle, R.; M. Wagalgave, S.; S. Bhosale, R.; V. Bhosale, S.; P. Pawar, R.; V. Bhosale, S. A Naphthalimide-Benzothiazole Conjugate as Colorimetric and Fluorescent Sensor for Selective Trinitrophenol Detection. Chemosensors 2019, 7, 38. https://doi.org/10.3390/chemosensors7030038
D. Jawale Patil P, D. Ingle R, M. Wagalgave S, S. Bhosale R, V. Bhosale S, P. Pawar R, V. Bhosale S. A Naphthalimide-Benzothiazole Conjugate as Colorimetric and Fluorescent Sensor for Selective Trinitrophenol Detection. Chemosensors. 2019; 7(3):38. https://doi.org/10.3390/chemosensors7030038
Chicago/Turabian StyleD. Jawale Patil, Pramod, Rajita D. Ingle, Sopan M. Wagalgave, Rajesh S. Bhosale, Sidhanath V. Bhosale, Rajendra P. Pawar, and Sheshanath V. Bhosale. 2019. "A Naphthalimide-Benzothiazole Conjugate as Colorimetric and Fluorescent Sensor for Selective Trinitrophenol Detection" Chemosensors 7, no. 3: 38. https://doi.org/10.3390/chemosensors7030038
APA StyleD. Jawale Patil, P., D. Ingle, R., M. Wagalgave, S., S. Bhosale, R., V. Bhosale, S., P. Pawar, R., & V. Bhosale, S. (2019). A Naphthalimide-Benzothiazole Conjugate as Colorimetric and Fluorescent Sensor for Selective Trinitrophenol Detection. Chemosensors, 7(3), 38. https://doi.org/10.3390/chemosensors7030038