Calcium Homeostasis and Muscle Energy Metabolism Are Modified in HspB1-Null Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Experimental Procedure
2.2. Muscle Samples
2.3. Proteomic Analysis
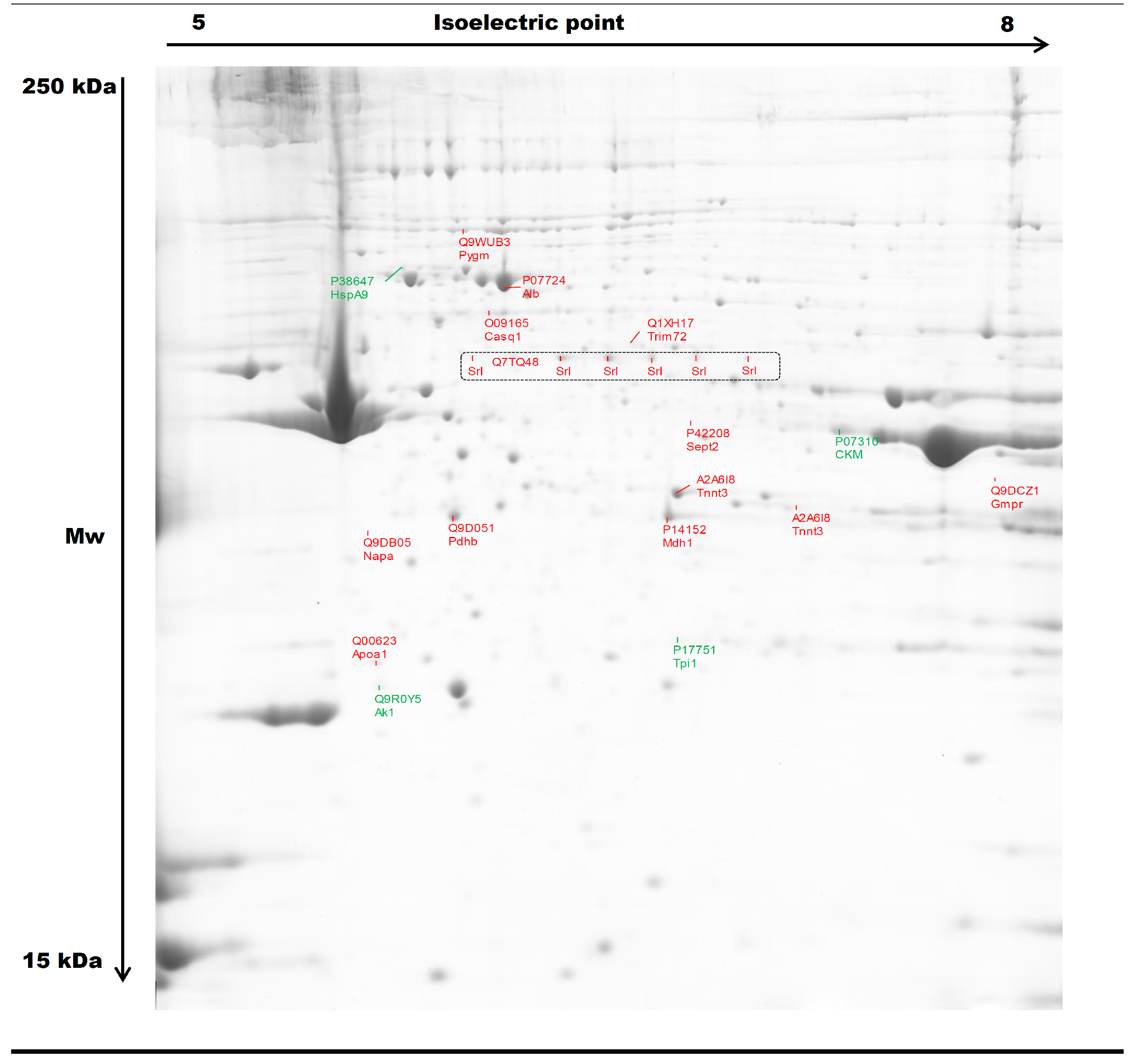
2.3.1. Two-Dimensional Gel Electrophoresis
2.3.2. Protein Identification by Mass Spectrometry LC-MS/MS
2.3.3. Western-Blotting Validation
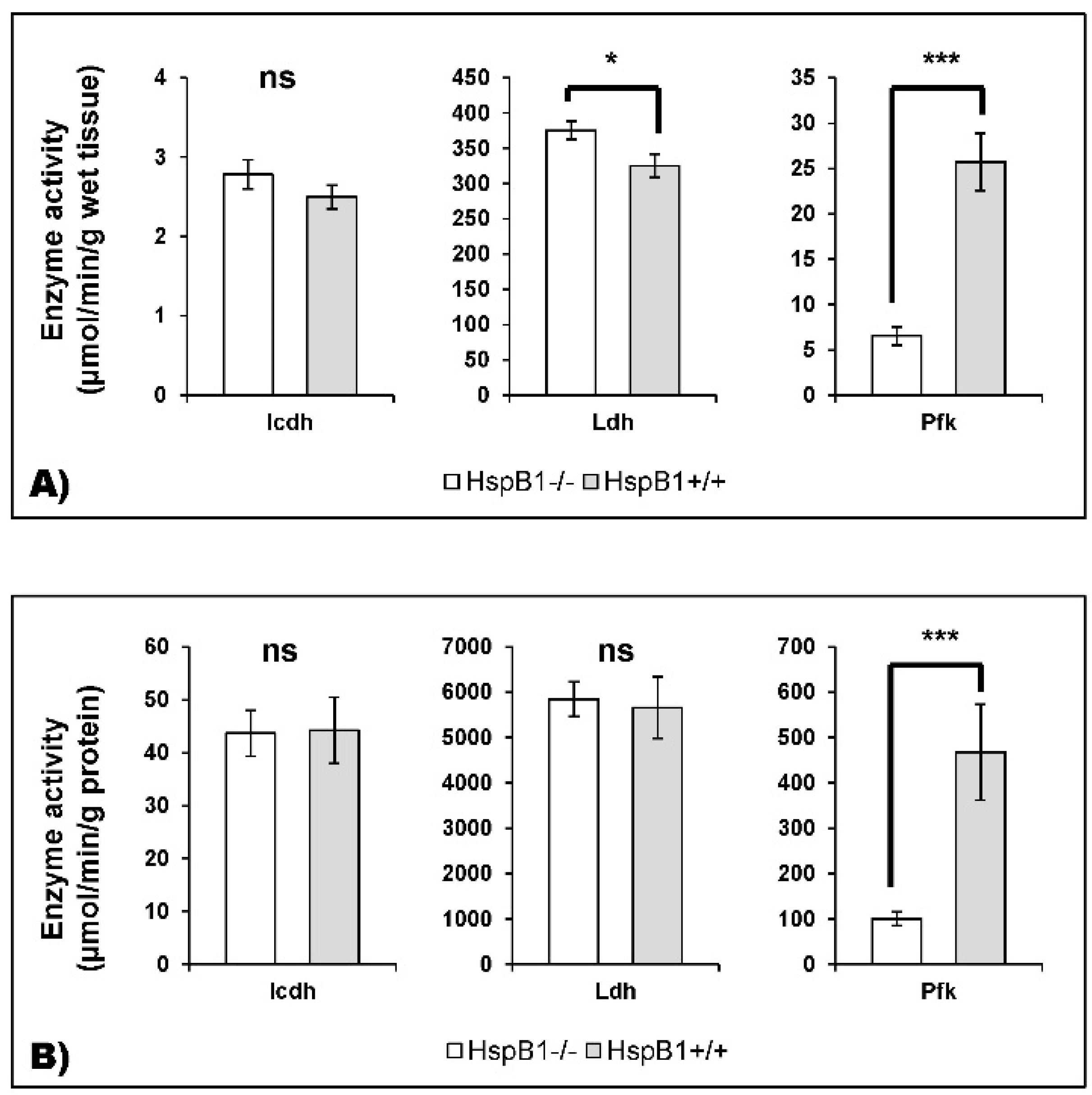
2.3.4. Enzyme Activities Measurement
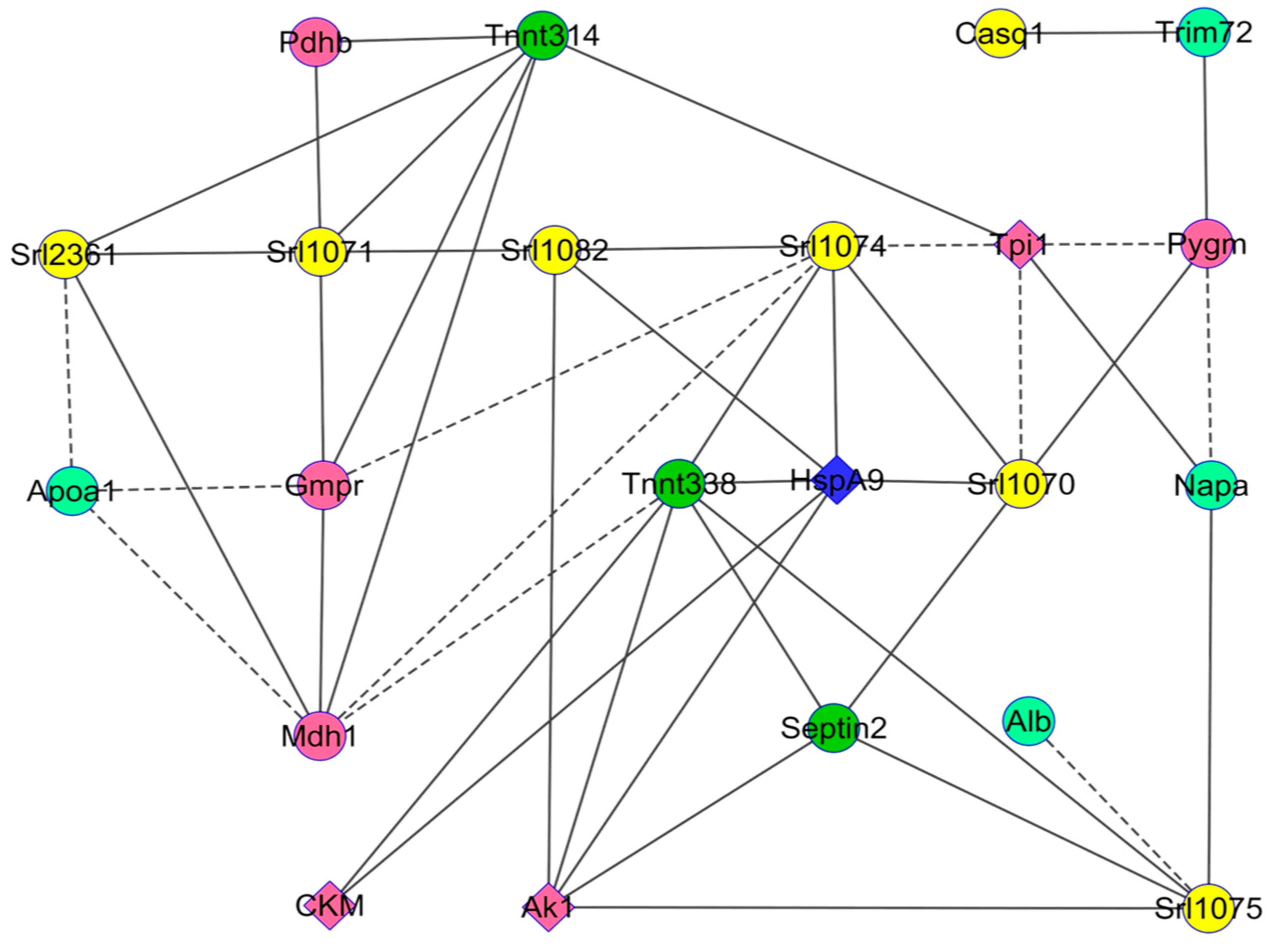
2.4. Bioinformatic Analyses
2.5. Statistical Analysis
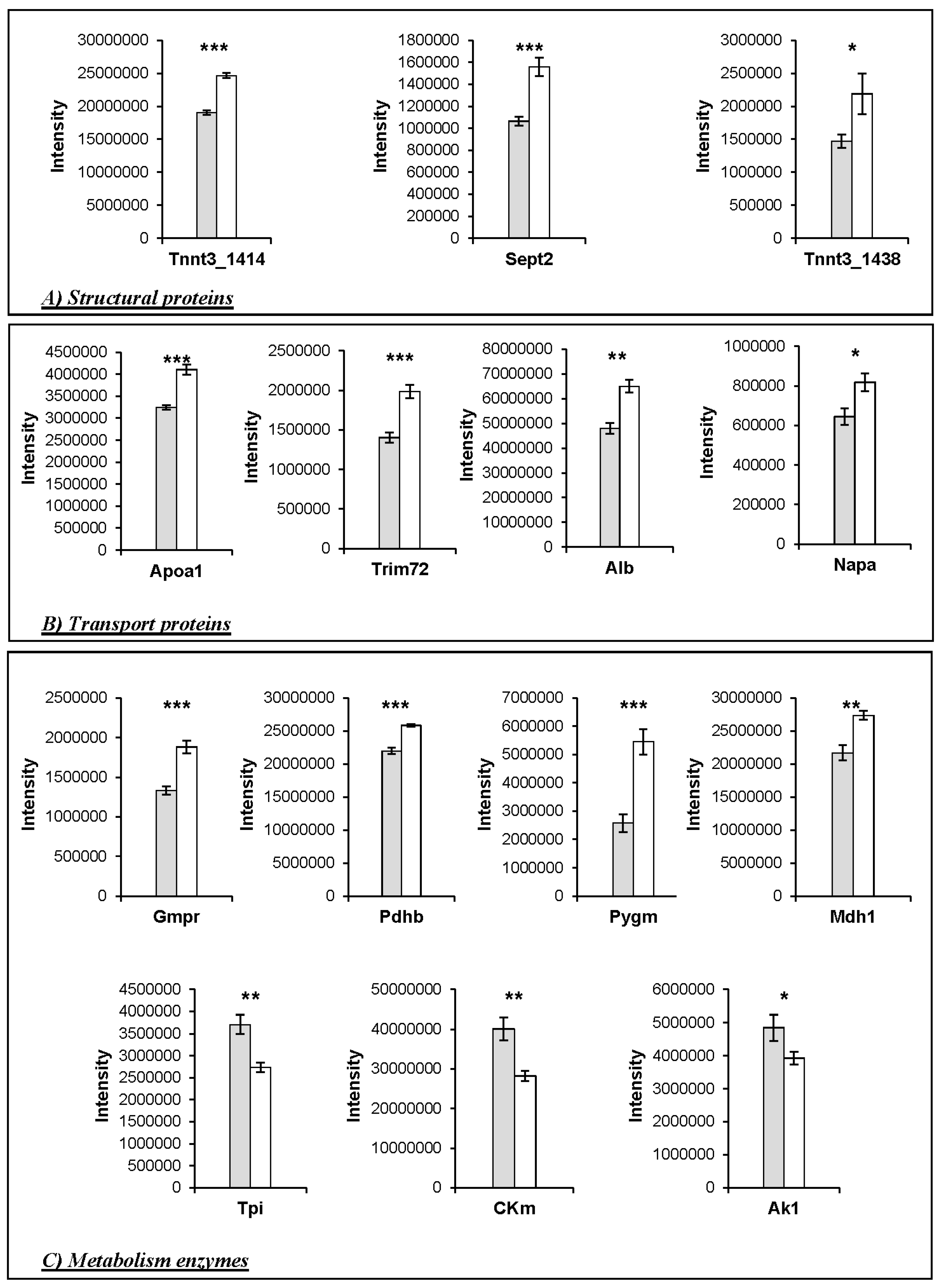
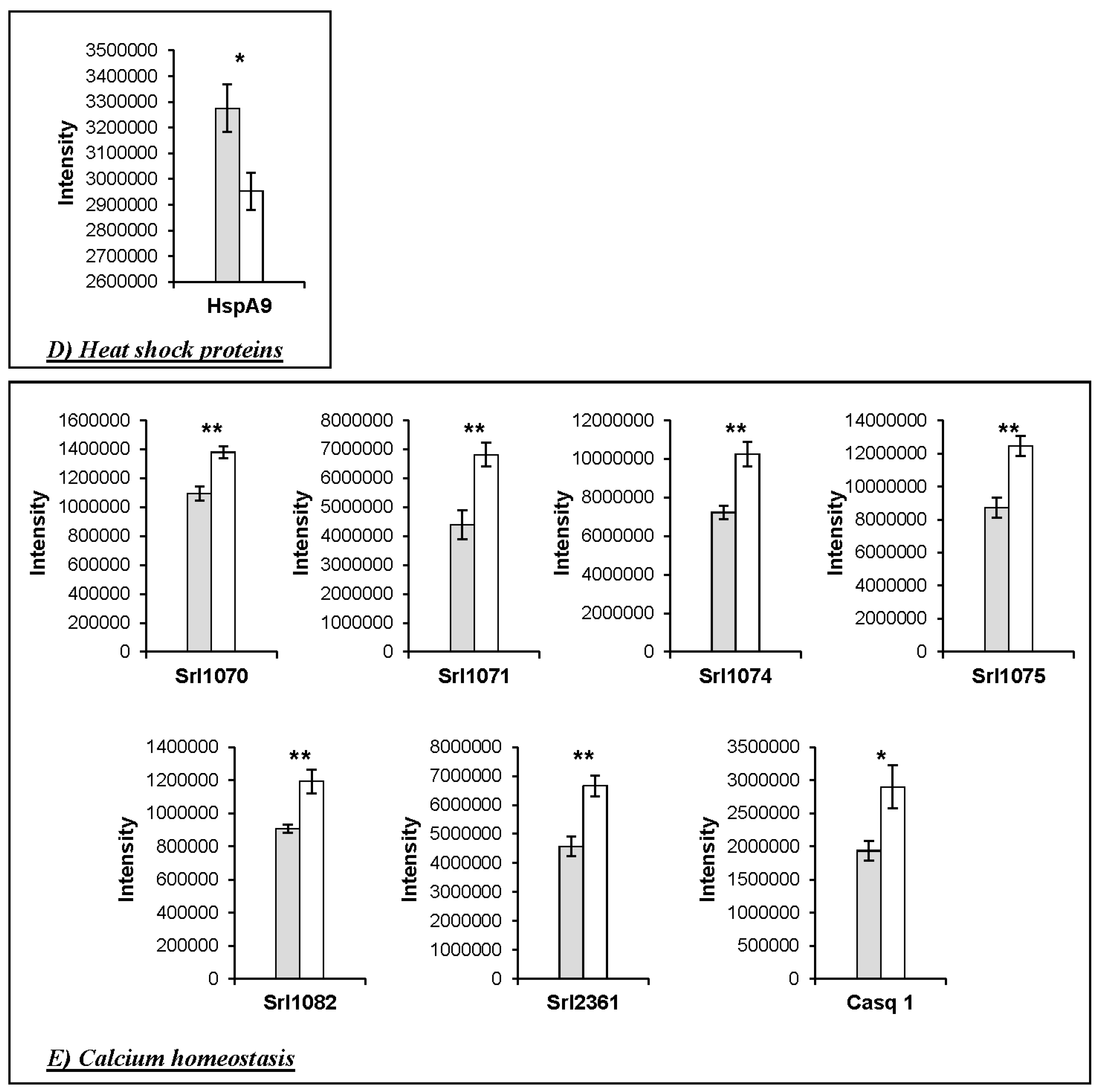
3. Results
Changes in Protein Profiles
4. Discussion
4.1. Changes in the Abundance of Proteins Involved in Calcium Homeostasis in the HspB1-NullMouse
4.2. Changes in the Abundance of Proteins Involved in Energy Metabolism in the HspB1-Null Mouse
4.3. Changes in the Abundance of Proteins Involved in Other Functions
4.4. Implications for Meat Quality
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
Appendix A
References
- Verbeke, W.; Van Wezemael, L.; de Barcellos, M.D.; Kügler, J.O.; Hocquette, J.-F.; Ueland, Ø.; Grunert, K.G. European beef consumers’ interest in a beef eating-quality guarantee. Appetite 2010, 54, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Lebret, B.; Cassar-Malek, I.; Liaubet, L.; Berri, C.; Le Bihan-Duval, E.; Hocquette, J.F.; Renand, G. Recent advances in genomics for meat quality management. Meat Sci. 2015, 109, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Picard, B. Relationship between Muscle Fibers, Growth Efficiency and Beef Quality. In Proceedings of the 8th SIMCORTE, Viçosa, Minas Gerais, Brasil, 28 May 2012.
- Picard, B.; Cassar-Malek, I.; Guillemin, N.; Bonnet, M. Quest for novel muscle pathway biomarkers by proteomics in beef production. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 395–405. [Google Scholar]
- Ouali, A.; Herrera-Mendez, C.H.; Coulis, G.; Becila, S.; Boudjellal, A.; Aubry, L.; Sentandreu, M.A. Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci. 2006, 74, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, M.; Picard, B.; Henry-Berger, J.; Cassar-Malek, I. A network-based approach for predicting Hsp27 knock-out targets in mouse skeletal muscles. Comput. Struct. Biotechnol. J. 2013, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassar-Malek, I.; Kammoun, M.; Astruc, T.; Chambon, C.; Barboiron, C.; Delavaud, A.; Picard, B. An hspb1-null mouse to depict the contribution of Hsp27 in beef tenderness. In Proceedings of the 61th International Congress of Meat Science and Technology (ICoMST), Clermont-Ferrand, France, 23–28 August 2015; INRA: Clermont-Ferrand, France, 2015; p. 97. [Google Scholar]
