Combinatorial Material Strategy: Parallel Synthesis and High-Throughput Screening of WO3 Nanoplates Decorated with Noble Metals for VOCs Sensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of WO3 Nanoplates
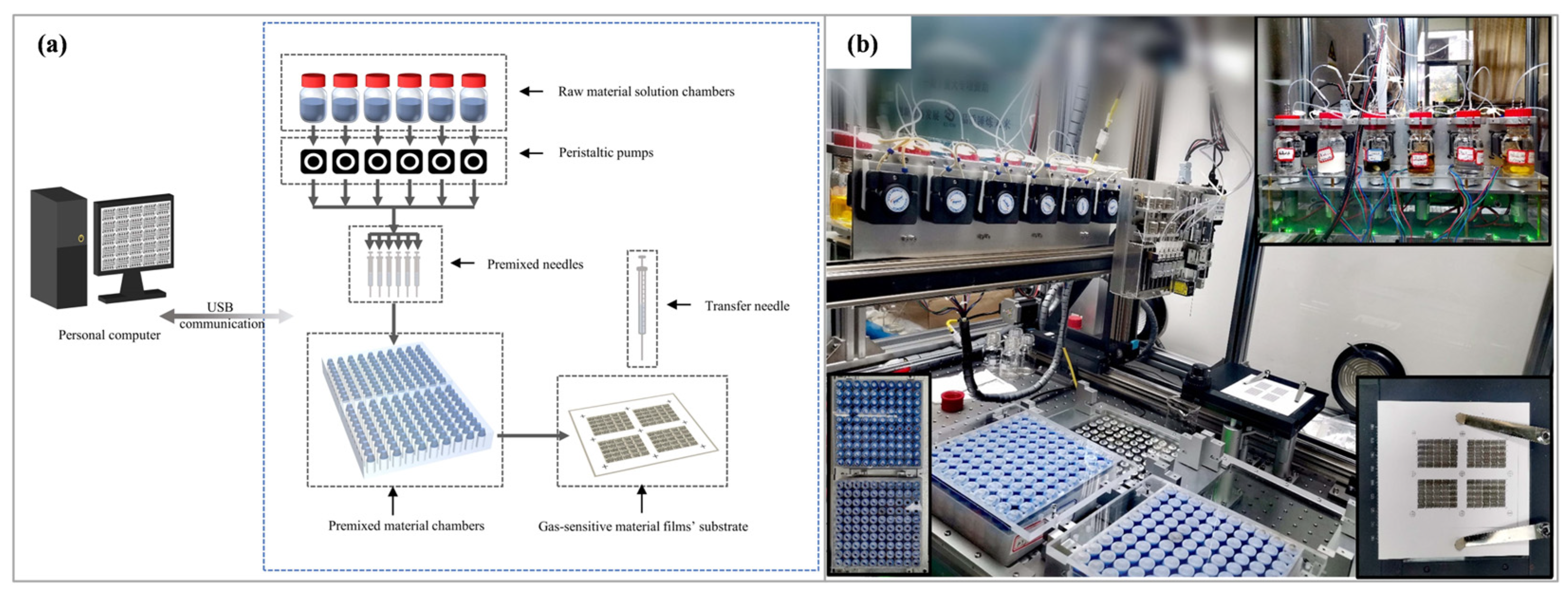
2.2. Preparation of Gas Sensors
2.3. Characterization
2.4. Measurements
3. Results and Discussion
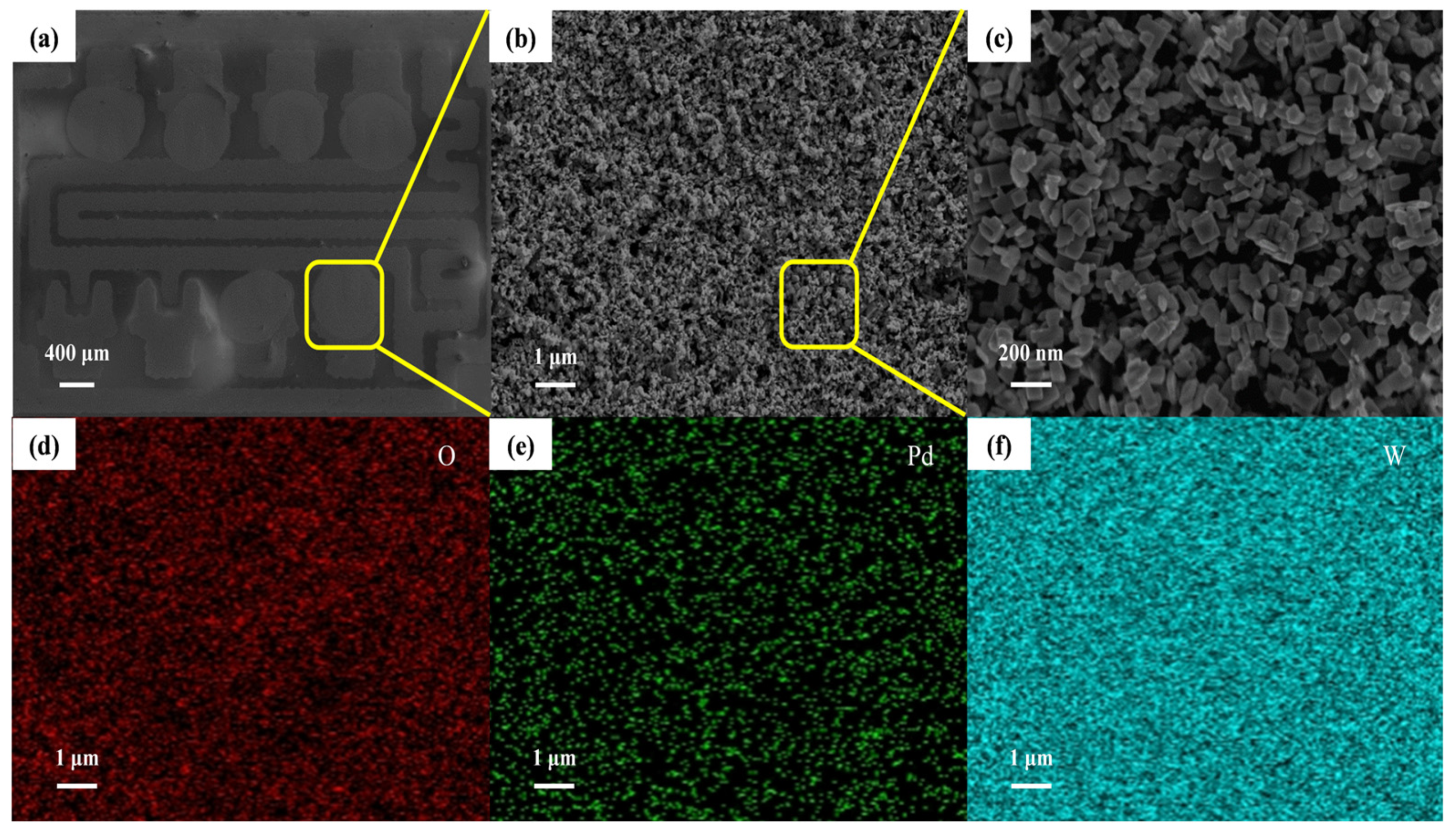
3.1. Structure and Morphology Characterization
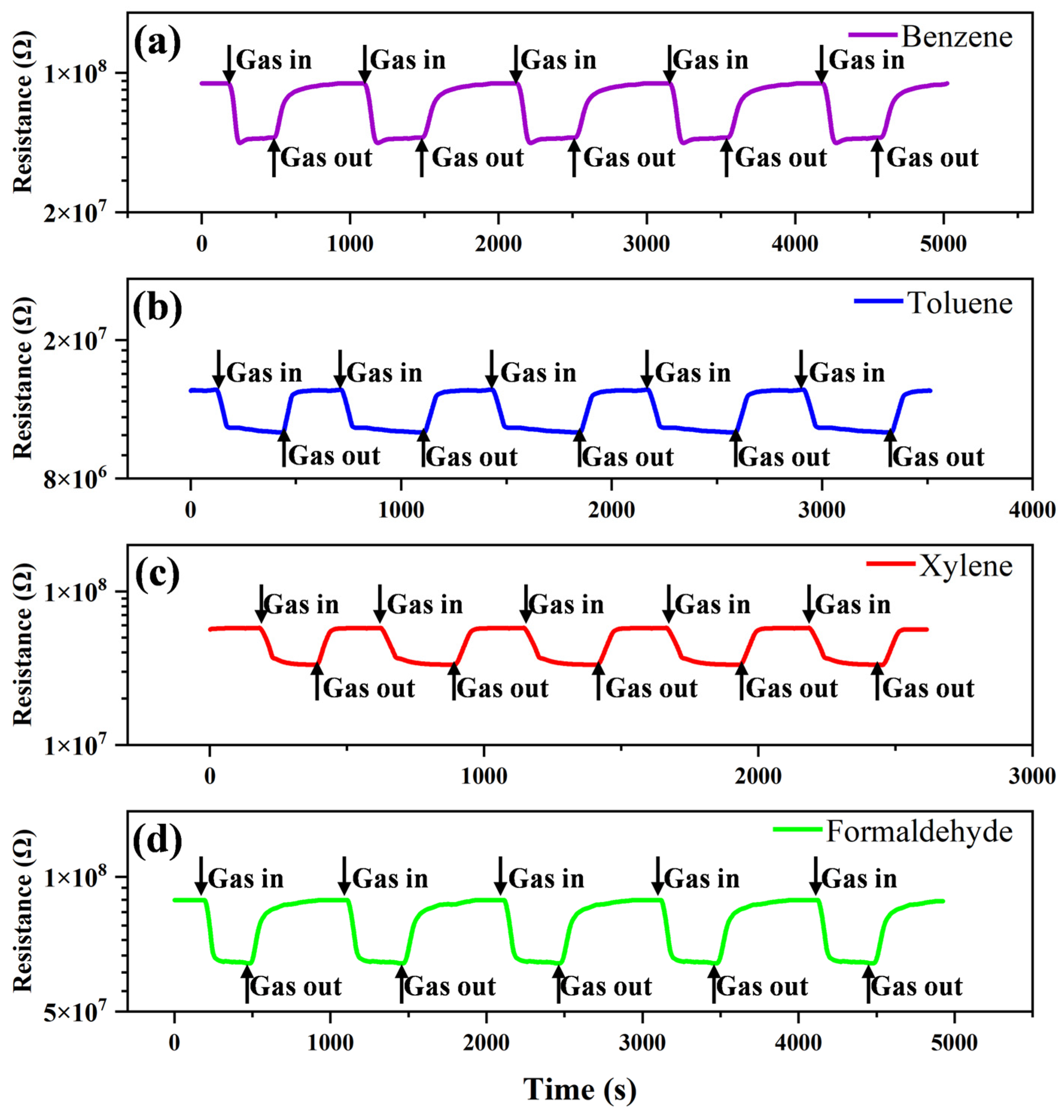
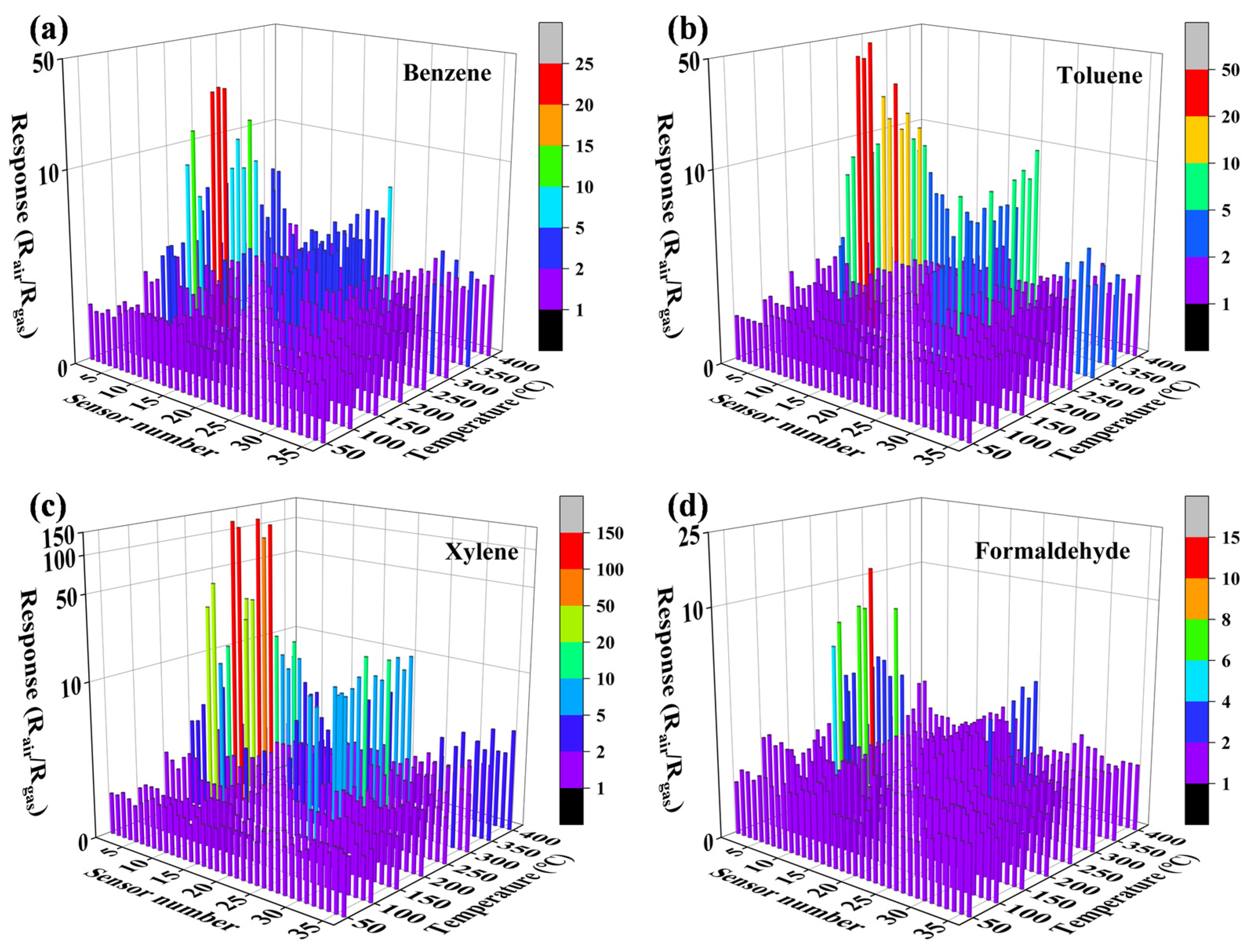
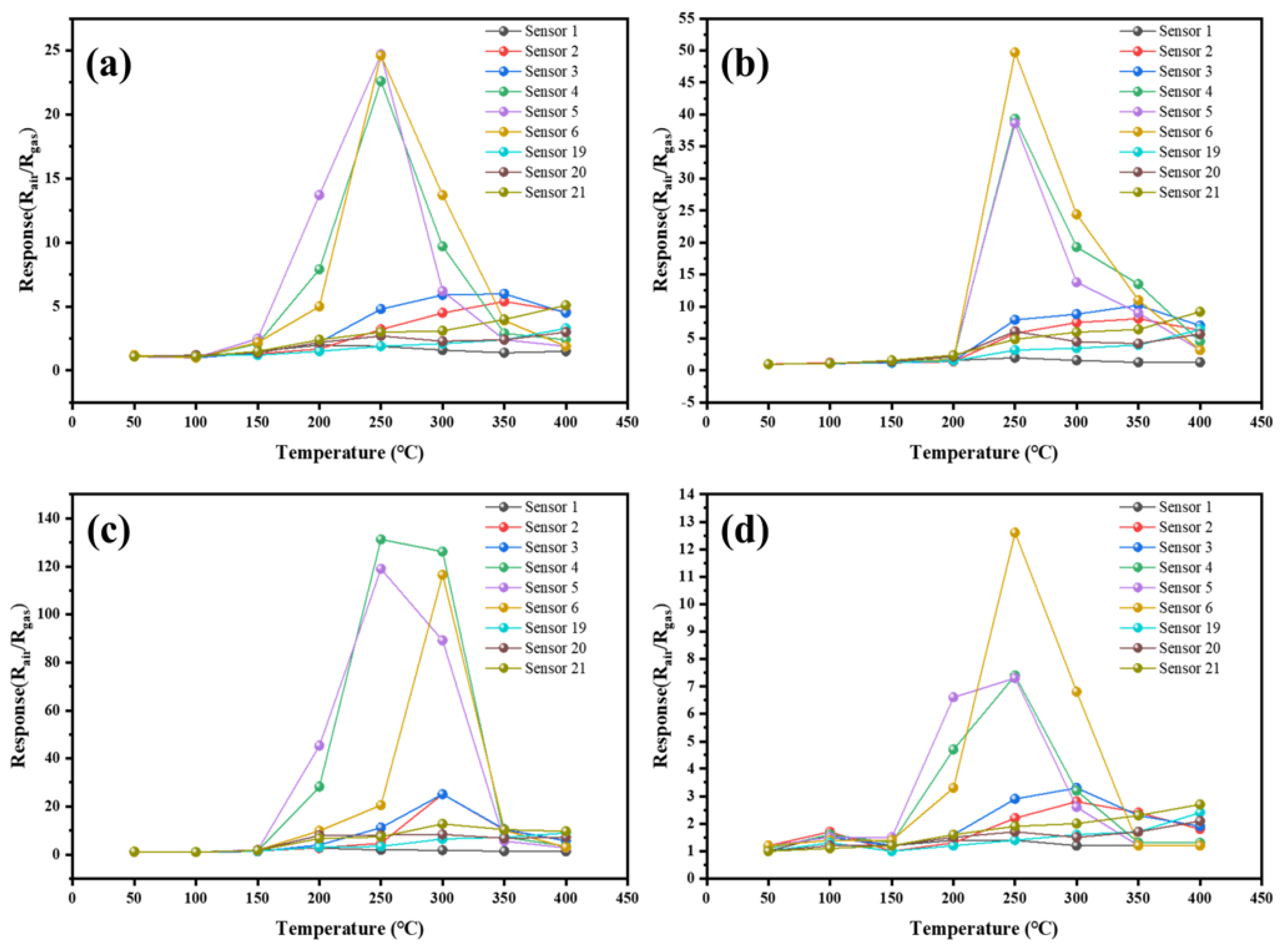
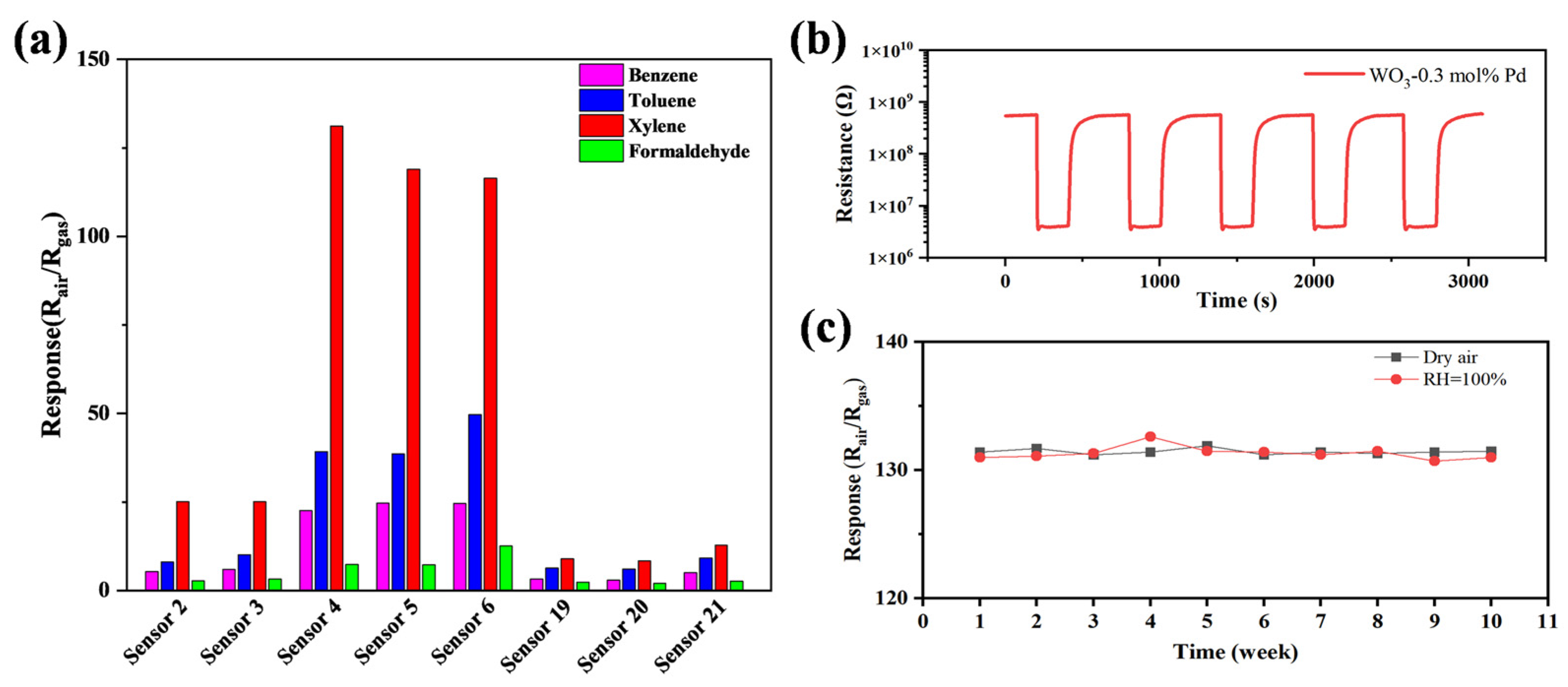
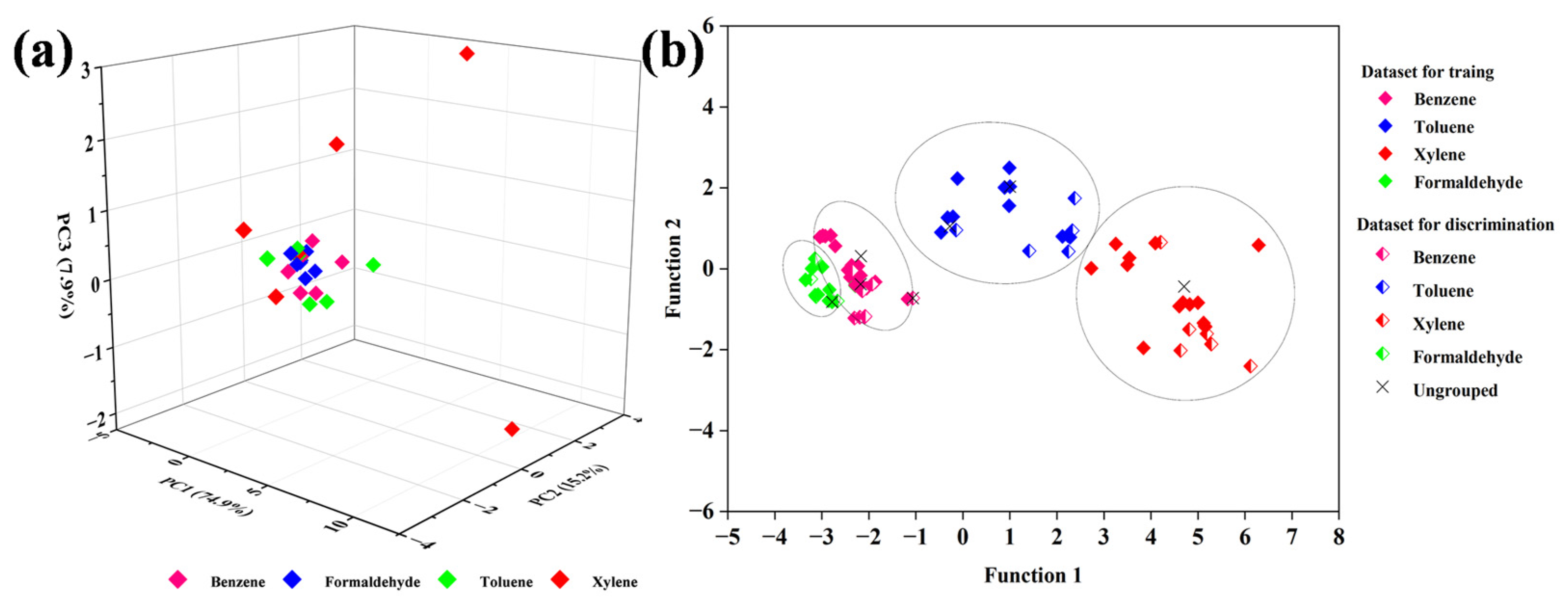
3.2. Gas-Sensing Properties
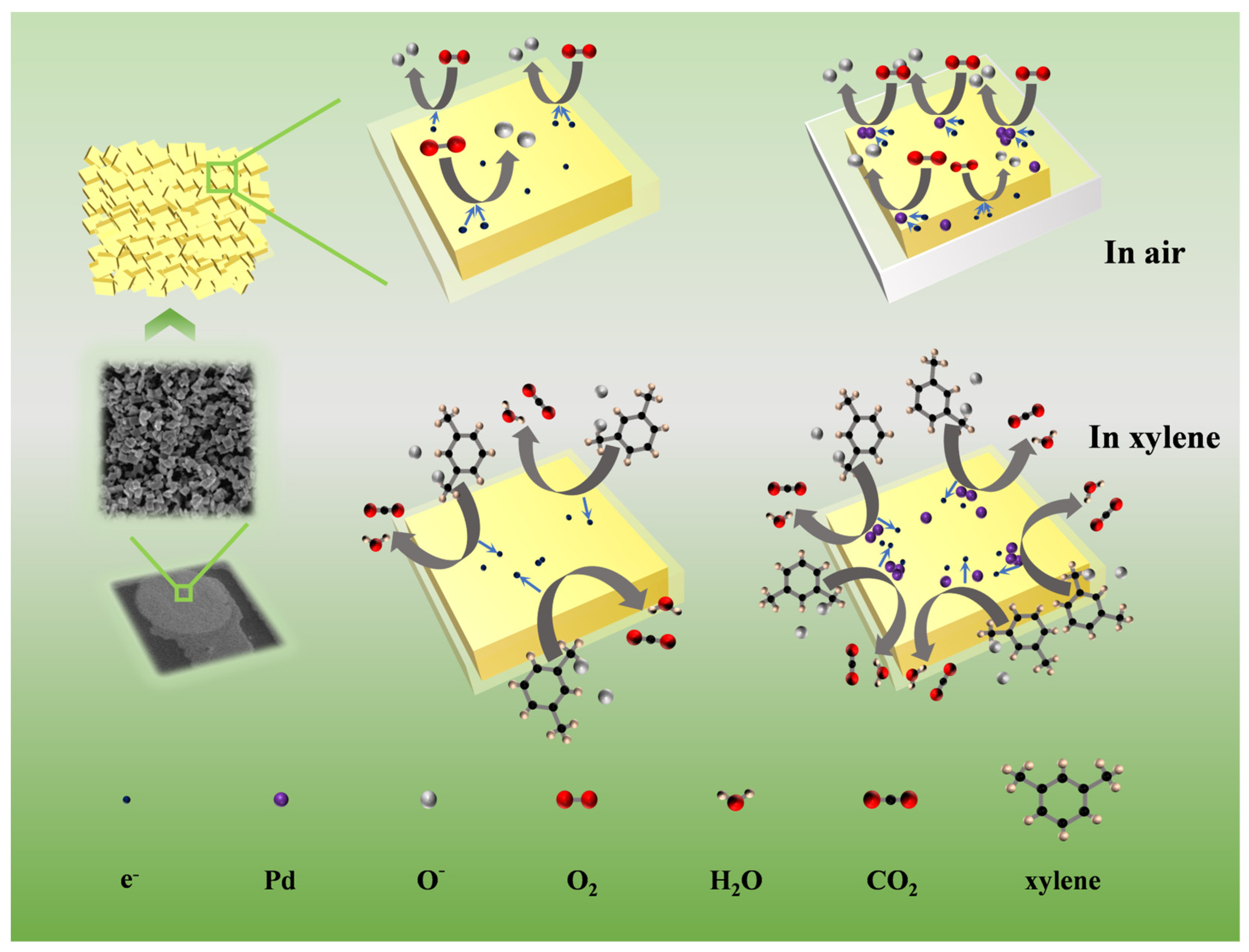
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, P.R.; Zeng, C.T.; Ke, M.T.; Lee, C.Y. Formaldehyde gas sensors: A review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Surya, S.G.; Babar, V.; Ming, F.; Sharma, S.; Alshareef, H.N.; Schwingenschlogl, U.; Salama, K.N. Selective Toluene Detection with Mo2CTx MXene at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 57218–57227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Liu, Y.; Li, Y.; Wang, C.; Bai, J.; Yang, L.; Wang, Y.; Liu, F.; Liang, X.; et al. Bimetallic PtRu alloy nanocrystal-functionalized flower-like WO3 for fast detection of xylene. Sens. Actuators B Chem. 2022, 351, 130950–130960. