Chemical Vapour Deposition of Gas Sensitive Metal Oxides
Abstract
:1. Introduction
- Precursor supply system
- CVD reactor
- Exhaust system.
- A single step for gas sensor processing which combines both materials synthesis and integration of the material with the sensor platform.
- Production of atomically mixed homogenous materials, including complex stoichiometries, with good reproducibility.
- Ability to influence crystal structure and surface morphology.
2. Gas Sensing Materials
2.1. Tungsten Oxide
2.2. Zinc Oxide
2.3. Tin Oxide
2.4. Complex Oxides
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B: Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide Materials for Development of Integrated Gas Sensors—A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Wang, H.; Liang, J.; Fan, H.; Xi, B.; Zhang, M.; Xiong, S.; Zhu, Y.; Qian, Y. Synthesis and gas sensitivities of SnO2 nanorods and hollow microspheres. J. Solid State Chem. 2008, 181, 122–129. [Google Scholar] [CrossRef]
- Siciliano, P. Preparation, characterisation and applications of thin films for gas sensors prepared by cheap chemical method. Sens. Actuators B: Chem. 2000, 70, 153–164. [Google Scholar] [CrossRef]
- Zhi-Peng, S.; Lang, L.; Li, Z.; Dian-Zeng, J. Rapid synthesis of ZnO nano-rods by one-step, room-temperature, solid-state reaction and their gas-sensing properties. Nanotechnology 2006, 17, 2266. [Google Scholar]
- Dai, Z.R.; Pan, Z.W.; Wang, Z.L. Novel Nanostructures of Functional Oxides Synthesized by Thermal Evaporation. Adv. Funct. Mater. 2003, 13, 9–24. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, T.-L.; Gu, Y.; Warren, J.; Osgood, R.M. Zinc Oxide Nanowires Grown by Vapor-Phase Transport Using Selected Metal Catalysts: A Comparative Study. Chem. Mater. 2005, 17, 4227–4234. [Google Scholar] [CrossRef]
- Choopun, S.; Hongsith, N.; Mangkorntong, P.; Mangkorntong, N. Zinc oxide nanobelts by RF sputtering for ethanol sensor. Phys. E: Low-Dimens. Syst. Nanostructures 2007, 39, 53–56. [Google Scholar] [CrossRef]
- Heo, Y.W.; Varadarajan, V.; Kaufman, M.; Kim, K.; Norton, D.P.; Ren, F.; Fleming, P.H. Site-specific growth of Zno nanorods using catalysis-driven molecular-beam epitaxy. Appl. Phys. Lett. 2002, 81, 3046–3048. [Google Scholar] [CrossRef]
- Marchand, P.; Hassan, I.A.; Parkin, I.P.; Carmalt, C.J. Aerosol-assisted delivery of precursors for chemical vapour deposition: Expanding the scope of CVD for materials fabrication. Dalton Trans. 2013, 42, 9406–9422. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Blackman, C. Growth mechanism of planar or nanorod structured tungsten oxide thin films deposited via aerosol assisted chemical vapour deposition (AACVD). Phys. Status Solidi C 2015, 12, 869–877. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Vallejos, S.; Stoycheva, T.; Blackman, C.; Llobet, E. Aerosol assisted chemical vapour deposition of gas-sensitive nanomaterials. Thin Solid Films 2013, 548, 703–709. [Google Scholar] [CrossRef]
- Meng, D.; Yamazaki, T.; Shen, Y.; Liu, Z.; Kikuta, T. Preparation of WO3 nanoparticles and application to NO2 sensor. Appl. Surface Sci. 2009, 256, 1050–1053. [Google Scholar] [CrossRef]
- Deb, B.; Desai, S.; Sumanasekera, G.U.; Sunkara, M.K. Gas sensing behaviour of mat-like networked tungsten oxide nanowire thin films. Nanotechnology 2007, 18, 285501. [Google Scholar] [CrossRef]
- Tesfamichael, T. Electron Beam Evaporation of Tungsten Oxide Films for Gas Sensors. Sens. J. IEEE 2010, 10, 1796–1802. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, W.; Yang, Z.; Yu, Z.; Zhang, X.; Chang, T.C.; Javey, A. Vertically aligned tungsten oxide nanorod film with enhanced performance in photoluminescence humidity sensing. Sens. Actuators B: Chem. 2014, 202, 708–713. [Google Scholar] [CrossRef]
- Ashraf, S.; Blackman, C.S.; Naisbitt, S.C.; Parkin, I.P. The gas-sensing properties of WO 3− x thin films deposited via the atmospheric pressure chemical vapour deposition (APCVD) of WCl 6 with ethanol. Meas. Sci. Technol. 2008, 19, 025203. [Google Scholar] [CrossRef]
- Tong, M.; Dai, G.; Wu, Y.; He, X.; Gao, D. WO3 thin film prepared by PECVD technique and its gas sensing properties to NO2. J. Mater. Sci. 2001, 36, 2535–2538. [Google Scholar] [CrossRef]
- Davazoglou, D.; Georgouleas, K. Low Pressure Chemically Vapor Deposited WO 3 Thin Films for Integrated Gas Sensor Applications. J. Electrochem. Soc. 1998, 145, 1346–1350. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Gràcia, I.; Figueras, E.; Llobet, E.; Cané, C.; Vallejos, S. Localized aerosol-assisted CVD of nanomaterials for the fabrication of monolithic gas sensor microarrays. Sens. Actuators B: Chem. 2015, 216, 374–383. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Vallejos, S.; Umek, P.; Guttmann, P.; Bittencourt, C.; Llobet, E. Aerosol-Assisted CVD-Grown WO3 Nanoneedles Decorated with Copper Oxide Nanoparticles for the Selective and Humidity-Resilient Detection of H2S. ACS Appl. Mater. Interfaces 2015, 7, 6842–6851. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Umek, P.; Stoycheva, T.; Annanouch, F.; Llobet, E.; Correig, X.; De Marco, P.; Bittencourt, C.; Blackman, C. Single-Step Deposition of Au- and Pt-Nanoparticle-Functionalized Tungsten Oxide Nanoneedles Synthesized Via Aerosol-Assisted CVD, and Used for Fabrication of Selective Gas Microsensor Arrays. Adv. Funct. Mater. 2013, 23, 1313–1322. [Google Scholar] [CrossRef]
- Naik, A.J.T.; Bowman, C.; Panjwani, N.; Warwick, M.E.A.; Binions, R. Electric field assisted aerosol assisted chemical vapour deposition of nanostructured metal oxide thin films. Thin Solid Films 2013, 544, 452–456. [Google Scholar] [CrossRef]
- Ashraf, S.; Blackman, C.S.; Palgrave, R.G.; Parkin, I.P. Aerosol-assisted chemical vapour deposition of WO3 thin films using polyoxometallate precursors and their gas sensing properties. J. Mater. Chem. 2007, 17, 1063–1070. [Google Scholar] [CrossRef]
- Vallejos, S.; Gràcia, I.; Figueras, E.; Sánchez, J.; Mas, R.; Beldarrain, O.; Cané, C. Microfabrication of flexible gas sensing devices based on nanostructured semiconducting metal oxides. Sens. Actuators A: Phys. 2014, 219, 88–93. [Google Scholar] [CrossRef]
- Vallejos, S.; Stoycheva, T.; Llobet, E.; Correig, X.; Umek, P.; Gracia, I.; Blackman, C. Benzene detection on nanostructured tungsten oxide MEMS based gas sensors. In Proceedings of the 2012 12th IEEE Conference on the Nanotechnology (IEEE-NANO), Birmingham, UK, 20–23 August 2012; pp. 1–5.
