Building Selectivity for NO Sensing in a NOx Mixture with Sonochemically Prepared CuO Structures
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of CuO
2.2. Structural Characterization
2.3. NOx Equilibration Studies
2.4. Sensing Experiments
3. Results
3.1. Structural Investigations
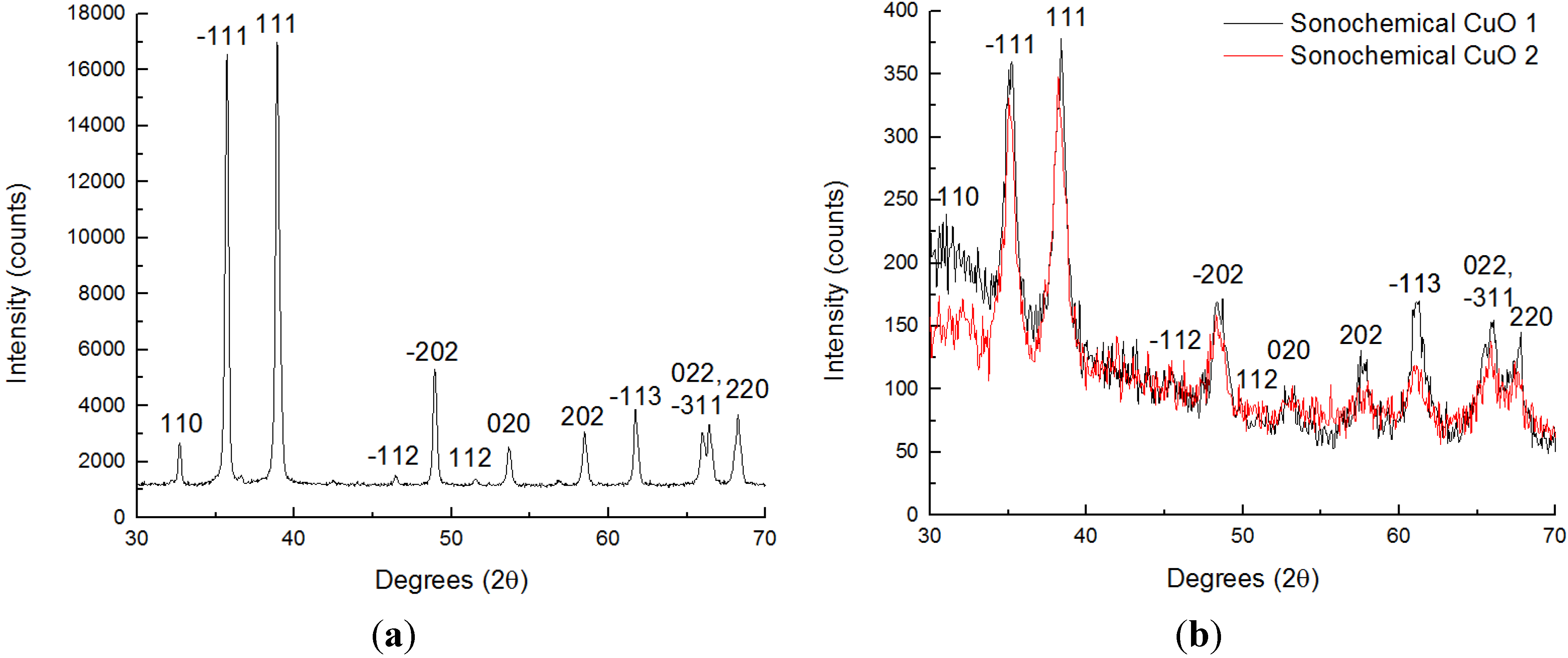
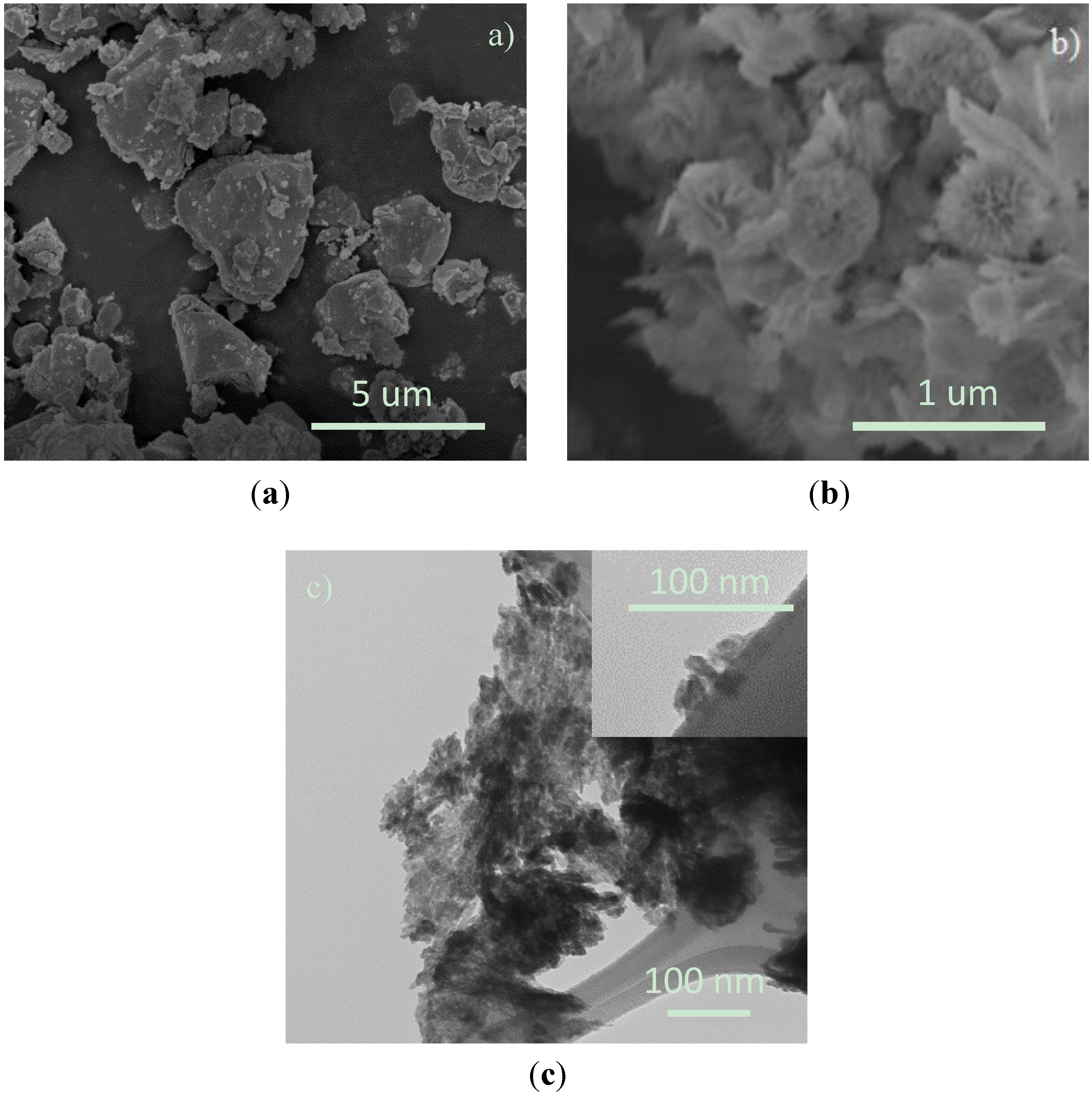
3.2. NOx Equilibration Experiments

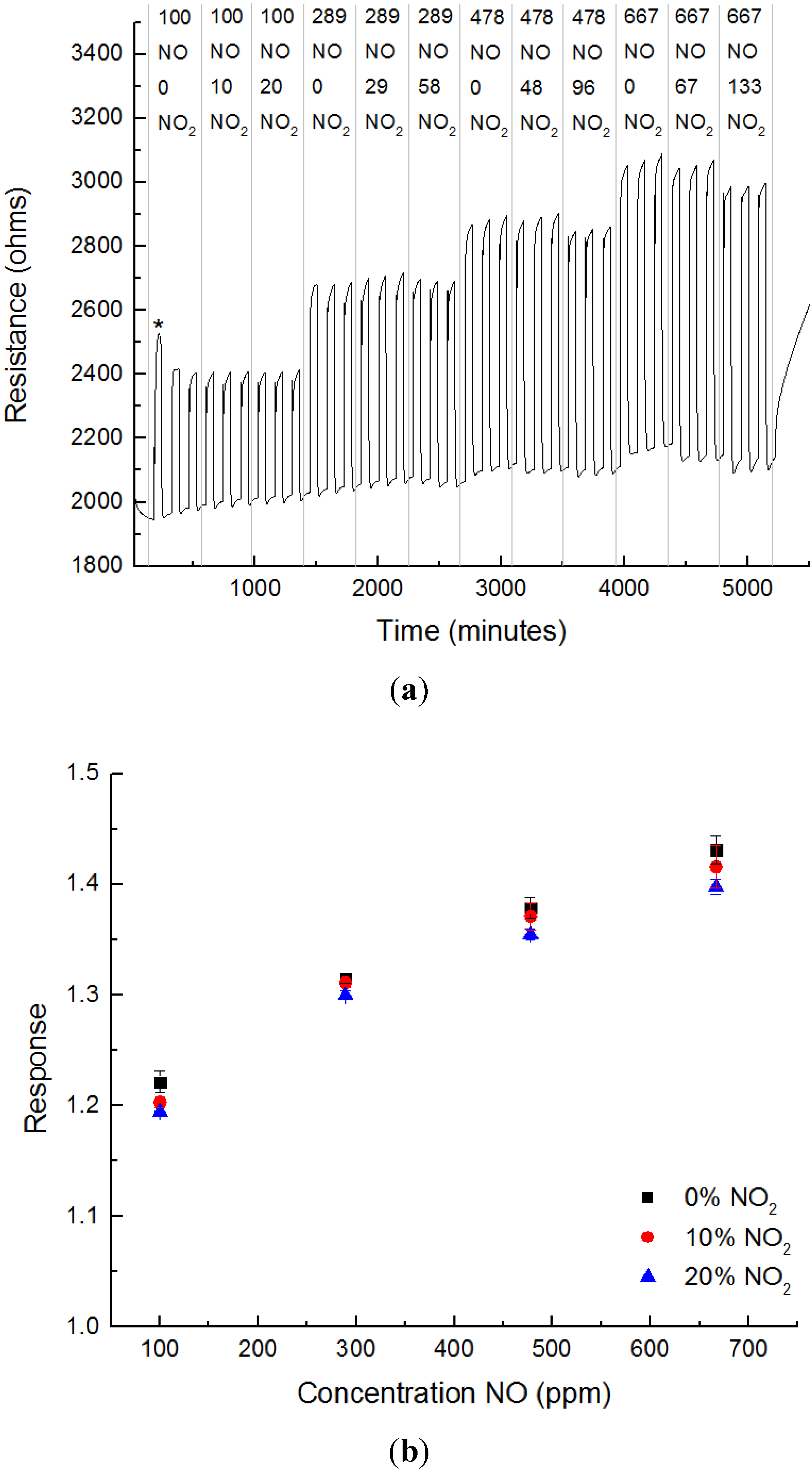
3.3. Sensing Experiments
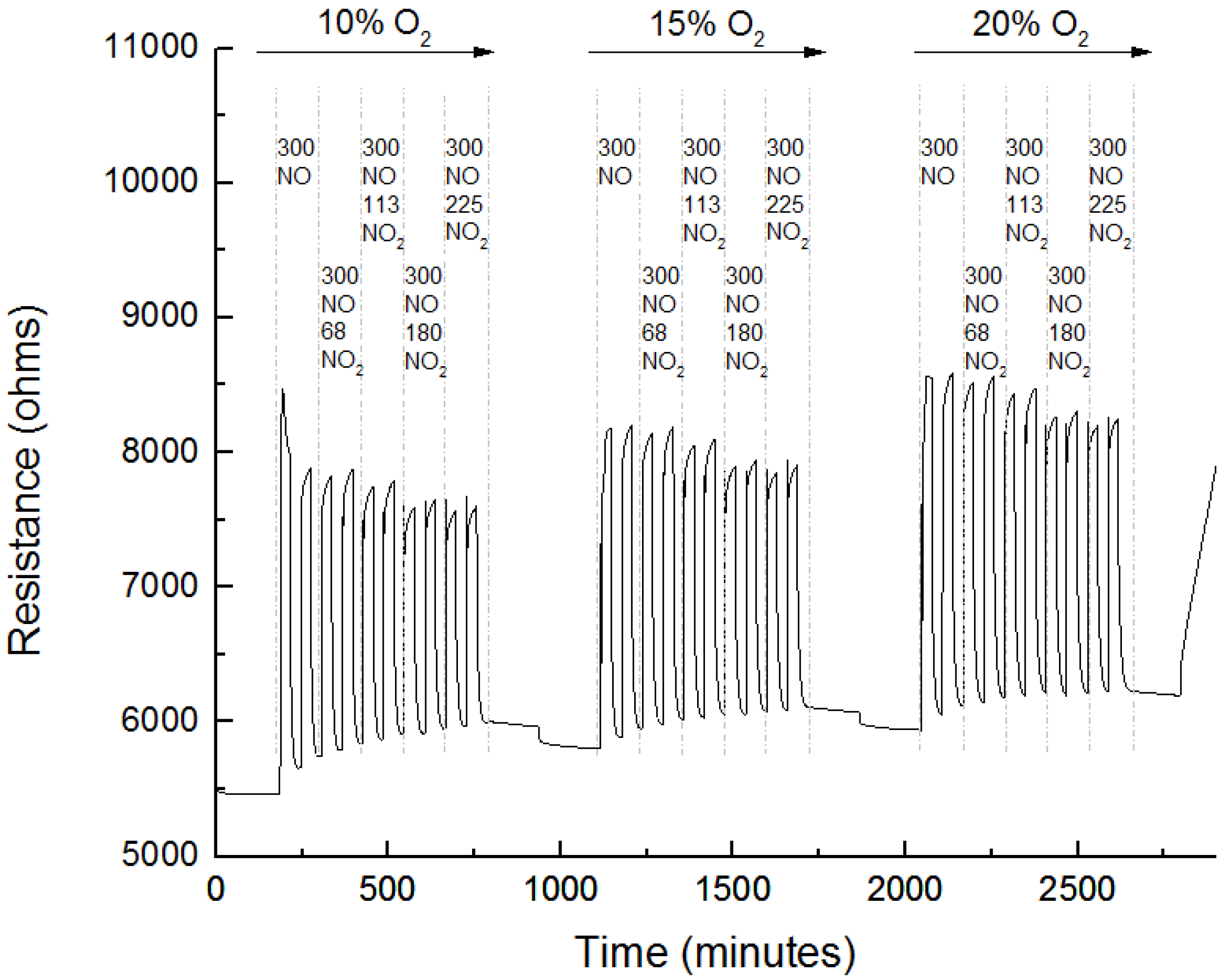
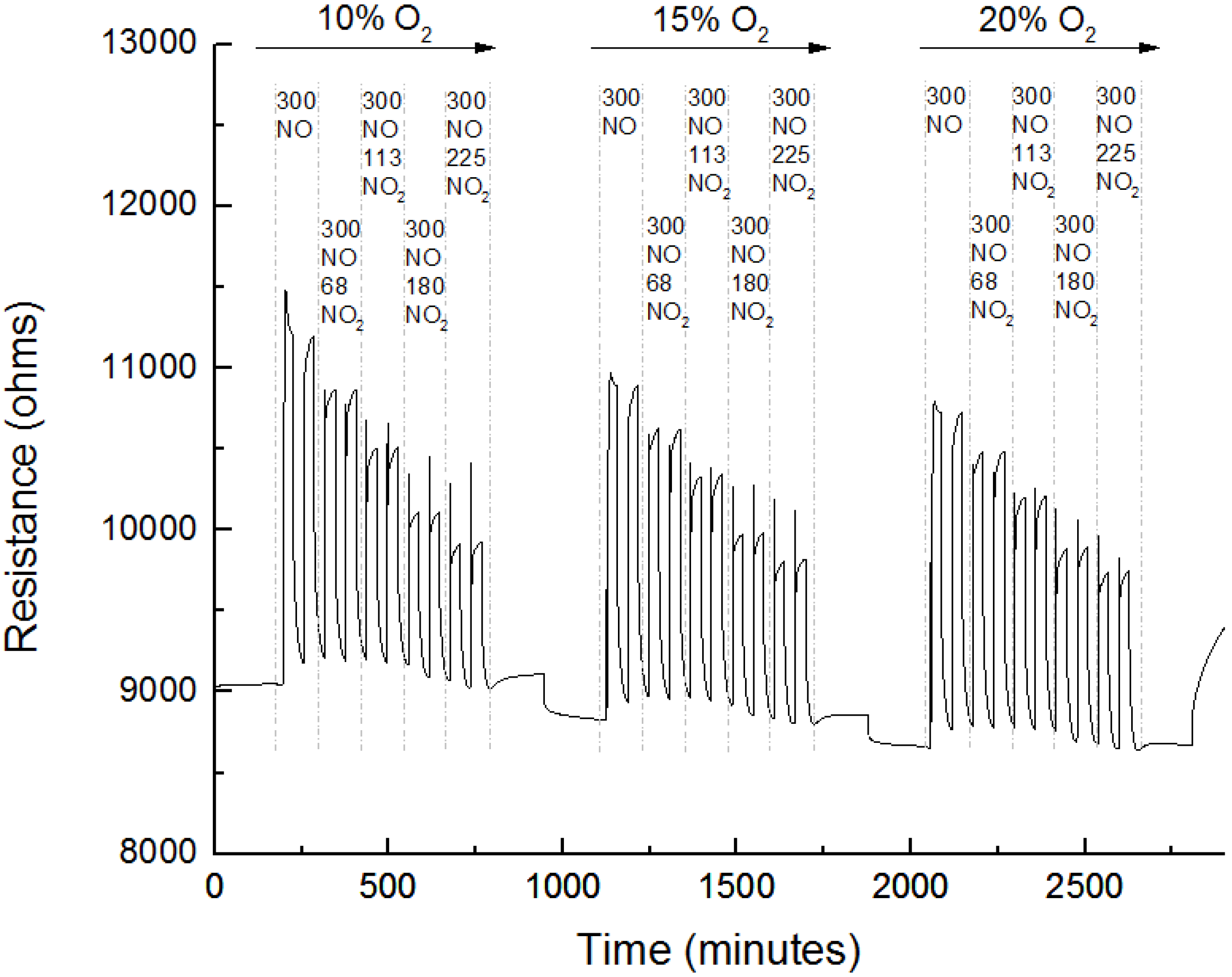

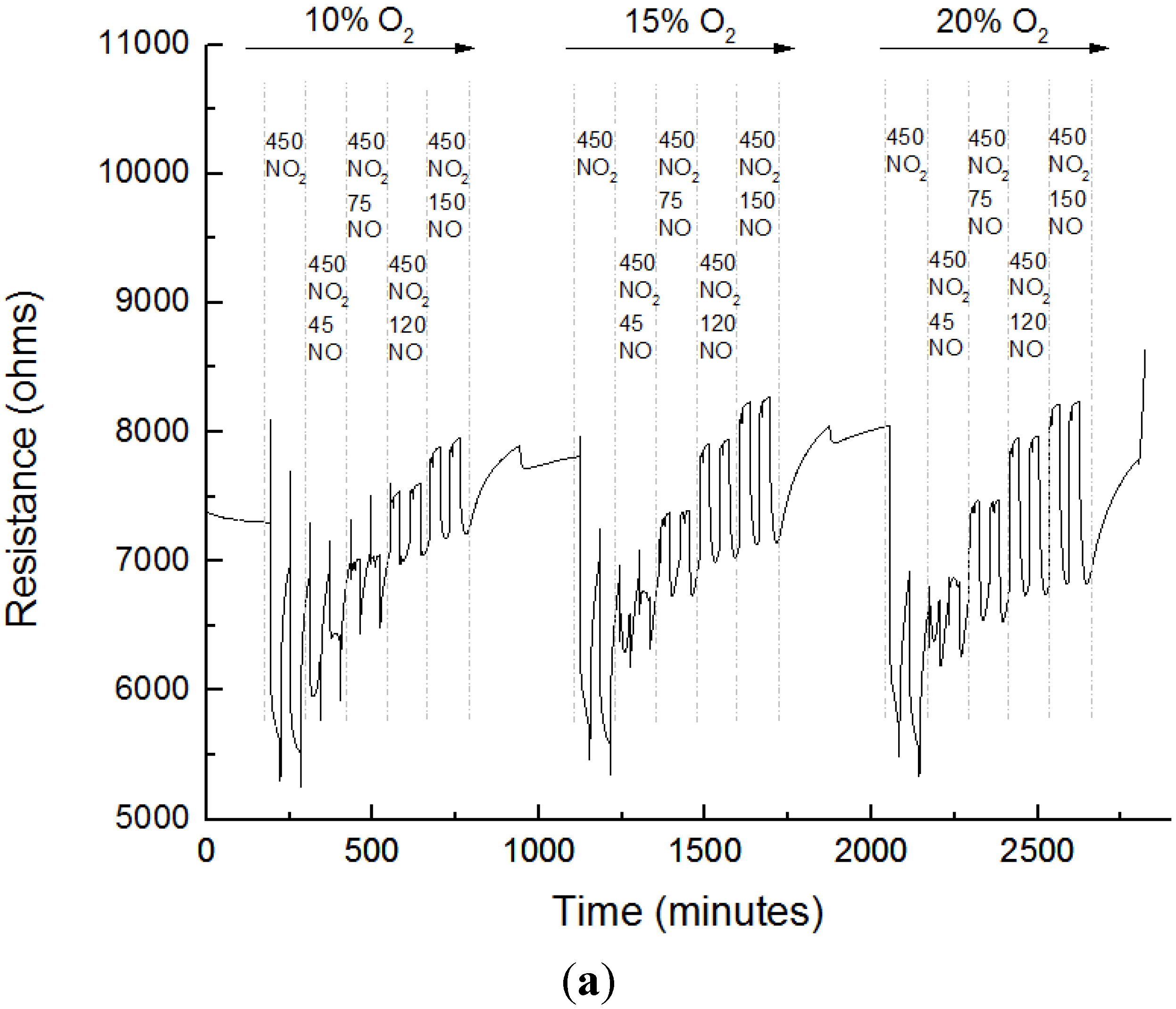
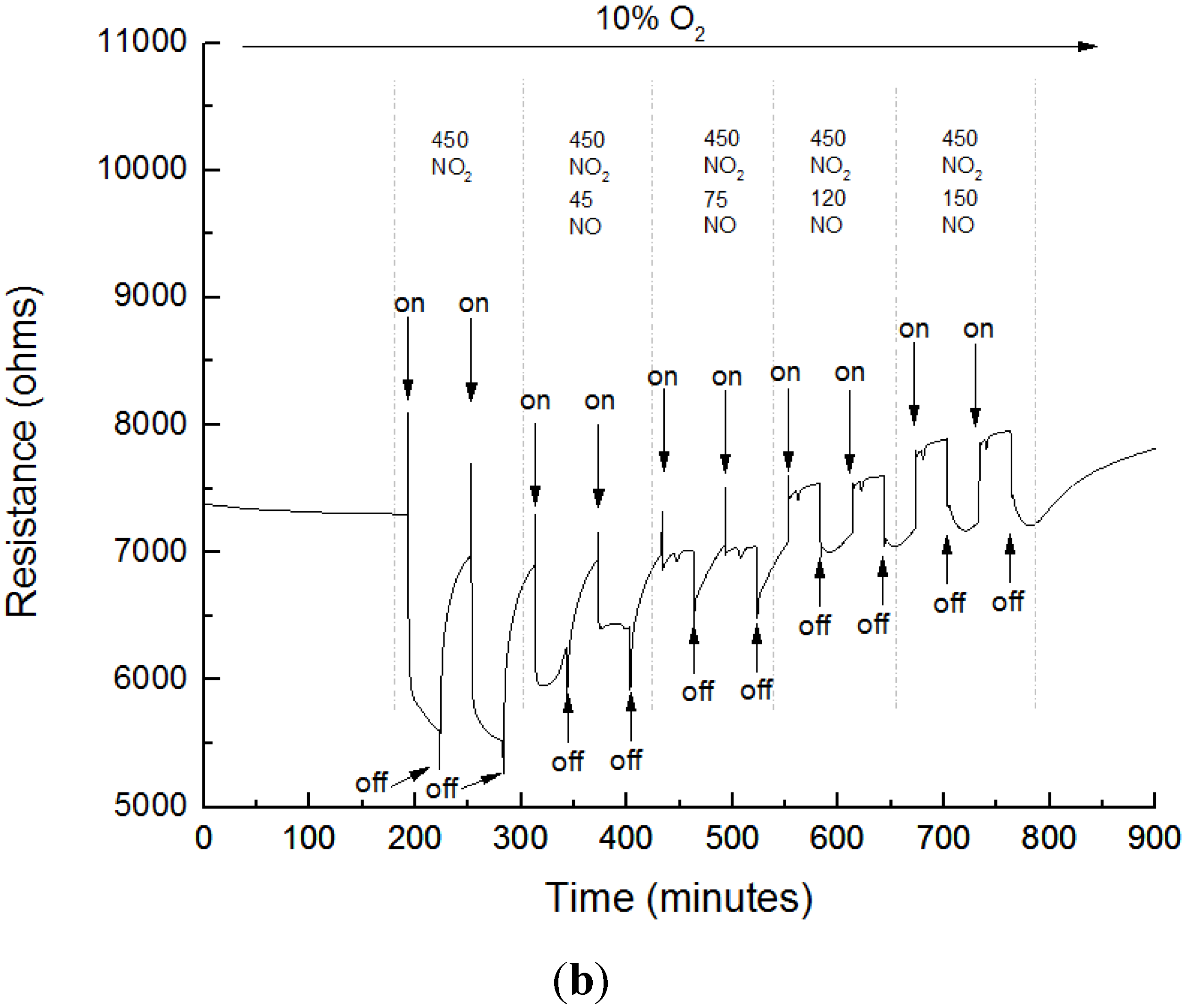
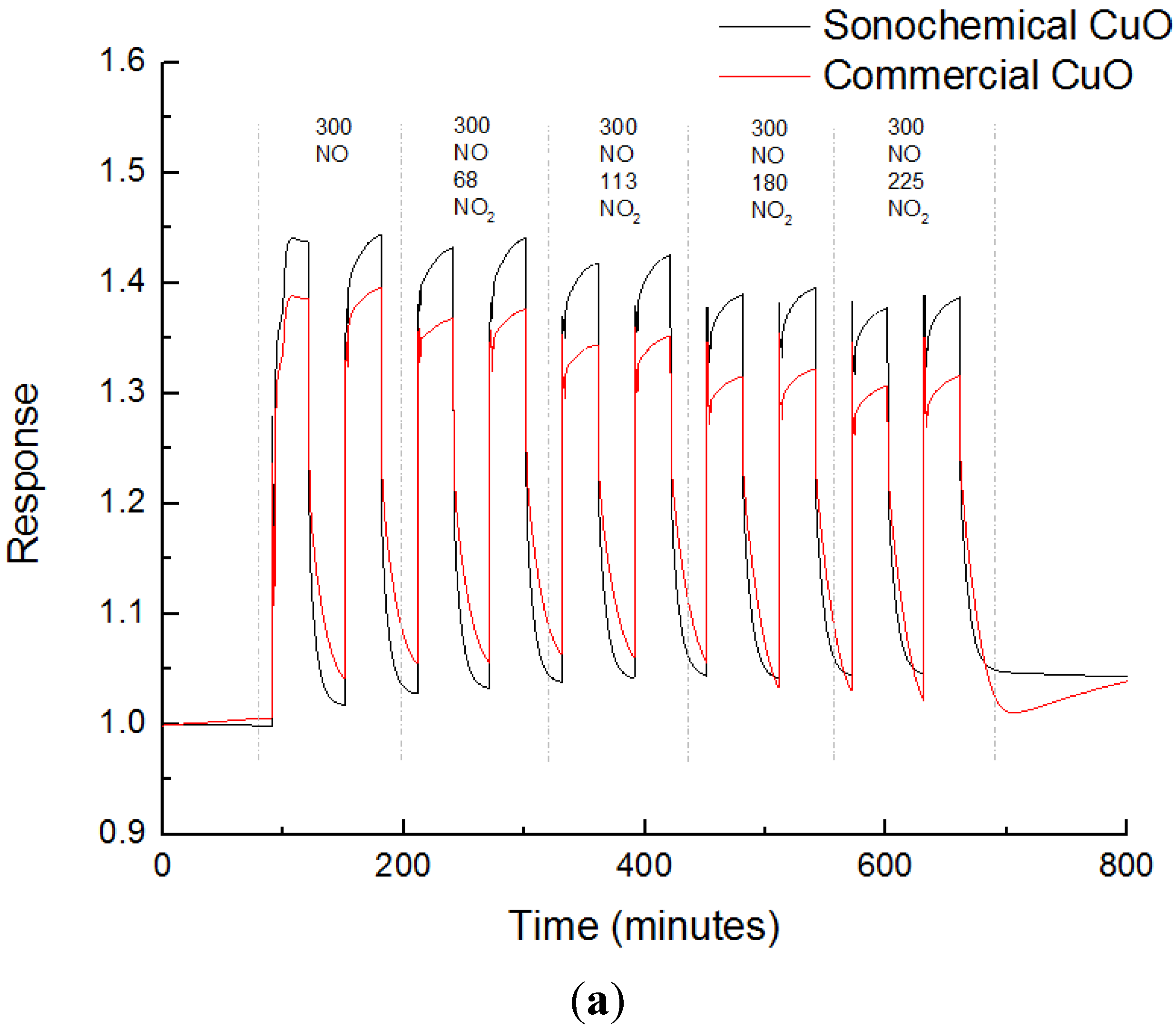
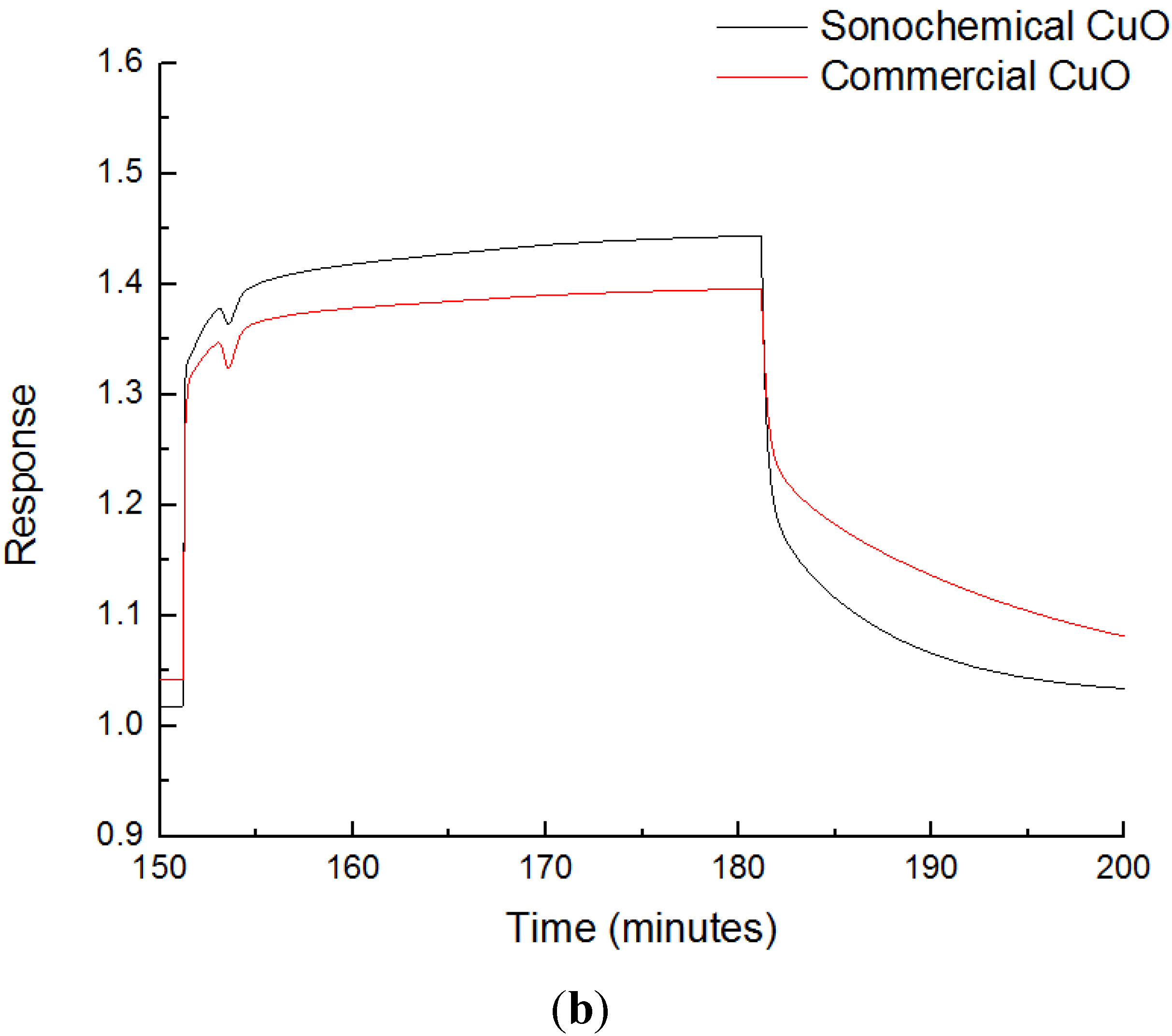
3.4. Interference to CO
| Pulse (NO ppm) | Response | Pulse (CO ppm) | Response | Pulse (NO:CO ppm) | Response |
|---|---|---|---|---|---|
| 200 NO | 1.31 | 10 | 1.12 | 200:10 | 1.16 |
| 400 NO | 1.37 | 20 | 1.12 | 400:10 | 1.19 |
| 600 NO | 1.41 | 30 | 1.11 | 600:10 | 1.20 |
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Fergus, J.W. Materials for high temperature electrochemical NOx gas sensors. Sens. Actuators B Chem. 2007, 121, 652–663. [Google Scholar] [CrossRef]