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Zhang, N.; Liu, C.; Zhou, J.; Liu, D.; Ruan, S. Self-assembled Co3O4@WO3 hollow microspheres with oxygen vacancy defects for fast and selective detection of toluene. Sens. Actuators B Chem. 2022, 351, 130931–130943. [Google Scholar] [CrossRef]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Korotcenkov, G. Practical aspects in design of one-electrode semiconductor gas sensors: Status report. Sens. Actuators B Chem. 2007, 121, 664–678. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-Temperature Wafer-Scale Production of ZnO Nanowire Arrays†. Angew. Chem. Int. Ed. Engl. 2003, 42, 3031–3034. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, S.; Hu, M.; Xie, C. Nanostructural ZnO based coplanar gas sensor arrays from the injection of metal chloride solutions: Device processing, gas-sensing properties and selectivity in liquors applications. Sens. Actuators B Chem. 2011, 153, 415–420. [Google Scholar] [CrossRef]
- Xie, C.; Xiao, L.; Hu, M.; Bai, Z.; Xia, X.; Zeng, D. Fabrication and formaldehyde gas-sensing property of ZnO–MnO2 coplanar gas sensor arrays. Sens. Actuators B Chem. 2010, 145, 457–463. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Lin, H.M.; Hsua, C.M.; Yang, H.Y.; Lee, P.Y.; Yang, C.C. Nanocrystalline WO3-based H2S sensors. Sens. Actuators B Chem. 1994, 22, 63–68. [Google Scholar] [CrossRef]