- Vallejos, S.; Gràcia, I.; Figueras, E.; Cané, C. Nanoscale Heterostructures Based on Fe2O3@WO3-x Nanoneedles and Their Direct Integration into Flexible Transducing Platforms for Toluene Sensing. ACS Appl. Mater. Interfaces 2015, 7, 18638–18649. [Google Scholar] [CrossRef] [PubMed]
- Boulmani, R.; Bendahan, M.; Lambert-Mauriat, C.; Gillet, M.; Aguir, K. Correlation between rf-sputtering parameters and WO3 sensor response towards ozone. Sens. Actuators B: Chem. 2007, 125, 622–627. [Google Scholar] [CrossRef]
- Stoycheva, T.; Annanouch, F.E.; Gràcia, I.; Llobet, E.; Blackman, C.; Correig, X.; Vallejos, S. Micromachined gas sensors based on tungsten oxide nanoneedles directly integrated via aerosol assisted CVD. Sens. Actuators B: Chem. 2014, 198, 210–218. [Google Scholar] [CrossRef]
- Stoycheva, T.; Vallejos, S.; Blackman, C.; Moniz, S.J.A.; Calderer, J.; Correig, X. Important considerations for effective gas sensors based on metal oxide nanoneedles films. Sens. Actuators B: Chem. 2012, 161, 406–413. [Google Scholar] [CrossRef]
- Kozuka, Y.; Tsukazaki, A.; Kawasaki, M. Challenges and opportunities of ZnO-related single crystalline heterostructures. Appl. Phys. Rev. 2014, 1, 011303. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanostructures of zinc oxide. Mater. Today 2004, 7, 26–33. [Google Scholar] [CrossRef]
- Zou, A.L.; Hu, L.Z.; Qiu, Y.; Cao, G.Y.; Yu, J.J.; Wang, L.N.; Zhang, H.Q.; Yin, B.; Xu, L.L. High performance of 1-D ZnO microwire with curve-side hexagon as ethanol gas sensor. J. Mater. Sci. Mater. Electron. 2015, 26, 4908–4912. [Google Scholar] [CrossRef]
- Hung, S.C.; Woon, W.Y.; Lan, S.M.; Ren, F.; Pearton, S.J. Characteristics of carbon monoxide sensors made by polar and nonpolar zinc oxide nanowires gated AlGaN/GaN high electron mobility transistor. Appl. Phys. Lett. 2013, 103, 083506. [Google Scholar] [CrossRef]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B: Chem. 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Chien, F.S.-S.; Wang, C.-R.; Chan, Y.-L.; Lin, H.-L.; Chen, M.-H.; Wu, R.-J. Fast-response ozone sensor with ZnO nanorods grown by chemical vapor deposition. Sens. Actuators B: Chem. 2010, 144, 120–125. [Google Scholar] [CrossRef]
- Han, N.; Hu, P.; Zuo, A.; Zhang, D.; Tian, Y.; Chen, Y. Photoluminescence investigation on the gas sensing property of ZnO nanorods prepared by plasma-enhanced CVD method. Sens. Actuators B: Chem. 2010, 145, 114–119. [Google Scholar] [CrossRef]
- Zhang, H.-D.; Long, Y.-Z.; Li, Z.-J.; Sun, B. Fabrication of comb-like ZnO nanostructures for room-temperature CO gas sensing application. Vacuum 2014, 101, 113–117. [Google Scholar] [CrossRef]