- Blackburn, B.M.; Wachsman, E.D. Performance of Thermally Modified, Potentiometric Gas Sensor Array in Gas Mixtures. ECS Trans. 2009, 16, 339–354. [Google Scholar]
- Yang, J.-C.; Dutta, P.K. Promoting selectivity and sensitivity for a high temperature YSZ-based electrochemical total NOx sensor by using a Pt-loaded zeolite Y filter. Sens. Actuators B Chem. 2007, 125, 30–39. [Google Scholar] [CrossRef]
- Docquier, N.; Candel, S. Combustion control and sensors: A review. Prog. Energy Combust. Sci. 2002, 28, 107–150. [Google Scholar] [CrossRef]
- Park, J.; Yoon, B.Y.; Park, C.O. The mixed potentials of NiO(+ YSZ) and CuO electrodes in a mixture gas of NO and NO2. Sens. Lett. 2008, 6, 844–847. [Google Scholar] [CrossRef]
- Mondal, S.P.; Dutta, P.K.; Hunter, G.W.; Ward, B.J.; Laksowski, D.; Dweik, R. Development of high sensitivity potentiometric NOx sensor and its application to breath analysis. Sens. Actuators B Chem. 2011, 158, 292–298. [Google Scholar] [CrossRef]
- Sun, C.; Maduraiveeran, G.; Dutta, P. Nitric oxide sensors using combination of p- and n-type semiconducting oxides and its application for detecting NO in human breath. Sens. Actuators B Chem. 2013, 186, 117–125. [Google Scholar] [CrossRef]
- Can, F.; Courtois, X.; Berland, S.; Seneque, M.; Royer, S.; Duprez, D. Composition dependent performance of alumina-based oxide supported WO3 catalysts for the NH3-SCR reaction and the NSR + SCR coupled process. Catal. Today 2015, 257, 41–50. [Google Scholar] [CrossRef]
- Choi, S.-W.; Park, J.Y.; Kim, S.S. Growth behavior and sensing properties of nanograins in CuO nanofibers. Chem. Eng. J. 2011, 172, 550–556. [Google Scholar] [CrossRef]
- Hansen, B.J.; Kouklin, N.; Ganhua, L.; Lin, I.-K.; Chen, J.; Zhang, X. Transport, analyte detection, and opto-electronic response of p-type CuO nanowires. J. Phys. Chem. C 2010, 114, 2440–2447. [Google Scholar] [CrossRef]
- Kim, K.-M.; Jeong, H.-M.; Kim, H.-R.; Choi, K.-I.; Kim, H.-J.; Lee, J.-H. Selective Detection of NO(2) Using Cr-Doped CuO Nanorods. Sensors 2012, 12, 8013–8025. [Google Scholar] [CrossRef] [PubMed]
- Cruccolini, A.; Narducci, R.; Palombari, R. Gas adsorption effects on surface conductivity of nonstoichiometric CuO. Sens. Actuators B Chem. 2004, 98, 227–232. [Google Scholar] [CrossRef]