- Cai, Z.X.; Li, H.Y.; Ding, J.C.; Guo, X. Hierarchical flowerlike WO3 nanostructures assembled by porous nanoflakes for enhanced NO gas sensing. Sens. Actuators B Chem. 2017, 246, 225–234. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Xie, W.; Li, H.; Zhou, R.; Li, Q.; Wang, T. Enhanced selective acetone sensing characteristics based on Co-doped WO3 hierarchical flower-like nanostructures assembled with nanoplates. Sens. Actuators B Chem. 2016, 235, 614–621. [Google Scholar] [CrossRef]
- Feng, C.; Teng, F.; Xu, Y.; Zhang, Y.; Fan, T.; Lin, T. Au-nanoparticle-decorated SnO2 nanorod sensor with enhanced xylene-sensing performance. Int. J. Appl. Ceram. Technol. 2017, 15, 742–750. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425–129435. [Google Scholar] [CrossRef]
- Abbas, A.M.; Naif, H.A.; Hassan, E.S. Silver Loading Tin Oxide Nanostructure for Gas Sensing Application. Braz. J. Phys. 2021, 51, 618–624. [Google Scholar] [CrossRef]
- Horprathum, M.; Srichaiyaperk, T.; Samransuksamer, B.; Wisitsoraat, A.; Eiamchai, P.; Limwichean, S.; Chananonnawathorn, C.; Aiempanakit, K.; Nuntawong, N.; Patthanasettakul, V.; et al. Ultrasensitive Hydrogen Sensor Based on Pt-Decorated WO3 Nanorods Prepared by Glancing-Angle dc Magnetron Sputtering. ACS Appl. Mater. Interfaces 2014, 6, 22051–22060. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Sun, X.; Sun, Y.; Liu, F.; Yan, C.; Wang, X.; Sun, P.; Lu, G. Preparation of Pd/PdO loaded WO3 microspheres for H2S detection. Sens. Actuators B Chem. 2020, 321, 128629–128637. [Google Scholar] [CrossRef]