- Pan, X.; Liu, X.; Bermak, A.; Fan, Z. Self-Gating Effect Induced Large Performance Improvement of ZnO Nanocomb Gas Sensors. ACS Nano 2013, 7, 9318–9324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Han, N.; Chen, Y. Chemical vapor deposition preparation of nanostructured ZnO particles and their gas-sensing properties. J. Nanopart Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Pati, S.; Banerji, P.; Majumder, S.B. MOCVD grown ZnO thin film gas sensors: Influence of microstructure. Sens. Actuators A: Phys. 2014, 213, 52–58. [Google Scholar] [CrossRef]
- Sanchez-Valencia, J.R.; Alcaire, M.; Romero-Gómez, P.; Macias-Montero, M.; Aparicio, F.J.; Borras, A.; Gonzalez-Elipe, A.R.; Barranco, A. Oxygen Optical Sensing in Gas and Liquids with Nanostructured ZnO Thin Films Based on Exciton Emission Detection. J. Phys. Chem. C 2014, 118, 9852–9859. [Google Scholar] [CrossRef]
- Pati, S.; Maity, A.; Banerji, P.; Majumder, S.B. Qualitative and quantitative differentiation of gases using ZnO thin film gas sensors and pattern recognition analysis. Analyst 2014, 139, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, S.-W.; Kim, S.S. Fabrication of a Highly Sensitive Chemical Sensor Based on ZnO Nanorod Arrays. Nanoscale Res. Lett. 2010, 5, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, J.; Hesketh, P.J.; Liu, M. Synthesis and gas sensing properties of ZnO single crystal flakes. J. Mater. Chem. 2005, 15, 2316–2320. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sada, C.; Sberveglieri, G.; Tondello, E. Urchin-like ZnO nanorod arrays for gas sensing applications. CrystEngComm 2010, 12, 3419–3421. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sberveglieri, G.; Tondello, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B: Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Hussain, M.; Mazhara, M.; Hussainb, T.; Khan, N.A. High efficiency ZnO nano sensor, fabrication and characterization. JICS 2010, 7, S59–S69. [Google Scholar] [CrossRef]
- Roy, S.; Basu, S. Improved zinc oxide film for gas sensor applications. Bull. Mater. Sci. 2002, 25, 513–515. [Google Scholar] [CrossRef]
- Kiasari, N.M.; Soltanian, S.; Gholamkhass, B.; Servati, P. Environmental Gas and Light Sensing Using ZnO Nanowires. IEEE Trans. Nanotechnol. 2014, 13, 368–374. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surface Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Mathur, S.; Ganesan, R.; Grobelsek, I.; Shen, H.; Ruegamer, T.; Barth, S. Plasma-Assisted Modulation of Morphology and Composition in Tin Oxide Nanostructures for Sensing Applications. Adv. Eng. Mater. 2007, 9, 658–663. [Google Scholar] [CrossRef]
- Trung, D.D.; Hoa, N.D.; Tong, P.V.; Duy, N.V.; Dao, T.D.; Chung, H.V.; Nagao, T.; Hieu, N.V. Effective decoration of Pd nanoparticles on the surface of SnO2 nanowires for enhancement of CO gas-sensing performance. J. Hazard. Mater. 2014, 265, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tonezzer, M.; Hieu, N.V. Size-dependent response of single-nanowire gas sensors. Sens. Actuators B: Chem. 2012, 163, 146–152. [Google Scholar] [CrossRef]