- Kammoun, M.; Picard, B.; Astruc, T.; Blanquet, V.; Cassar-Malek, I. The invalidation of Hspb1 gene does not impair mouse development but alters the ultrastructural phenotype of muscles. PLoS ONE 2016. submitted. [Google Scholar]
- Bouley, J.; Chambon, C.; Picard, B. Mapping of bovine skeletal muscle proteins using two-dimensional gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chevalier, F. Standard dyes for total protein staining in gel-based proteomic analysis. Materials 2010, 3, 4784–4792. [Google Scholar] [CrossRef]
- Chaze, T.; Meunier, B.; Chambon, C.; Jurie, C.; Picard, B. In vivo proteome dynamics during early bovine myogenesis. Proteomics 2008, 8, 4236–4248. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Jurie, C.; Ortigues-Marty, I.; Picard, B.; Micol, D.; Hocquette, J.F. The separate effects of the nature of diet and grazing mobility on metabolic potential of muscles from charolais steers. Livest. Sci. 2006, 104, 182–192. [Google Scholar] [CrossRef]
- Kaspric, N.; Picard, B.; Reichstadt, M.; Tournayre, J.; Bonnet, M. Proteinside to easily investigate proteomics data from ruminants: Application to mine proteome of adipose and muscle tissues in bovine foetuses. PLoS ONE 2015, 10, e0128086. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Claudia Terlouw, E.M.; Boudjellal, A.; Picard, B. Coherent correlation networks among protein biomarkers of beef tenderness: What they reveal. J. Proteom. 2015, 128, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, M.W.; Brinkmeier, H.; Muntener, M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [PubMed]
- Leberer, E.; Timms, B.G.; Campbell, K.P.; Maclennan, D.H. Purification, calcium-binding properties, and ultrastructural-localization of the 53,000-dalton and 160,000 (sarcalumenin)-dalton glycoproteins of the sarcoplasmic-reticulum. J. Biol. Chem. 1990, 265, 10118–10124. [Google Scholar] [PubMed]
- Mahaney, J.E.; Weis, C.P.; Grisham, C.M.; Kutchai, H. Antibodies against the 53 kDa glycoprotein inhibit the rotational dynamics of both the 53 kDa glycoprotein and the Ca2+-atpase in the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta BBA Biomembr. 1991, 1064, 55–68. [Google Scholar] [CrossRef]