- Xiang, X.D.; Sun, X.; Briceño, G.; Lou, Y.; Wang, K.A.; Chang, H.; WallaceFreedman, W.G.; Chen, S.W.; Schultz, P.G. A Combinatorial Approach to Materials Discovery. Science 1995, 268, 1738–1740. [Google Scholar] [CrossRef]
- Frantzen, A.; Scheidtmann, J.; Frenzer, G.; Maier, W.F.; Jockel, J.; Brinz, T.; Sanders, D.; Simon, U. High-Throughput Method for the Impedance Spectroscopic Characterization of Resistive Gas Sensors†. Angew. Chem. Int. Ed. Engl. 2004, 43, 752–754. [Google Scholar] [CrossRef]
- Siemons, M.; Leifert, A.; Simon, U. Preparation and Gas Sensing Characteristics of Nanoparticulate p-Type Semiconducting LnFeO3 and LnCrO3 Materials†. Adv. Funct. Mater. 2007, 17, 2189–2197. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Zhang, C.; Zhang, Q.; Li, Z.; Zhang, S. A high throughput platform screening of ppb-level sensitive materials for hazardous gases. Sens. Actuators B Chem. 2018, 276, 189–203. [Google Scholar] [CrossRef]
- Briceño, G.; Chang, H.; Sun, X.; Schultz, P.G.; Xiang, X.D. A Class of Cobalt Oxide Magnetoresistance Materials Discovered with Combinatorial Synthesis. Science 1995, 270, 273–275. [Google Scholar] [CrossRef]