- Jung, T.-H.; Kwon, S.-I.; Park, J.-H.; Lim, D.-G.; Choi, Y.-J.; Park, J.-G. SnO2 nanowires bridged across trenched electrodes and their gas-sensing characteristics. Appl. Phys. A 2008, 91, 707–710. [Google Scholar] [CrossRef]
- Bolotov, V.V.; Roslikov, V.E.; Roslikova, E.A.; Ivlev, K.E.; Knyazev, E.V.; Davletkildeev, N. A Formation of two-layer composite-on-insulator structures based on porous silicon and SnO x. Study of their electrical and gas-sensing properties. Semiconductors 2014, 48, 397–401. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Espinós, J.P.; González-Elipe, A.R. Effect of texture and annealing treatments in SnO2 and Pd/SnO2 gas sensor materials. Sens. Actuators B: Chem. 1999, 61, 23–32. [Google Scholar] [CrossRef]
- Du, X.; George, S.M. Thickness dependence of sensor response for CO gas sensing by tin oxide films grown using atomic layer deposition. Sens. Actuators B: Chem. 2008, 135, 152–160. [Google Scholar] [CrossRef]
- Ansari, S.G.; Gosavi, S.W.; Gangal, S.A.; Karekar, R.N.; Aiyer, R.C. Characterization of SnO2-based H2 gas sensors fabricated by different deposition techniques. J. Mater. Sci.: Mater. Electron. 1997, 8, 23–27. [Google Scholar]
- Brown, J.R.; Haycock, P.W.; Smith, L.M.; Jones, A.C.; Williams, E.W. Response behaviour of tin oxide thin film gas sensors grown by MOCVD. Sens. Actuators B: Chem. 2000, 63, 109–114. [Google Scholar] [CrossRef]
- Benkstein, K.; Martinez, C.; Li, G.; Meier, D.; Montgomery, C.; Semancik, S. Integration of nanostructured materials with MEMS microhotplate platforms to enhance chemical sensor performance. J. Nanopart Res. 2006, 8, 809–822. [Google Scholar] [CrossRef]
- Stoycheva, T.T.; Vallejos, S.; Pavelko, R.G.; Popov, V.S.; Sevastyanov, V.G.; Correig, X. Aerosol-Assisted CVD of SnO2 Thin Films for Gas-Sensor Applications. Chem. Vapor Depos. 2011, 17, 247–252. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Szuber, J. Comparative analysis of physico-chemical and gas sensing characteristics of two different forms of SnO2 films. Appl. Surface Sci. 2015, 326, 27–31. [Google Scholar] [CrossRef]
- Larciprete, R.; Borsella, E.; De Padova, P.; Perfetti, P.; Faglia, G.; Sberveglieri, G. Organotin films deposited by laser-induced CVD as active layers in chemical gas sensors. Thin Solid Films 1998, 323, 291–295. [Google Scholar] [CrossRef]
- Liu, Y.; Koep, E.; Liu, M. A Highly Sensitive and Fast-Responding SnO2 Sensor Fabricated by Combustion Chemical Vapor Deposition. Chem. Mater. 2005, 17, 3997–4000. [Google Scholar] [CrossRef]
- Hui, H.; Lee, Y.C.; Tan, O.K.; Zhou, W.; Peng, N.; Zhang, Q. High sensitivity SnO 2 single-nanorod sensors for the detection of H 2 gas at low temperature. Nanotechnology 2009, 20, 115501. [Google Scholar]
- Moseley, P.T.; Williams, D.E. A selective ammonia sensor. Sens. Actuators B: Chem. 1990, 1, 113–115. [Google Scholar] [CrossRef]
- Dawson, D.H.; Henshaw, G.S.; Williams, D.E. Description and characterization of a hydrogen sulfide gas sensor based on Cr2-yTiyO3+x. Sens. Actuators B: Chem. 1995, 26, 76–80. [Google Scholar] [CrossRef]