- Das, A.; Venkataramana, B.; Partheephan, D.; Prasad, A.K.; Dhara, S.; Tyagi, A.K. Facile synthesis of nanostructured CuO for low temperature NO2 sensing. Phys. E Low-Dimens. Syst. Nanostructures 2013, 54, 40–44. [Google Scholar] [CrossRef]
- Li, Y.; Liang, J.; Tao, Z.; Chen, J. CuO particles and plates: Synthesis and gas-sensor application. Mater. Res. Bull. 2008, 43, 2380–2385. [Google Scholar] [CrossRef]
- Yu, M.-R.; Wu, R.-J.; Chavali, M. Effect of ‘Pt’ loading in ZnO–CuO hetero-junction material sensing carbon monoxide at room temperature. Sens. Actuators B Chem. 2011, 153, 321–328. [Google Scholar] [CrossRef]
- Jun, S.T.; Choi, G.M. CO gas-sensing properties of ZnO/CuO contact ceramics. Sens. Actuators B Chem. 1994, 17, 175–178. [Google Scholar] [CrossRef]
- Hüebner, M.; Simion, C.E.; Tomescu-Stanoiu, A.; Pokhrel, S.; Barsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Nguyen, V.Q.; Jung, H.; Kim, D.; Kim, H.; Hong, S.-K. Synthesis of porous CuO nanowires and its application to hydrogen detection. Sens. Actuators B Chem. 2010, 146, 266–272. [Google Scholar]