- Brocchini, S.; James, K.; Kohn, J.J. Non-Bonding Interaction of Neighboring Fe and Ni Single-Atom Pairs on MOF-Derived N-Doped Carbon for Enhanced CO2 Electroreduction. Am. Chem. Soc. 1997, 119, 4553–4554. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Ciaridge, T.D.W.; Culshaw, A.J.; Heupel, F.A.; Lee, V.; Spring, D.R.; Whitehead, R.C.; Boughtflower, R.J.; Mutton, I.M.; Upton, R.J. Investigations into the Manzamine Alkaloid Biosynthetic Hypothesis. Angew. Chem. Int. Ed. Engl. 1998, 37, 2661–2663. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Yang, J.; Guo, W.; Zhu, L.; Zheng, W. Temperature and acidity effects on WO3 nanostructures and gas-sensing properties of WO3 nanoplates. Mater. Res. Bullet. 2014, 57, 260–267. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, L.; Yu, Y.; Qiu, M.; Gao, H.; Chen, D.J. WO3 nanoplates for sensitive and selective detections of both acetone and NH3 gases at different operating temperatures. J. Alloy. Compd. 2021, 858, 157638–157647. [Google Scholar] [CrossRef]
- Li, D.; Lei, T.; Zhang, S.; Shao, X.; Xie, C. A novel headspace integrated E-nose and its application in discrimination of Chinese medical herbs. Sens. Actuators B Chem. 2015, 221, 556–563. [Google Scholar] [CrossRef]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced Gas Sensing by Individual SnO2 Nanowires and Nanobelts Functionalized with Pd Catalyst Particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef]