- Hadad, N.; Meyer, H.E.; Varsanyi, M.; Fleischer, S.; Shoshan-Barmatz, V. Cardiac sarcalumenin: Phosphorylation, comparison with the skeletal muscle sarcalumenin and modulation of ryanodine receptor. J. Membr. Biol. 1999, 170, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Beard, N.A.; Laver, D.R.; Dulhunty, A.F. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog. Biophys. Mol. Biol. 2004, 85, 33–69. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, M.; Furukawa, K.I.; Fujimori, H.; Kuruma, A.; Kawano, S.; Hiraoka, M.; Kuniyasu, A.; Nakayama, H.; Ohizumi, Y. Dual regulation of the skeletal muscle ryanodine receptor by triadin and calsequestrin. Biochemistry 1998, 37, 12987–12993. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, M.; Canato, M.; Paolini, C.; Dainese, M.; Reggiani, C.; Volpe, P.; Protasi, F.; Nori, A. Calsequestrin (CASQ1) rescues function and structure of calcium release units in skeletal muscles of CASQ1-null mice. Am. J. Physiol. Cell Physiol. 2012, 302, C575–C586. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Demaurex, N. Cell biology: Apoptosis—The calcium connection. Science 2003, 300, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef] [PubMed]
- McNeil, P.L.; Kirchhausen, T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 2005, 6, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Masumiya, H.; Weisleder, N.; Matsuda, N.; Nishi, M.; Hwang, M.; Ko, J.-K.; Lin, P.; Thornton, A.; Zhao, X.; et al. Mg53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2009, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Drago, I.; Zampese, E.; Pozzan, T. Mitochondria: The calcium connection. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Endo, I.; Matsumoto, T.; Umaki, Y.; Akaike, M. Apoptosis-related changes in skeletal muscles of patients with mitochondrial diseases. Acta Neuropathol. 2002, 103, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.M.; Eppenberger, H.M.; Volpe, P.; Cotrufo, R.; Wallimann, T. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J. Biol. Chem. 1990, 265, 5258–5266. [Google Scholar] [PubMed]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Livisay, S.A.; Zhou, S. Mechanisms of endogenous skeletal muscle antioxidants: Chemical and physical aspects. In Antioxidants in Muscle Foods: Nutritional Strategies to Improve Quality; Decker, E.A., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 25–60. [Google Scholar]