- Pokhrel, S.; Li, X.; Huo, L.; Zhao, H.; Cheng, X. Investigation and characterization of radio frequency sputtered Cr1.8Ti0.2O3−δ thin films derived by citrate, sol–gel and solid state routes. Thin Solid Films 2007, 515, 7053–7058. [Google Scholar] [CrossRef]
- Pokhrel, S.; Huo, L.; Zhao, H.; Gao, S. Sol–gel derived polycrystalline Cr1.8Ti0.2O3 thick films for alcohols sensing application. Sens. Actuators B: Chem. 2007, 120, 560–567. [Google Scholar] [CrossRef]
- Nartowski, A.M.; Atkinson, A. Sol-Gel Synthesis of Sub-Micron Titanium-Doped Chromia Powders for Gas Sensing. J. Sol-Gel. Sci. Technol. 2003, 26, 793–797. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Rizzo, G.; Galvagno, S. Low temperature sol-gel synthesis and humidity sensing properties of Cr2−xTixO3. J. Eur. Ceramic Soc. 2004, 24, 1435–1438. [Google Scholar] [CrossRef]
- Chabanis, G.; Parkin, I.P.; Williams, D.E. Microspheres of the gas sensor material Cr2-xTixO3 prepared by the sol-emulsion-gel route. J. Mater. Chem. 2001, 11, 1651–1655. [Google Scholar] [CrossRef]
- Niemeyer, D.; Williams, D.E.; Smith, P.; Pratt, K.F.E.; Slater, B.; Catlow, C.R.A.; Stoneham, A.M. Experimental and computational study of the gas-sensor behaviour and surface chemistry of the solid-solution Cr2-xTixO3 (x ≤0.5). J. Mater. Chem. 2002, 12, 667–675. [Google Scholar] [CrossRef]
- Peter, C.; Kneer, J.; Schmitt, K.; Eberhardt, A.; Wöllenstein, J. Preparation, material analysis, and morphology of Cr2-xTi xO3+z for gas sensors. Phys. Status Solidi (A) Appl. Mater. Sci. 2013, 210, 403–407. [Google Scholar] [CrossRef]
- Jayaraman, V.; Gnanasekar, K.I.; Prabhu, E.; Gnanasekaran, T.; Periaswami, G. Preparation and characterisation of Cr2−xTixO3+δ and its sensor properties. Sens. Actuators B: Chem. 1999, 55, 175–179. [Google Scholar] [CrossRef]
- Li, C.C.; Yin, X.M.; Wang, T.H.; Zeng, H.C. Morphogenesis of Highly Uniform CoCO3 Submicrometer Crystals and Their Conversion to Mesoporous Co3O4 for Gas-Sensing Applications. Chem. Mater. 2009, 21, 4984–4992. [Google Scholar] [CrossRef]
- Pokhrel, S.; Ming, Y.; Huo, L.; Zhao, H.; Gao, S. Cr2-xTixO3 (x ≤ 0.5) as CH3COCH3 sensitive resistors. Sens. Actuators, B: Chem. 2007, 125, 550–555. [Google Scholar] [CrossRef]
- Conde-Gallardo, A.; Cruz-Orea, A.; Zelaya-Angel, O.; Bartolo-Pèrez, P. Electrical and optical properties of Cr2-xTixO 3 thin films. J. Phys. D: Appl. Phys. 2008, 41, 1–6. [Google Scholar] [CrossRef]
- Shaw, G.A.; Parkin, I.P.; Williams, D.E. Atmospheric pressure chemical vapour deposition of Cr2-xTixO3 (CTO) thin films ([less-than-or-equal]3 [small micro]m) on to gas sensing substrates. J. Mater. Chem. 2003, 13, 2957–2962. [Google Scholar] [CrossRef]
- Shaw, G.A.; Pratt, K.F.E.; Parkin, I.P.; Williams, D.E. Gas sensing properties of thin film (≤3 μm) Cr2−xTixO3 (CTO) prepared by atmospheric pressure chemical vapour deposition (APCVD), compared with that prepared by thick film screen-printing. Sens. Actuators B: Chem. 2005, 104, 151–162. [Google Scholar] [CrossRef]