- Ionescu, R.; Llobet, E.; Vilanova, X.; Brezmes, J.; Sueiras, J.E.; Calderer, J.; Correig, X. Quantitative analysis of NO2 in the presence of CO using a single tungsten oxide semiconductor sensor and dynamic signal processing†. Analyst 2002, 127, 1237–1246. [Google Scholar] [CrossRef]
- Bai, S.; Hu, J.; Li, D.; Luo, R.; Chen, A.; Liu, C.C. Quantum-sized ZnO nanoparticles: Synthesis, characterization and sensing properties for NO2. J. Mater. Chem. 2011, 21, 12286–12294. [Google Scholar] [CrossRef]
- You, L.; Sun, Y.F.; Ma, J.; Guan, Y.; Sun, J.M.; Du, Y.; Lu, G.Y. Highly sensitive NO2 sensor based on square-like tungsten oxide prepared with hydrothermal treatment. Sens. Actuators B Chem. 2011, 157, 401–407. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760–3781. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.W.; Kang, D.W.; Lee, D.S.; Huh, J.S.; Lee, D.D. Heating power-controlled micro-gas sensor array. Sens. Actuators B Chem. 2001, 77, 139–144. [Google Scholar] [CrossRef]
- Deng, Q.; Gao, S.; Lei, T.; Ling, Y.; Zhang, S.; Xie, C. Temperature & light modulation to enhance the selectivity of Pt-modified zinc oxide gas sensor. Sens. Actuators B Chem. 2017, 247, 903–915. [Google Scholar] [CrossRef]
- Ricardo, G.O. Ultrasensitive and Selective Bacteria Sensors Based on Functionalized Graphene Transistors. IEEE Sens. J. 2002, 2, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.J.; Park, C.H.; Han, M.R.; Lee, S.; Ohn, J.H.; Kim, J.; Kim, J.; Kim, J.H. ArrayXPath II: Mapping and