- Mayer, M.P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 2013, 38, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, N.; Frazier, A.E.; Pfanner, N. The protein import machinery of mitochondria. J. Biol. Chem. 2004, 279, 14473–14476. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Terlouw, E.M.C.; Micol, D.; Boudjellal, A.; Hocquette, J.-F.; Picard, B. Understanding early post-mortem biochemical processes underlying meat color and pH decline in the longissimus thoracis muscle of young blond d’aquitaine bulls using protein biomarkers. J. Agric. Food Chem. 2015, 63, 6799–6809. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.C.; Shieh, C.-C. Medicinal chemistry of Ca2+-activated K+ channel modulators. In Triggle: Voltage-Gated Ion Channels as Drug Targets O-Bk; Wiley-Blackwell: Hoboken, NJ, USA, 2006; pp. 310–334. [Google Scholar]
- Sausbier, M.; Hu, H.; Arntz, C.; Feil, S.; Kamm, S.; Adelsberger, H.; Sausbier, U.; Sailer, C.A.; Feil, R.; Hofmann, F.; et al. Cerebellar ataxia and purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc. Natl. Acad. Sci. USA 2004, 101, 9474–9478. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.-Y.; Lyu, M.S.; Kozak, C.A.; Jeang, K.-T. Function of 14-3-3 proteins. Nature 1996, 382, 308–308. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Isobe, T.; Ichimura, T.; Taoka, M.; Funaki, M.; Sakoda, H.; Onishi, Y.; Inukai, K.; Anai, M.; Fukushima, Y.; et al. 14-3-3 protein binds to insulin receptor substrate-1, one of the binding sites of which is in the phosphotyrosine binding domain. J. Biol. Chem. 1997, 272, 25267–25274. [Google Scholar] [CrossRef] [PubMed]
- Gohla, A.; Bokoch, G.M. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr. Biol. 2002, 12, 1704–1710. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Gusev, N.B. 14-3-3 proteins and regulation of cytoskeleton. Biochem. Moscow 2010, 75, 1528–1546. [Google Scholar] [CrossRef]
- Chernik, I.S.; Seit-Nebi, A.S.; Marston, S.B.; Gusev, N.B. Small heat shock protein Hsp20 (Hspb6) as a partner of 14-3-3γ. Mol. Cell. Biochem. 2006, 295, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dreiza, C.M.; Komalavilas, P.; Furnish, E.J.; Flynn, C.R.; Sheller, M.R.; Smoke, C.C.; Lopes, L.B.; Brophy, C.M. The small heat shock protein, hspb6, in muscle function and disease. Cell Stress Chaperones 2009, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.-I.; Onoue, H.; Arima, K.; Yamamura, T. The 14-3-3 protein forms a molecular complex with heat shock protein Hsp60 and cellular prion protein. J. Neuropathol. Exp. Neurol. 