- Volanti, D.P.; Felix, A.A.; Orlandi, M.O.; Whitfield, G.; Yang, D.-J.; Longo, E.; Tuller, H.L.; Varela, J.A. The role of hierarchical morphologies in the superior gas sensing performance of CuO-based chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kim, D.-H.; Hong, S.-H.; Hong, K.S. H2 and C2H5OH sensing characteristics of mesoporous p-type CuO films prepared via a novel precursor-based ink solution route. Sens. Actuators B Chem. 2013, 178, 395–403. [Google Scholar] [CrossRef]
- Gopalakrishna, D.; Vijayalakshmi, K.; Ravidhas, C. Effect of pyrolytic temperature on the properties of nano-structured Cuo optimized for ethanol sensing applications. J. Mater. Sci. Mater. Electron. 2013, 24, 1004–1011. [Google Scholar] [CrossRef]
- Yu, M.-R.; Suyambrakasam, G.; Wu, R.-J.; Chavali, M. Performance evaluation of ZnO-CuO hetero junction solid state room temperature ethanol sensor. Mater. Res. Bull. 2012, 47, 1713–1718. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Kang, Y.; Wu, S.; Wang, S. Brochantite tabular microspindles and their conversion to wormlike CuO structures for gas sensing. CrystEngComm 2012, 14, 620–625. [Google Scholar] [CrossRef]