visualizing microarray gene-expression data with biomedical ontologies and integrated biological pathway resources using Scalable Vector Graphics. Nucleic Acids Res. 2005, 33, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Macias, M.M.; Agudo, J.E.; Manso, A.G.; Orellana, C.J.G.; Velasco, H.M.G.; Caballero, R.G. A compact and low cost electronic nose for aroma detection. Sensors 2013, 13, 5528–5541. [Google Scholar] [CrossRef] [Green Version]
- Tshabalala, Z.P.; Mokoena, T.P.; Hillie, K.T.; Swart, H.C. Improved BTEX gas sensing characteristics of thermally treated TiO2 hierarchical spheres manifested by high-energy {001} crystal facets. Motaung 2021, 338, 129774–129789. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Qiu, L.; Rasaki, S.A.; Qu, F.; Thomas, T.; Liu, M.; Yang, Y.J. Ru-decorated WO3 nanosheets for efficient xylene gas sensing application. J. Alloy. Compd. 2020, 826, 154196–154203. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Yang, W.; Wang, J.; Wei, X. Hierarchical Pt-decorated In2O3 microspheres with highly enhanced isoprene sensing properties. Ceram. Int. 2021, 47, 9477–9485. [Google Scholar] [CrossRef]




| Element | Chemical Reagents | Concentration (g/mL) |
|---|---|---|
| Au | AuCl3 | 0.01 |
| Ag | AgNO3 | |
| Pd | PdCl2 | |
| Pt | H2PtCl6·6H2O | |
| Ru | RuCl3·3H2O | |
| Rh | RhCl3·3H2O | 0.002 |
| Ir | IrCl3 |