2005, 64, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, N.; Jurie, C.; Cassar-Malek, I.; Hocquette, J.; Renand, G.; Picard, B. Variations in the abundance of 24 proteins biomarkers of beef tenderness according to muscle and animal type. Animal 2011, 6, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Zoubeidi, A.; Zardan, A.; Beraldi, E.; Fazli, L.; Sowery, R.; Rennie, P.; Nelson, C.; Gleave, M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007, 67, 10455–10465. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Biswas, M.H.; Zhang, C.; Du, C.; Balaji, K.C. Heat shock protein 27 mediates repression of androgen receptor function by protein kinase d1 in prostate cancer cells. Oncogene 2009, 28, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Jayasena, D.D.; Jung, Y.; Jung, S.; Kang, B.S.; Heo, K.N.; Lee, J.H.; Jo, C. Differential proteome analysis of breast and thigh muscles between korean native chickens and commercial broilers. Asian Aust. J. Anim. Sci. 2012, 25, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M.; Micol, D.; Cassar-Malek, I.; Hocquette, J.-F.; Terlouw, C. Inverse relationships between biomarkers and beef tenderness according to contractile and metabolic properties of the muscle. J. Agric. Food Chem. 2014, 62, 9808–9818. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Hollung, K.; Therkildsen, M.; Hildrum, K.I.; Bendixen, E. Proteome analysis of early post-mortem changes in two bovine muscle types: M. longissimus dorsi and M. semitendinosus. Proteomics 2006, 6, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.R. Postmortem changes in muscle. In The Structure and Function of Muscle; Elsevier BV: Amsterdam, The Netherlands, 1973; pp. 243–309. [Google Scholar]
- Vignon, X.; Beaulaton, J.; Ouali, A. Ultrastructural localization of calcium in post-mortem bovine muscle: A cytochemical and X-ray microanalytical study. Histochem. J. 1989, 21, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Sporer, K.R.B.; Zhou, H.R.; Linz, J.E.; Booren, A.M.; Strasburg, G.M. Differential expression of calcium-regulating genes in heat-stressed turkey breast muscle is associated with meat quality. Poult. Sci. 2012, 91, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, R.L.; Owens, C.M.; Hirschler, E.M.; Martinez-Dawson, R.; Sams, A.R. The characterization and incidence of pale, soft, and exudative broiler meat in a commercial processing plant. Poult. Sci. 2002, 81, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Greaser, M.L.; Cassens, R.G.; Briskey, E.J.; Hoekstra, W.G. Post-mortem changes in subcellular fractions from normal and pale, soft, exudative porcine muscle. 1. Calcium accumulation and adenosine triphosphatase activities. J. Food Sci. 1969, 34, 120–124. [Google Scholar] [CrossRef]