- Yang, C.; Su, X.; Xiao, F.; Jian, J.; Wang, J. Gas sensing properties of CuO nanorods synthesized by a microwave-assisted hydrothermal method. Sens. Actuators B Chem. 2011, 158, 299–303. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly Monodisperse Cu2O and CuO Nanospheres: Preparation and Applications for Sensitive Gas Sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Radlik, M.; Adamowska, M.; Lamacz, A.; Krzton, A.; Da Costa, P.; Turek, W. Study of the surface evolution of nitrogen species on CuO/CeZrO2 catalysts. React. Kinet. Mech. Catal. 2013, 109, 43–56. [Google Scholar] [CrossRef]
- Chi, Y.; Chuang, S.S.C. Infrared Study of NO Adsorption and Reduction with C3H6 in the Presence of O2 over CuO/Al2O3. J. Catal. 2000, 190, 75–91. [Google Scholar] [CrossRef]
- Xiao, G.; Gao, P.; Wang, L.; Chen, Y.; Wang, Y.; Zhang, G. Ultrasonochemical-assisted Synthesis of CuO Nanorods with High Hydrogen Storage Ability. J Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Choi, G.M. Nonstoichiometry and electrical conduction of CuO. J. Phys. Chem. Solids 1996, 57, 81–84. [Google Scholar] [CrossRef]