| Date of Gas for Training | Date of Gas for Discrimination | Classification Rates (%) | ||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | All Samples | ||
| 1–25, 26–50, 51–75, | 76–100 | 92.65 | 100 | 97.37 | 100 | 97.51 |
| 1–25, 26–50, 76–100, | 51–75 | 97.37 | 94.32 | 98.75 | 100 | 97.61 |
| 1–25, 51–75, 76–100, | 26–50 | 90.48 | 98.81 | 93.75 | 100 | 95.76 |
| 26–50, 51–75, 76–100, | 1–25 | 91.67 | 100 | 100 | 96.25 | 96.98 |
| The average classification rates | 93.04 | 98.28 | 97.47 | 99.06 | 96.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Hou, M.; Yang, L.; Gao, J.; Zhang, G.; Guo, R.; Guo, S. Combinatorial Material Strategy: Parallel Synthesis and High-Throughput Screening of WO3 Nanoplates Decorated with Noble Metals for VOCs Sensor. Chemosensors 2023, 11, 239. https://doi.org/10.3390/chemosensors11040239
Ma Y, Hou M, Yang L, Gao J, Zhang G, Guo R, Guo S. Combinatorial Material Strategy: Parallel Synthesis and High-Throughput Screening of WO3 Nanoplates Decorated with Noble Metals for VOCs Sensor. Chemosensors. 2023; 11(4):239. https://doi.org/10.3390/chemosensors11040239
Chicago/Turabian StyleMa, Yanjia, Ming Hou, Li Yang, Jiyun Gao, Guozhu Zhang, Ronghui Guo, and Shenghui Guo. 2023. "Combinatorial Material Strategy: Parallel Synthesis and High-Throughput Screening of WO3 Nanoplates Decorated with Noble Metals for VOCs Sensor" Chemosensors 11, no. 4: 239. https://doi.org/10.3390/chemosensors11040239