- Chai, J.; Xiong, Q.; Zhang, P.P.; Shang, Y.Y.; Zheng, R.; Peng, J.; Jiang, S.W. Evidence for a new allele at the serca1 locus affecting pork meat quality in part through the imbalance of Ca2+ homeostasis. Mol. Biol. Rep. 2009, 37, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Malila, Y.; Tempelman, R.J.; Sporer, K.R.B.; Ernst, C.W.; Velleman, S.G.; Reed, K.M.; Strasburg, G.M. Differential gene expression between normal and pale, soft, and exudative turkey meat. Poult. Sci. 2013, 92, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yu, X.; Khan, M.A.; Shao, J.; Xiang, Y.; Zhou, G. The effect of calcium chloride injection on shear force and caspase activities in bovine longissimus muscles during postmortem conditioning. Animal 2011, 6, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Chaze, T.; Hocquette, J.-F.; Meunier, B.; Renand, G.; Jurie, C.; Chambon, C.; Journaux, L.; Rousset, S.; Denoyelle, C.; Lepetit, J.; et al. Biological markers for meat tenderness of the three main french beef breeds using 2-DE and MS approach. In Proteomics in Foods; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2012; pp. 127–146. [Google Scholar]
- Krischek, C.; Natter, R.; Wigger, R.; Wicke, M. Adenine nucleotide concentrations and glycolytic enzyme activities in longissimus muscle samples of different pig genotypes collected before and after slaughter. Meat Sci. 2011, 89, 217–220. [Google Scholar] [CrossRef] [PubMed]


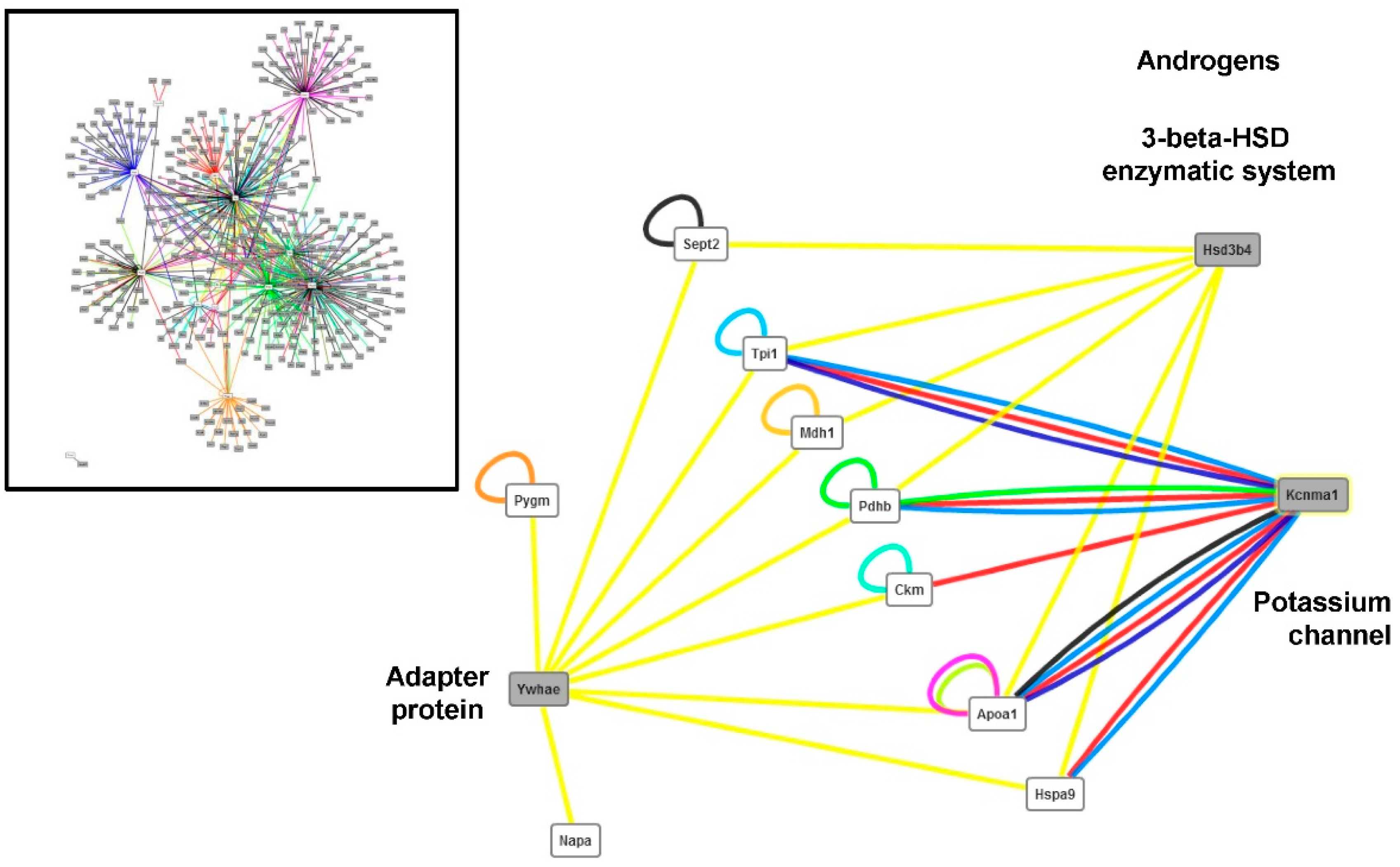
| Protein | Protein Name | Primary Antibody Type | References | Dilution |
|---|---|---|---|---|
| Pygm | Glycogen phosphorylase | Polyclonal | Santa Cruz: SC-46347 | 1/1000 |
| Casq1 | Calsequestrin-1 | Monoclonal | Santa Cruz: SC-137080 | 1/1000 |
| Srl | Sarcalumenin | Monoclonal | Santa Cruz: SC-58845 | 1/1000 |
| HspA9 | Stress-70 protein, mitochondrial | Monoclonal | R & D Systems: MAB3584 | 1/200 |
| Mdh1 | Malate dehydrogenase | Monoclonal | Rockland 100-601-145 | 1/1000 |
| Alb | Albumin | Polyclonal | Polycsiences: 23715-5 | 1/100 |
| Park7 | Parkinson disease protein 7 homolog | Polyclonal | Santa Cruz: SC-32874 | 1/250 |
| HspA8 | Heat shock cognate 71 kDa (kilodalton) protein | Monoclonal | Santa Cruz: SC-59572 | 1/250 |