- Du, J.; Wu, Y.; Choy, K.-L. Controlled synthesis of gas sensing Cr2−xTixO3 films by electrostatic spray assisted vapour deposition and their structural characterisation. Thin Solid Films 2006, 497, 42–47. [Google Scholar] [CrossRef]
- Du, J.; Wu, Y.; Choy, K.-L.; Shipway, P.H. Structure, properties and gas sensing behavior of Cr2 − xTixO3 films fabricated by electrostatic spray assisted vapour deposition. Thin Solid Films 2010, 519, 1293–1299. [Google Scholar] [CrossRef]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 Nanomaterials in Lithium-Ion Batteries and Gas Sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, H.-R.; Kim, K.-M.; Liu, D.; Cao, G.; Lee, J.-H. C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction. Sens. Actuators B: Chem. 2010, 146, 183–189. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Junge, H.; Pohl, M.-M.; Radnik, J.; Brückner, A.; Beller, M. Selective Oxidation of Alcohols to Esters Using Heterogeneous Co3O4–N@C Catalysts under Mild Conditions. J. Am. Chem. Soc. 2013, 135, 10776–10782. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Gavagnin, M.; Maccato, C.; Sada, C.; Sberveglieri, G.; et al. Plasma enhanced-CVD of undoped and fluorine-doped Co3O4 nanosystems for novel gas sensors. Sens. Actuators B: Chem. 2011, 160, 79–86. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Das, S.; Kundoo, S.; Chattopadhyay, K.K. Effect of Fluorine Doping on Semiconductor to Metal-Like Transition and Optical Properties of Cadmium Oxide Thin Films Deposited by Sol–Gel Process. J. Sol-Gel. Sci. Technol. 2005, 34, 173–179. [Google Scholar] [CrossRef]
- Jinling, Y.; Jingkui, L.; Duo, J.; Shi, Y.; Weihua, T.; Guanghui, R. The influence of fluorine on the structures and properties of Pr2-xSrxCuO4-y (X = 0.0, 0.4, 1.0). J. Phys.: Condens. Matter 1997, 9, 1249. [Google Scholar]
- Liu, B.; Gu, M.; Liu, X.; Huang, S.; Ni, C. First-principles study of fluorine-doped zinc oxide. Appl. Phys. Lett. 2010, 97, 122101. [Google Scholar] [CrossRef]
- Singh, N.; Yan, C.; Lee, P.S. Room temperature CO gas sensing using Zn-doped In2O3 single nanowire field effect transistors. Sens. Actuators B: Chem. 2010, 150, 19–24. [Google Scholar] [CrossRef]
- Bloor, L.G.; Manzi, J.; Binions, R.; Parkin, I.P.; Pugh, D.; Afonja, A.; Blackman, C.S.; Sathasivam, S.; Carmalt, C.J. Tantalum and Titanium doped In2O3 Thin Films by Aerosol-Assisted Chemical Vapor Deposition and their Gas Sensing Properties. Chem. Mater. 2012, 24, 2864–2871. [Google Scholar] [CrossRef]
- Salehi, A.; Gholizade, M. Gas-sensing properties of indium-doped SnO2 thin films with variations in indium concentration. Sens. Actuators B: Chem. 2003, 89, 173–179. [Google Scholar] [CrossRef]
- Salehi, A. Selectivity enhancement of indium-doped SnO2 gas sensors. Thin Solid Films 2002, 416, 260–263. [Google Scholar] [CrossRef]