- Suda, S.; Fujitsu, S.; Koumoto, K.; Yanagida, H. Effect of atmosphere and doping on electrical conductivity of CuO. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 1992, 31, 2488–2491. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Bacher, V.; Perbandt, C.; Schwefer, M.; Siefert, R.; Turek, T. Kinetics of the NO/NO2 equilibrium reaction over an iron zeolite catalyst. Appl. Catal. B Environ. 2013, 134–135, 55–59. [Google Scholar] [CrossRef]
- Hu, J.; Li, D.; Lu, J.G.; Wu, R. Effects on Electronic Properties of Molecule Adsorption on CuO Surfaces and Nanowires. J. Phys. Chem. C 2010, 114, 17120–17126. [Google Scholar] [CrossRef]
- Ishihara, T.; Shiokawa, K.; Eguchi, K.; Arai, H. The mixed oxide A12O3-V2O5 as a semiconductor gas sensor for NO and NO2. Sens. Actuators 1989, 19, 259–265. [Google Scholar] [CrossRef]
- Yin, X.; Han, H.; Kubo, M.; Miyamoto, A. Adsorption of NH3, NO2 and NO on copper-aluminate catalyst: an ab initio density functional study. Theor. Chem. Acc. 2003, 109, 190–194. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, K.; Xie, X. Theoretical studies of CO and NO on CuO and Cu2O(110) surfaces. Surf. Sci. 1994, 321, L249–L254. [Google Scholar] [CrossRef]
- Hansen, K.K. Solid state electrochemical DeNOx—An overview. Appl. Catal. B Environ. 2010, 100, 427–432. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullen, M.R.; Dutta, P.K. Building Selectivity for NO Sensing in a NOx Mixture with Sonochemically Prepared CuO Structures. Chemosensors 2016, 4, 1. https://doi.org/10.3390/chemosensors4010001
Mullen MR, Dutta PK. Building Selectivity for NO Sensing in a NOx Mixture with Sonochemically Prepared CuO Structures. Chemosensors. 2016; 4(1):1. https://doi.org/10.3390/chemosensors4010001
Chicago/Turabian StyleMullen, Max R., and Prabir K. Dutta. 2016. "Building Selectivity for NO Sensing in a NOx Mixture with Sonochemically Prepared CuO Structures" Chemosensors 4, no. 1: 1. https://doi.org/10.3390/chemosensors4010001
APA StyleMullen, M. R., & Dutta, P. K. (2016). Building Selectivity for NO Sensing in a NOx Mixture with Sonochemically Prepared CuO Structures. Chemosensors, 4(1), 1. https://doi.org/10.3390/chemosensors4010001