| Spot_ID | UniprotKB ID | Protein | Protein Name | Score | ΣCoverage | Mr [kDa] | Calculated. pI | Anova (p) | Fold | Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| Structural proteins | ||||||||||
| 1414 | A2A6I8 | Tnnt3 | Troponin T fast | 4530.73 | 43.51 | 28.3 | 9.36 | 0.0000 | 1.30 | KO > WT |
| 1438 | A2A6I8 | Tnnt3 | Troponin T fast | 1575.36 | 41.84 | 28.3 | 9.36 | 0.0571 | 1.49 | KO > WT |
| 1259 | P42208 | Sept2 | Septin-2 | 1415.13 | 48.20 | 41.5 | 6.55 | 0.0006 | 1.46 | KO > WT |
| Transport proteins | ||||||||||
| 2338 | P07724 | Alb | Serum albumin | 7254.76 | 72.53 | 68.6 | 6.07 | 0.0011 | 1.35 | KO > WT |
| 1761 | Q00623 | ApoA1 | Apolipoprotein A1 | 1249.41 | 46.59 | 30.6 | 5.73 | 0.0001 | 1.26 | KO > WT |
| 1016 | Q1XH17 | Trim72 | Tripartite motif-containing protein 72 | 760.45 | 37.46 | 32.2 | 5.74 | 0.0006 | 1.41 | KO > WT |
| 1511 | Q9DB05 | Napa | α-soluble NSF attachment protein | 2396.08 | 81.02 | 33.2 | 5.45 | 0.0221 | 1.27 | KO > WT |
| Heat shock proteins | ||||||||||
| 2362 | P38647 | HspA9 | Stress-70 protein, mitochondrial | 1005.69 | 35.79 | 73.4 | 6.07 | 0.0253 | 1.11 | WT > KO |
| Metabolism | ||||||||||
| 1786 | Q9R0Y5 | Ak1 | Adenylate kinase isoenzyme 1 | 2595.48 | 62.37 | 21.5 | 5.81 | 0.0675 | 1.24 | WT > KO |
| 1273 | P07310 | Ckm | Creatine kinase M | 4104.48 | 53.02 | 43.0 | 7.06 | 0.0061 | 1.42 | WT > KO |
| 1723 | P17751 | Tpi1 | Triosephosphate isomerase 1 | 1694.75 | 51.51 | 32.2 | 5.74 | 0.0040 | 1.35 | WT > KO |
| 2422 | P14152 | Mdh1 | Malate dehydrogenase | 2973.62 | 48.50 | 36.5 | 6.58 | 0.0027 | 1.26 | KO > WT |
| 462 | Q9WUB3 | Pygm | Glycogen phosphorylase | 5946.10 | 55.70 | 97.2 | 7.11 | 0.0008 | 2.12 | KO > WT |
| 2374 | Q9D051 | Pdhb | Pyruvate dehydrogenase E1 component subunit β | 4353.89 | 55.71 | 38.9 | 6.87 | 0.0000 | 1.18 | KO > WT |
| 1382 | Q9DCZ1 | Gmpr | Guanosine 5′-monophosphate oxidoreductase 1 | 1127.11 | 59.71 | 37.5 | 7.09 | 0.0004 | 1.41 | KO > WT |
| Calcium homeostasis | ||||||||||
| 902 | O09165 | Casq1 | Calsequestrin-1 | 1176.41 | 26.17 | 46.3 | 4.12 | 0.027 | 1.50 | KO > WT |
| 1070 | Q7TQ48 | Srl | Isoform 2 of Sarcalumenin | 1260.25 | 55.30 | 54.3 | 6.64 | 0.0017 | 1.26 | KO > WT |
| 1071 | Q7TQ48 | Srl | Isoform 2 of Sarcalumenin | 4730.75 | 65.47 | 54.3 | 6.64 | 0.0051 | 1.55 | KO > WT |
| 1074 | Q7TQ48 | Srl | Isoform 2 of Sarcalumenin | 1576.43 | 57.20 | 54.3 | 6.64 | 0.0033 | 1.42 | KO > WT |
| 1075 | Q7TQ48 | Srl | Isoform 2 of Sarcalumenin | 4266.69 | 58.26 | 54.3 | 6.64 | 0.0026 | 1.43 | KO > WT |