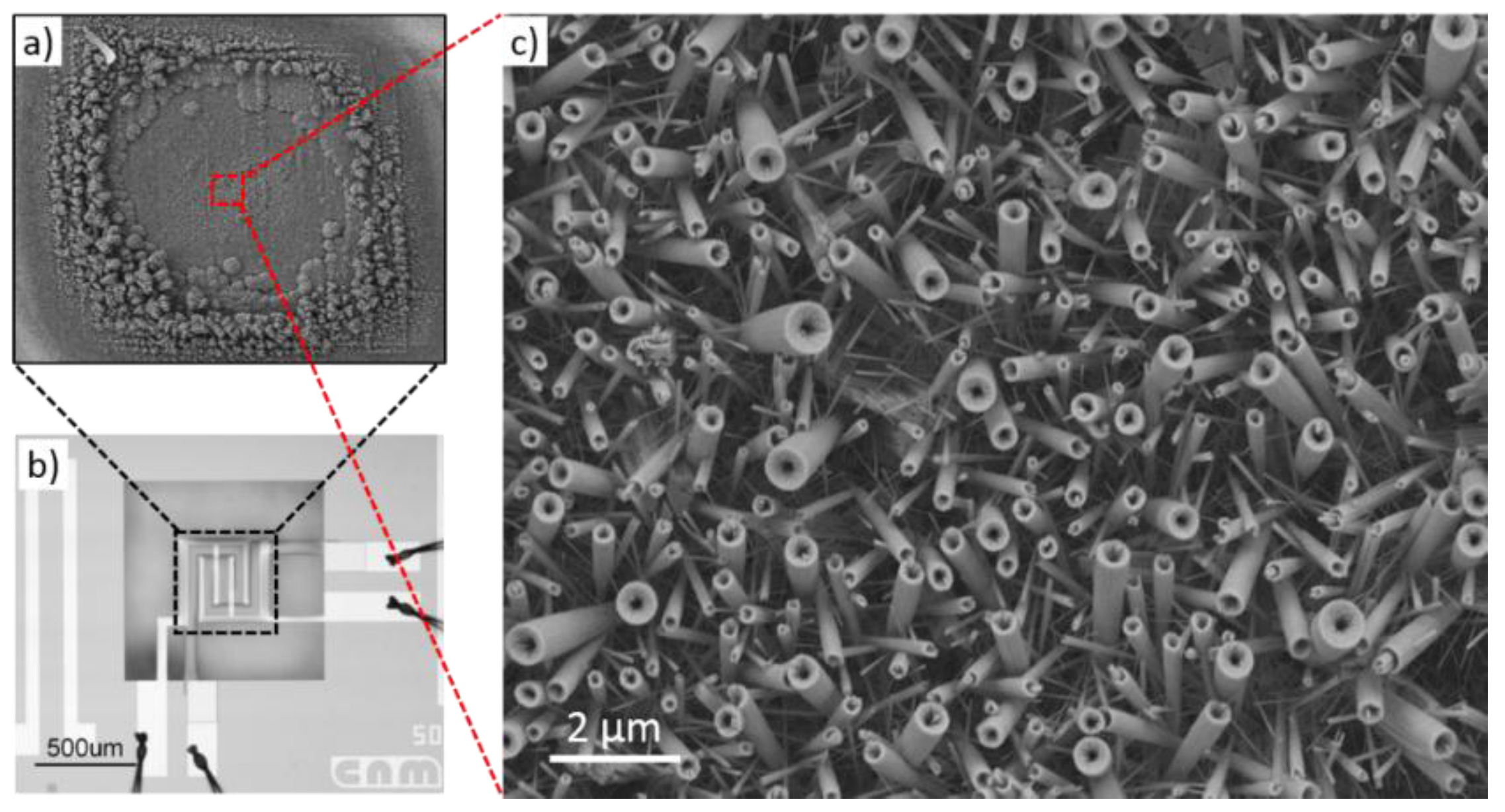
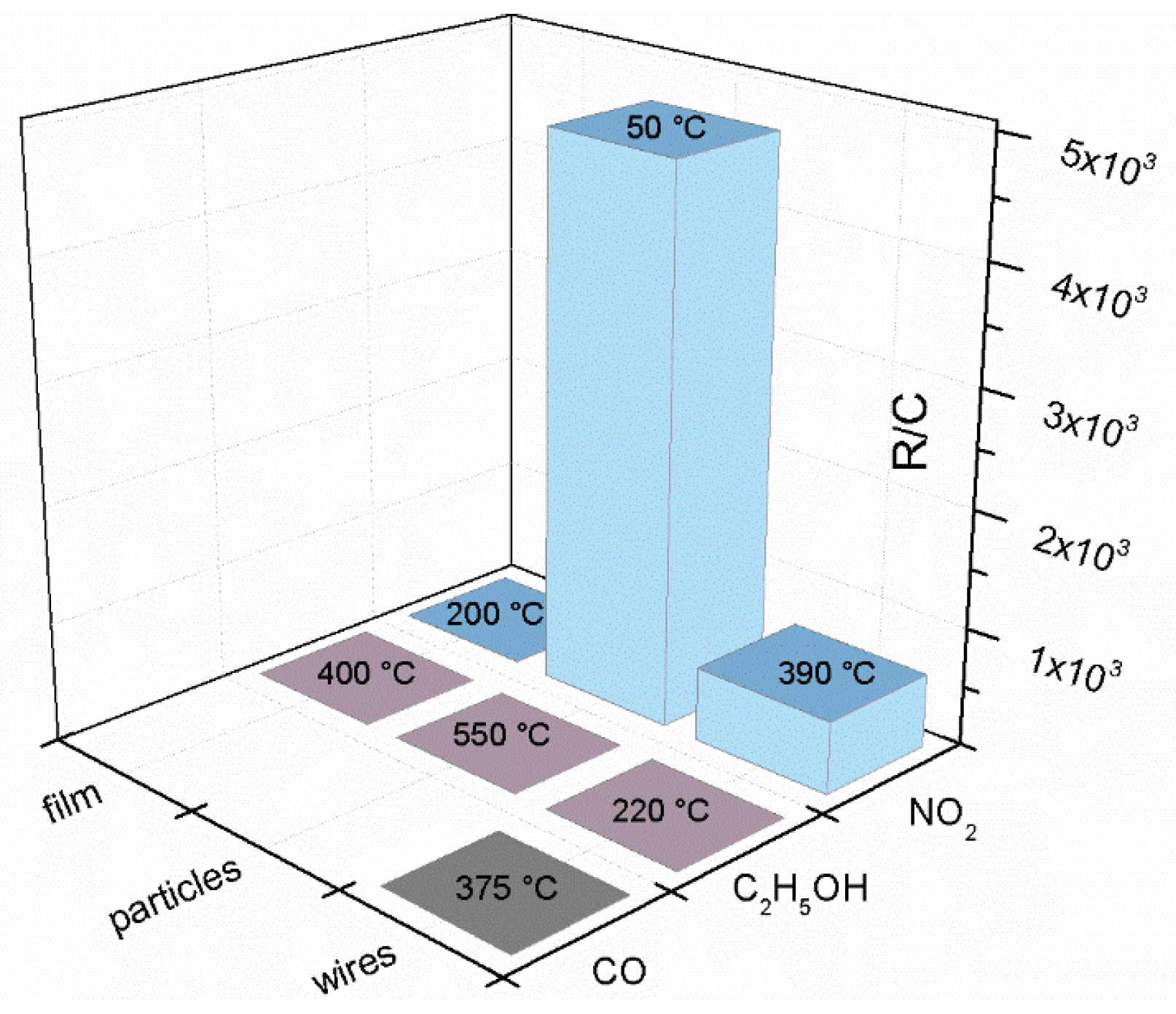
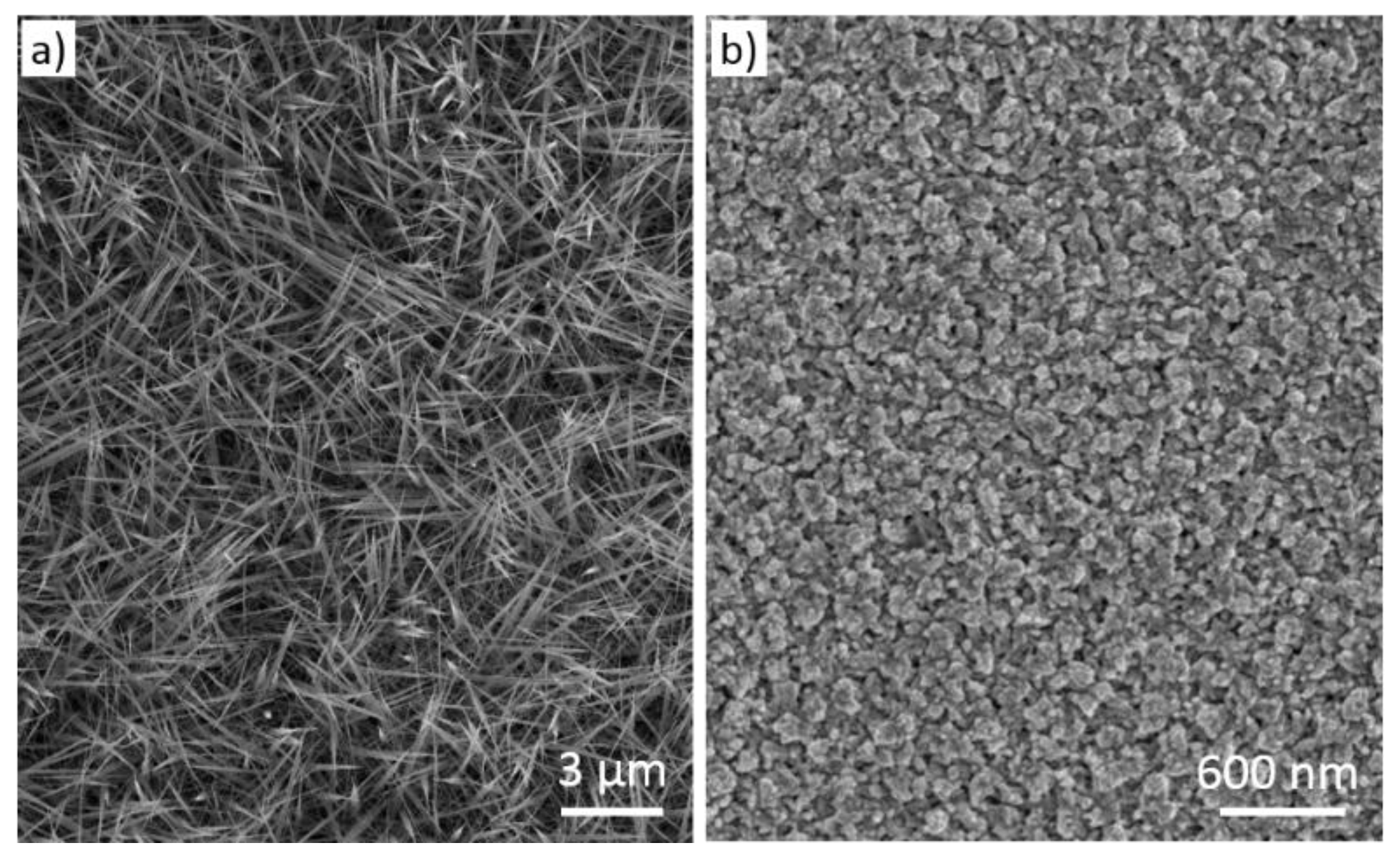
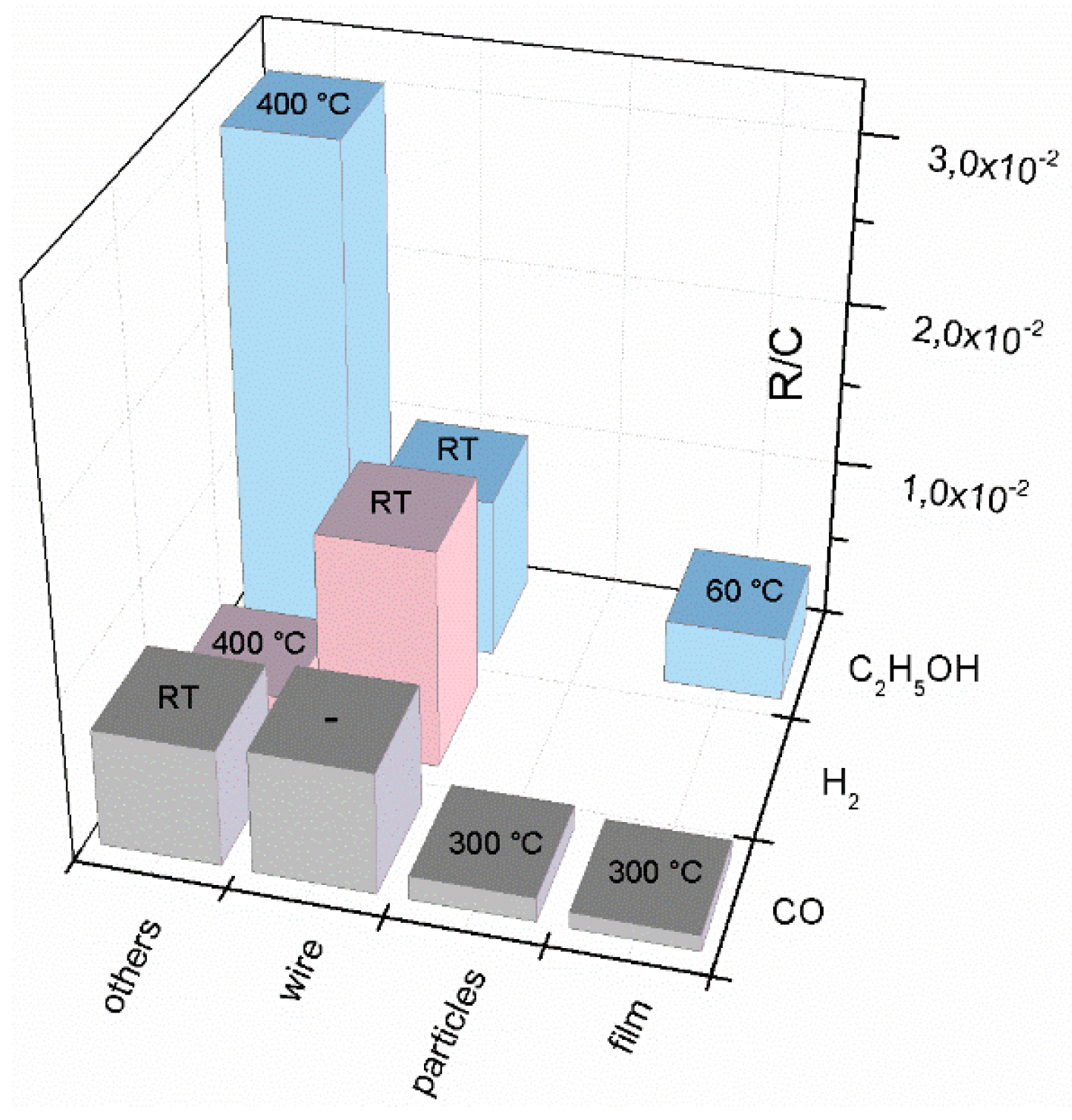
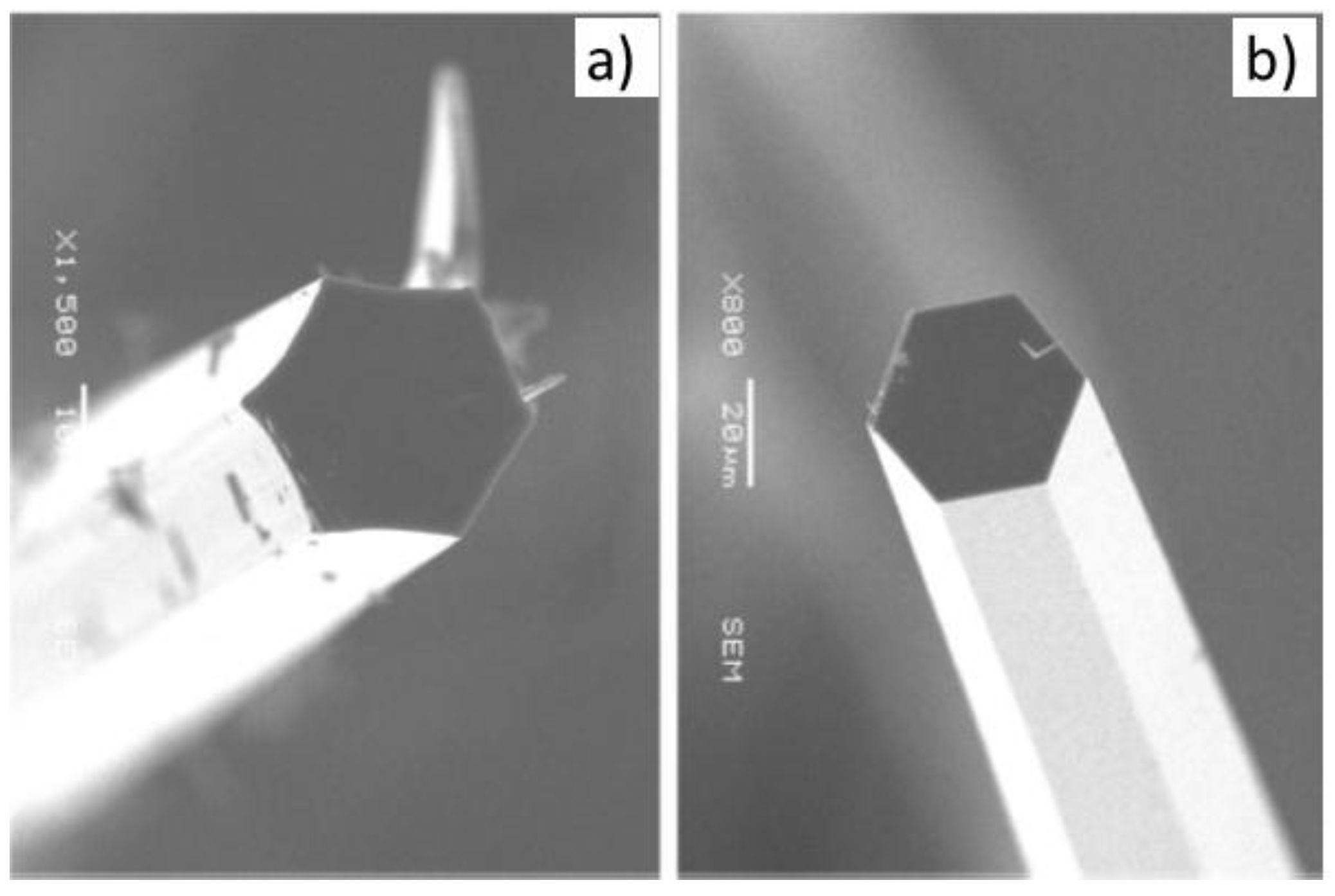
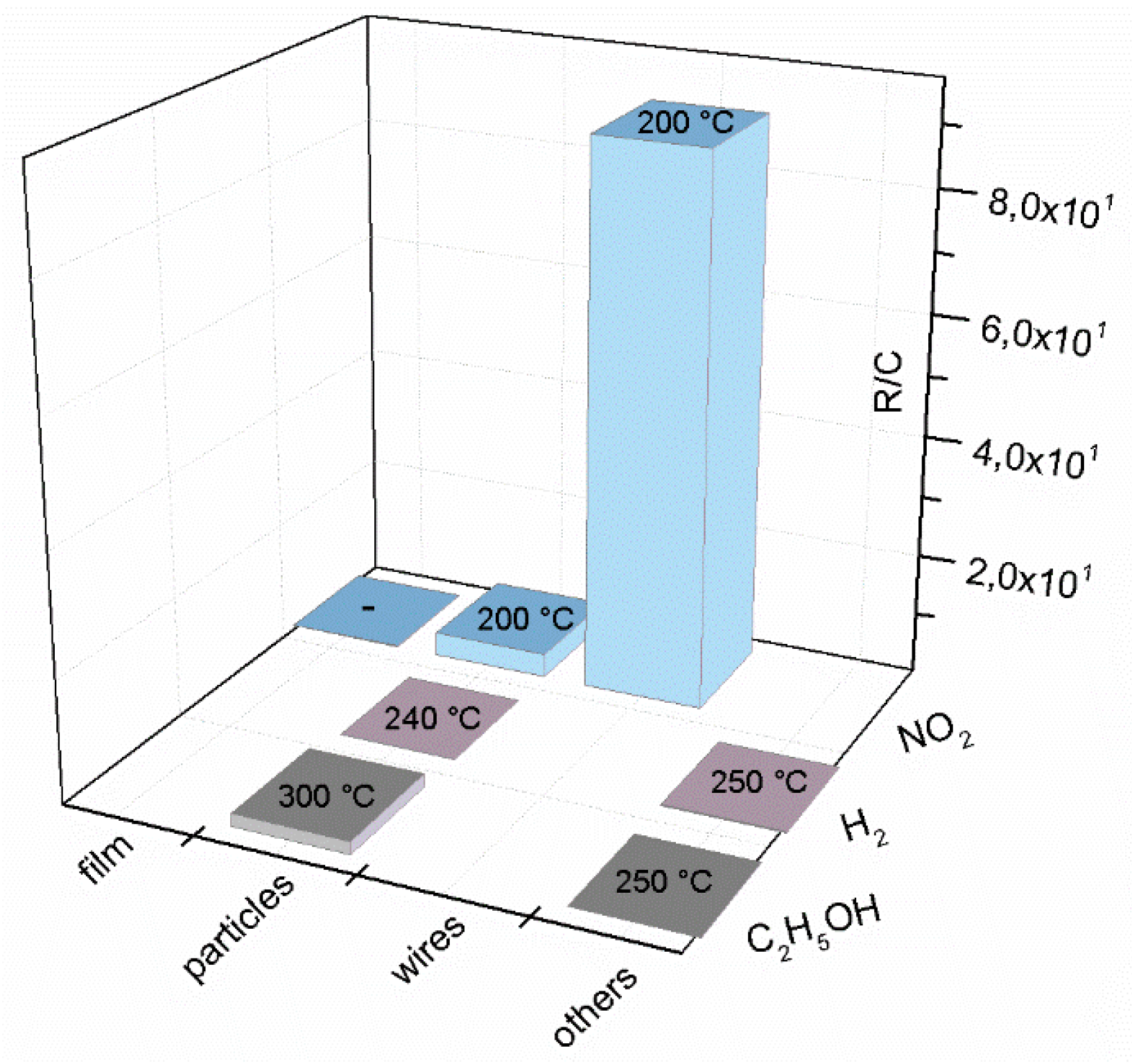
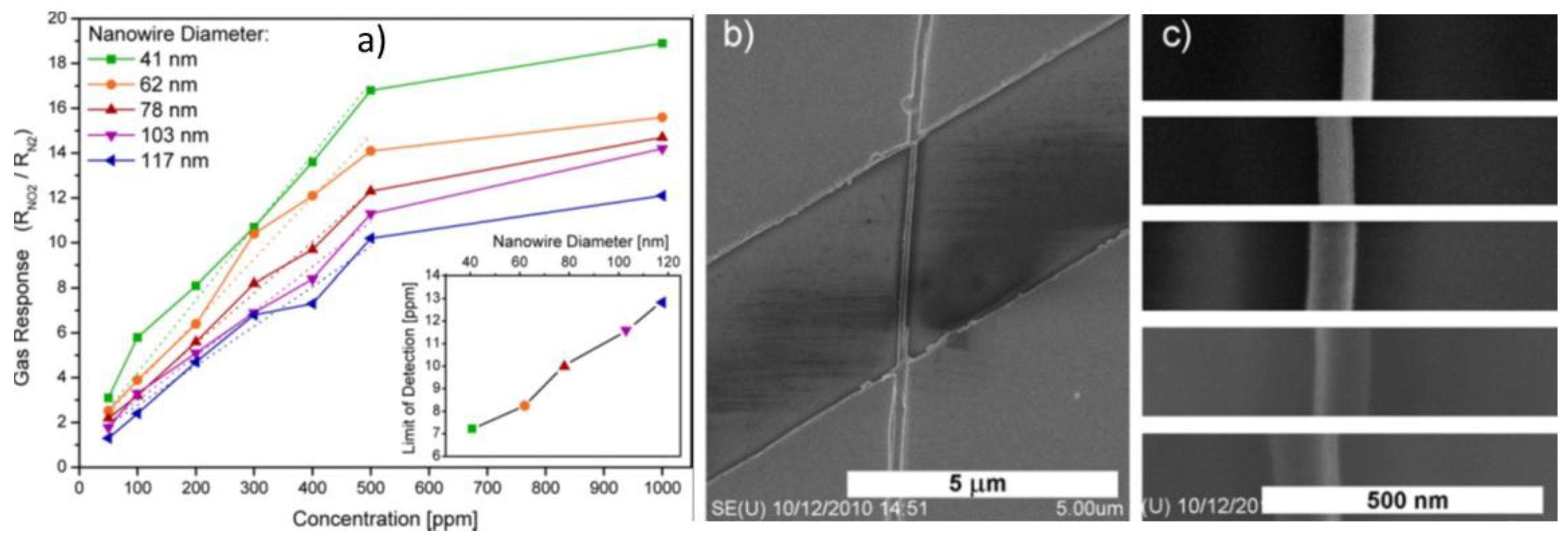
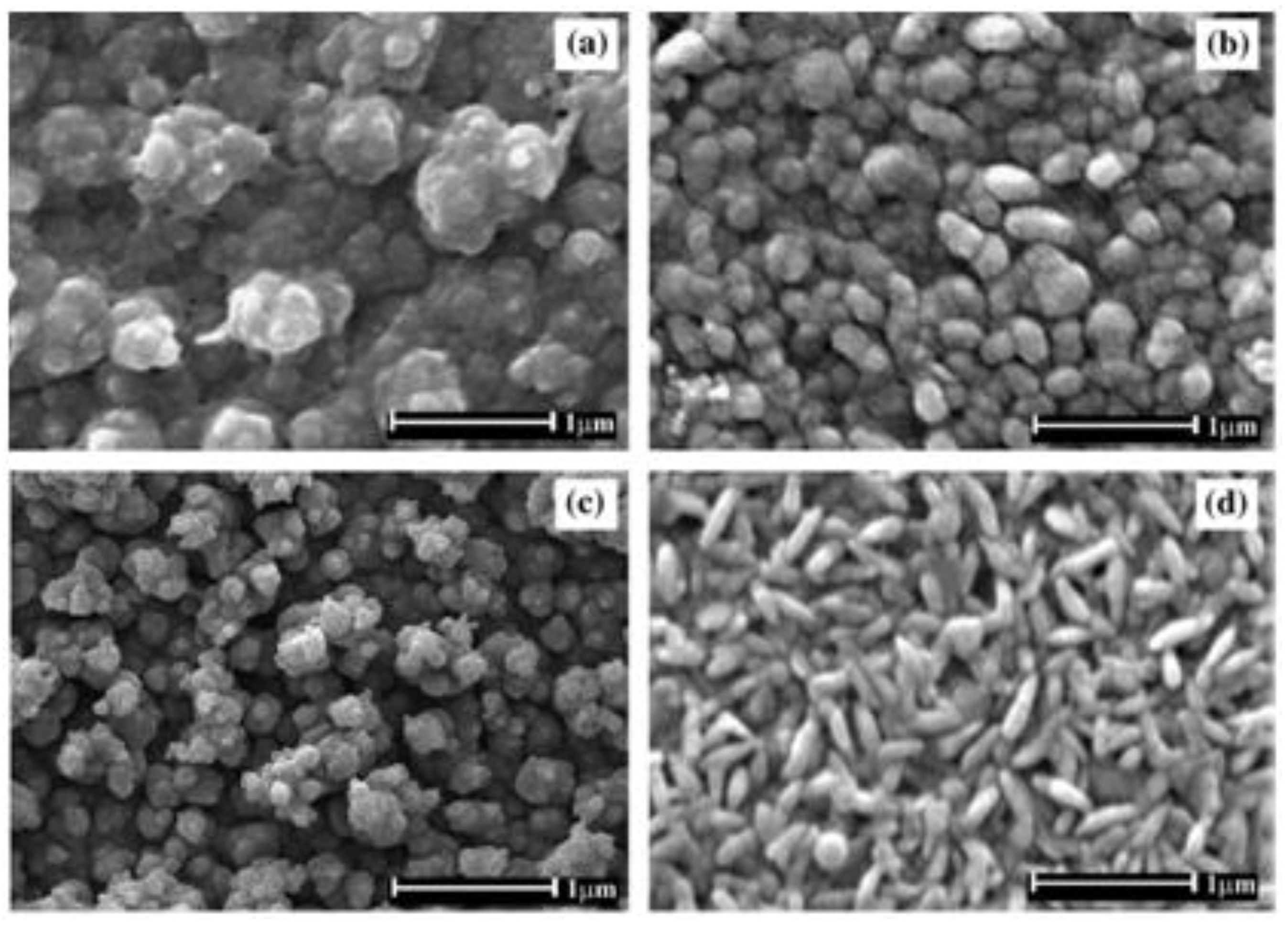


| Prec. | CVD method | Tdep °C | Form | Features nm | Sensor type | Top °C | ppm | Gas | R | tres s | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W(OCl4) | PE | - | film | - | Ω | 200 | 10 | NO2 | 48 | - | [21] |
| WCl6 | AP | 625 | film | 3600T | Ω | 400 | 20 | C2H5OH | 8.5 | - | [20] |
| W(CO)6 | LP | 500 | NPs | 140Ø | Ω | >450 | 5000 | H2 | *** | - | [22] |
| W | HF | - | NPs | 100Ø | Ω | 50 | 1 | NO2 | 4701 | - | [16] |
| WO3 | EB | - | NPs | 9Ø 200T | NS* | 100 | 10 | NH3 | - | - | [18] |
| WCMPLX | AA | 500 | P | 1000Ø 30,000T | Ω | 550 | 20 | C2H5OH | 5.1 | - | [27] |
| W | HF | 800 | NWs | - | Ω | 450 | 1 | N2O | 4.41 | 175 | [17] |
| W(OPh)6 | AAEF | - | NWs | − | Ω | 250 | 0.8 | NO2 | 120 | - | [26] |
| W(CO)6 | AAL°c | 580 | NWs | 100–400Ø | Ω | 375 | 80 | CO | 8 | - | [23] |
| W(OPh)6 | AA | 400 | NWs | 60–120Ø 7000L | Ω | 150 | 100 | CO | 5 | - | [25] |
| W(OPh)6 | AA | 400 | NWs | 60–120Ø 7000L | Ω | 200 | 1 | C6H6 | 2 | 1114 | [29] |
| W(CO)6 | AA | 500 | NWs | 50–100Ø 11000L | Ω | 390 | 0.4 | NO2 | 250 | - | [24] |
| W(CO)6 | AA | 390 | NWs | 50–100Ø 10,000L | Ω | 190 220 | 100 100 | C7H8 C2H5OH | 3 3.5 | 450 - | [30] |
| WO2.9 | CVD | 400 | NRs | 30–110Ø 1000T | O | RT | 65** | H2O | 2.16 | - | [19] |
| Prec. | CVD method | Tdep °C | Form | Features nm | Sensor Type | Top °C | ppm | gas | R | tres s | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Et2Zn | MO | - | Film | 130FT | Ω | 300 | 1660 | CO | 1.6 | - | [44] |
| Et2Zn | PE | - | Film | 500 FT 38 CZ | Ω +O | RT | 200000 | O2 | 1.8 | - | [45] |
| ZnCMPLX | AA | 450 | Film | 25CZ | Ω | 60 | 500 | C2H5OH | 2 | 10 | [51] |
| Et2Zn | MO | 450 | NPs | - | Ω | 300 | 1000 | CO | 1.53 | - | [46] |
| Zn(OAc)2 | AA | 350 | NPs | 12CZ | Ω | 300 | 10000 | DMA | 1.7 | 240 | [52] |
| Zn | CVD | 550 | NWs | 30000Ø | Ω+OSNW | RT | 200 | C2H5OH | 2 | - | [36] |
| ZnO | CVD | - | NWs | 130Ø 4000L | Ω+OSNW | 200 | 1×106 | CO | 4 | - | [53] |
| Zn | CVD | - | NWs | 80Ø 3500L | FETSNW | - | 400 | CO | 3 | - | [37] |
| Zn | UHV | 650 | NWs | 100Ø | Ω+OSNW | RT | 100 | H2 | 1.35 | 3 | [38] |
| Et2Zn | MO | 500 | NRs | 100Ø | Ω | 300 | 500 | O2 | 3.5 | - | [47] |
| Zn | CVD | 600 | NRs | - | Ω+O | RT | 2.5 | O3 | 1300 | 45 | [39] |
| Zn | PE | - | NRs | 100Ø 2000L | Ω | 400 | CH2O | 100 | - | [40] | |
| Zn | CVD | 700 | HS | - | Ω | RT | 250 | CO | 1.8 | - | [41] |
| Zn | VT | 700 | HS | 800TB 150TC | FETSNW | 200 | 1 | NO2 | 2 | - | [42] |
| ZnCMPLX | PE | 300 | HS | Ω | 100 | 0.28 | O3 | 1000 | - | [49] | |