| 1082 | Q7TQ48 | Srl | Isoform 2 of Sarcalumenin | 886.87 | 51.91 | 54.3 | 6.64 | 0.0052 | 1.32 | KO > WT |
| 2361 | Q7TQ48 | Srl | Isoform 2 of Sarcalumenin | 4731.14 | 60.17 | 54.3 | 6.64 | 0.0028 | 1.46 | KO > WT |
| UniprotKB ID | Gene Name | Peptide | SignalP Score | GO Related to Secretion | Number of GO | TargetP | TargetP Score |
|---|---|---|---|---|---|---|---|
| Q00623 | ApoA1 | noTM | 0.899 | GO:0072562 GO:0034361 GO:0034366 GO:0034364 GO:1903561 GO:0070062 GO:0042627 | 7 | Signal peptide | 1 |
| P07724 | Alb | noTM | 0.852 | GO:0005783 GO:0072562 GO:0070062 GO:0005794 | 4 | Signal peptide | 3 |
| Q7TQ48 | Srl | noTM | 0.897 | 0 | Signal peptide | 1 | |
| O09165 | Casq1 | noTM | 0.744 | 0 | Signal peptide | 3 | |
| Q9DB05 | Napa | - | - | GO:0070062 | 1 | Other secretory pathway | 2 |
| P17751 | Tpi1 | - | - | GO:0070062 | 1 | Other secretory pathway | 1 |
| P42208 | Sept2 | - | - | GO:0070062 | 1 | Other secretory pathway | 2 |
| Q9D051 | Pdhb | - | - | GO:0070062 | 1 | Mitochondrial | 1 |
| Q9WUB3 | Pygm | - | - | GO:0070062 | 1 | - | 4 |
| P38647 | HspA9 | - | - | GO:0070062 | 1 | Mitochondrial | 2 |
| Q9R0Y5 | Ak1 | - | - | GO:0070062 | 1 | Other secretory pathway | 2 |
| P14152 | Mdh1 | - | - | GO:0070062 | 1 | - | - |
| Proteins | HspB1-Null− | WT | SEM | Significance |
|---|---|---|---|---|
| Pygm | 61.072 | 64.897 | 4897 | ns |
| Casq1 | 107.586 | 103.184 | 5015 | ns |
| HspA9 | 20.946 | 40.914 | 3583 | ** |
| Mdh1 | 93.353 | 161.232 | 23.589 | t |
| Srl | 18.690 | 17.144 | 489 | t |
| Alb | 955.617 | 975.046 | 20.705 | ns |
| HspA8 | 233.744 | 404.037 | 32.292 | ** |
| Park7 | 328.440 | 383.780 | 17.540 | * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picard, B.; Kammoun, M.; Gagaoua, M.; Barboiron, C.; Meunier, B.; Chambon, C.; Cassar-Malek, I. Calcium Homeostasis and Muscle Energy Metabolism Are Modified in HspB1-Null Mice. Proteomes 2016, 4, 17. https://doi.org/10.3390/proteomes4020017
Picard B, Kammoun M, Gagaoua M, Barboiron C, Meunier B, Chambon C, Cassar-Malek I. Calcium Homeostasis and Muscle Energy Metabolism Are Modified in HspB1-Null Mice. Proteomes. 2016; 4(2):17. https://doi.org/10.3390/proteomes4020017
Chicago/Turabian StylePicard, Brigitte, Malek Kammoun, Mohammed Gagaoua, Christiane Barboiron, Bruno Meunier, Christophe Chambon, and Isabelle Cassar-Malek. 2016. "Calcium Homeostasis and Muscle Energy Metabolism Are Modified in HspB1-Null Mice" Proteomes 4, no. 2: 17. https://doi.org/10.3390/proteomes4020017