| Zn | VT | 410 | HS | 5000Ø | Ω | 400 | 205 | CH2O | 38 | - | [43] |
| ZnCMPLX | PE | 300 | HS | Ω | 400 | 5000 | H2 | 14 | - | [50] | |
| Zn(NO3)2 | C | 1100 | F | 20000 | Ω SF | 400 | 500 | C2H5OH | 15.3 | - | [48] |
| Prec. | CVD method | Tdep, °C | Form | Features, nm | Sensor type | Top °C | ppm | gas | R | tres s | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SnCl2.2H2O | CVD | - | Film | 100T | Ω | - | 6 | NO2 | 1.2 | - | [59] |
| TTB | MO | 350 | Film | 50T | Ω | RT | 5 | H2S | 1.1 | - | [63] |
| SnCl4 | IBI | RT | Film | 400T | Ω | 500 | - | H2 | - | - | [60] |
| SnCl4 | ALD | 250 | Film | 2.6T | Ω | 300 | - | CO | 43 | - | [61] |
| TMH | LE | - | NPs | ~15Ø | Ω | 200 | 20 | NO2 | 77 | 160 | [66,67] |
| Sn(NO3)4 | Loc | 375 | NPs | - | Ω | - | 200 | CH4O | 5 | - | [64] |
| T-crown | AA | 400 | NPs | 18–36Ø | Ω | 300 | 10 | NO2 | 1.7 | - | [65] |
| TEH | C | 850 | NPs | 1000Ø | Ω | 300 | 500 | C2H5OH | 1075 | 31 | [68] |
| SnCl2.2H2O | CVD | 375 | P | - | Ω | 240 | 300 | H2 | 1.03 | - | [62] |
| Sn | CVD | 750 | NWs | - | Ω | 400 | CO | 3.9 | 10 | [56] | |
| Sn | CVD | 800 | NWs | 41Ø | ΩSNW | 300 | 500 | NO2 | 17 | 3 | [57] |
| DBTA | PE | - | NRs | 1200L 45Øb-10Ø | ΩSNW | 250 | 100 | H2 | 13 | - | [69] |
| Sn | CVD | 800 | NWs | 60Ø 20,000L | Ω | 200 | 1 | NO2 | 90 | 8 | [58] |
| TTB | CVD | 700 | Plates | 30–40T | Ω | 250 | 100 | C2H5OH | 1.5 | 10 | [55] |
| Material | Prec. | CVD Method | Tdep °C | FT nm | Top °C | ppm | gas | R | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Co3O4:F | Co(dpm)2 Co(hfa)2·TMEDA | PE | 200 300 400 | 200 | 100 | Acetone | [90] | ||
| Cr2O3:Ti | Cr(acac)3 Ti-butoxide | AA | 550 | 150-1000 | [82] | ||||
| Cr2O3:Ti | CrO2Cl2 TiCl4 | AP | 400 475 550 | 500 1000 1500 | 400 | 80 | CH3CH2OH | 3.1 1.7 1.1 | [83,84] |
| Cr2O3:Ti | Chromium acetate Ti(acac)2OiPr2 | ESAVD | 650 | 200 300400500 | 500 | NH3 | 1.05 1.18 1.22 1.46 | [85,86] | |
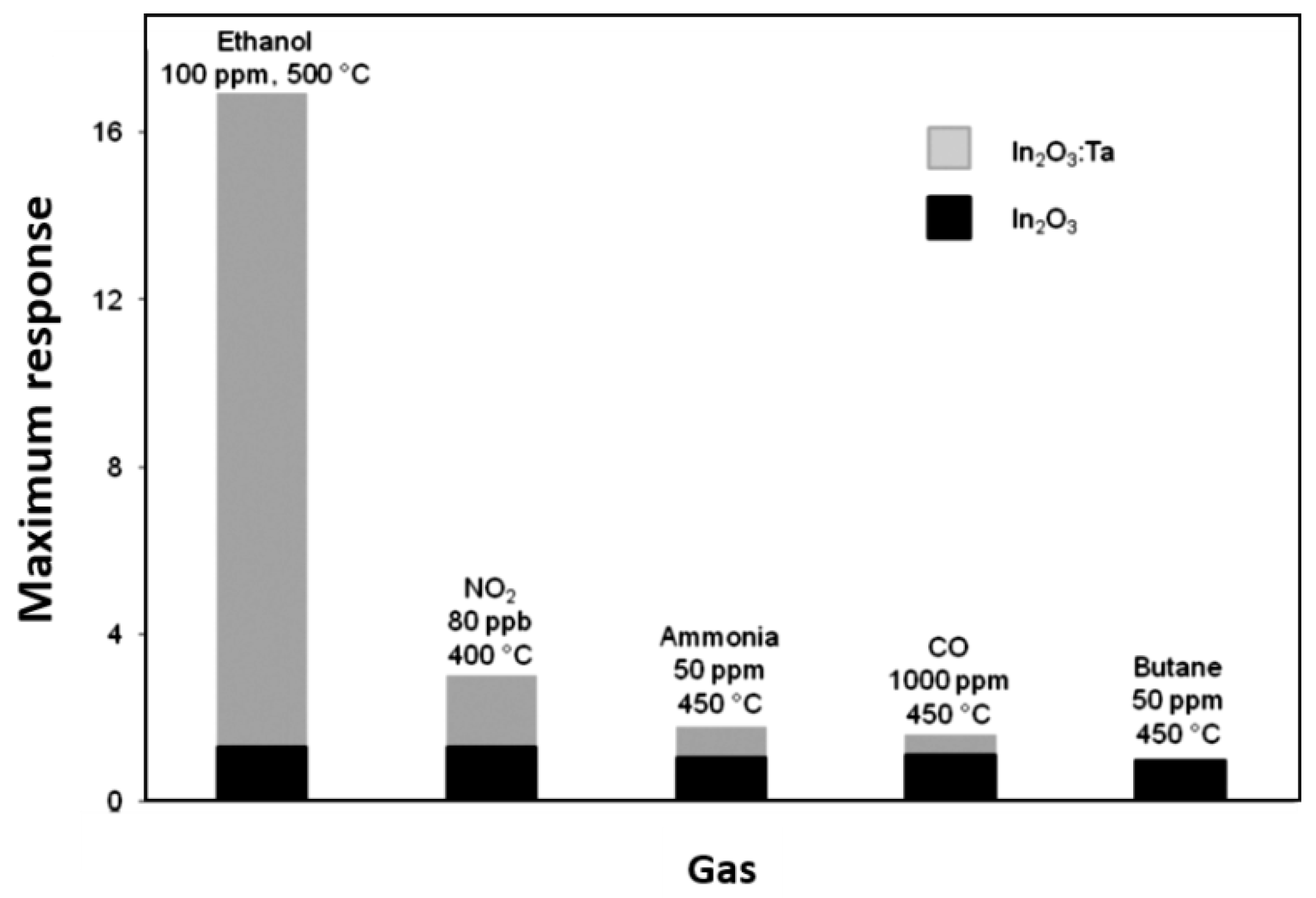
| In2O3:Ta | InMe3 Ta(NMe2)5 | AA | 550 | 650 | 500 | 100 0.08 | CH3CH2OH NO2 | 16.9 3.01 | [95] |
| In2O3:Ti | InMe3 Ti(NMe2)4 | AA | 550 | 790 | 500 | 100 0.08 | CH3CH2OH NO2 | 2.62 1.80 | [95] |
| In2O3:Zn | ZnO In2O3 | CVD | 400-550 | RT | 1-5 | CO | [94] | ||
| SnO2:In | SnCl4 InCl3 | CVD | 400 | 200 | 50-250 | 1000 | H2 Methanol CO | 1.14 1.23 1.20 | [96,97] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejos, S.; Di Maggio, F.; Shujah, T.; Blackman, C. Chemical Vapour Deposition of Gas Sensitive Metal Oxides. Chemosensors 2016, 4, 4. https://doi.org/10.3390/chemosensors4010004
Vallejos S, Di Maggio F, Shujah T, Blackman C. Chemical Vapour Deposition of Gas Sensitive Metal Oxides. Chemosensors. 2016; 4(1):4. https://doi.org/10.3390/chemosensors4010004
Chicago/Turabian StyleVallejos, Stella, Francesco Di Maggio, Tahira Shujah, and Chris Blackman. 2016. "Chemical Vapour Deposition of Gas Sensitive Metal Oxides" Chemosensors 4, no. 1: 4. https://doi.org/10.3390/chemosensors4010004