1. Introduction
Musculoskeletal ageing is characterized by age-related muscle fiber atrophy, as a corollary of motor unit (MU) loss [
1]. Each MU comprises a presynaptic motor neuron and a postsynaptic population of mono-innervated skeletal muscle fibers [
2]. MUs are, typically but not always [
3], defined by uniform skeletal muscle fiber isoform (Type I, Type II) content [
4]. Type II MUs are preferentially lost with age, owing to failed re-innervation [
2]. Functionally, type II MU loss underpins age-related phenotypical alterations (e.g., reduced skeletal muscle power [
5]), owing to the distinct contractile and metabolic properties associated with each fiber type (reviewed in [
4,
6]). Therefore, classification of skeletal muscle fiber type can provide insight into the nature of the phenotypical alterations that define musculoskeletal ageing [
7].
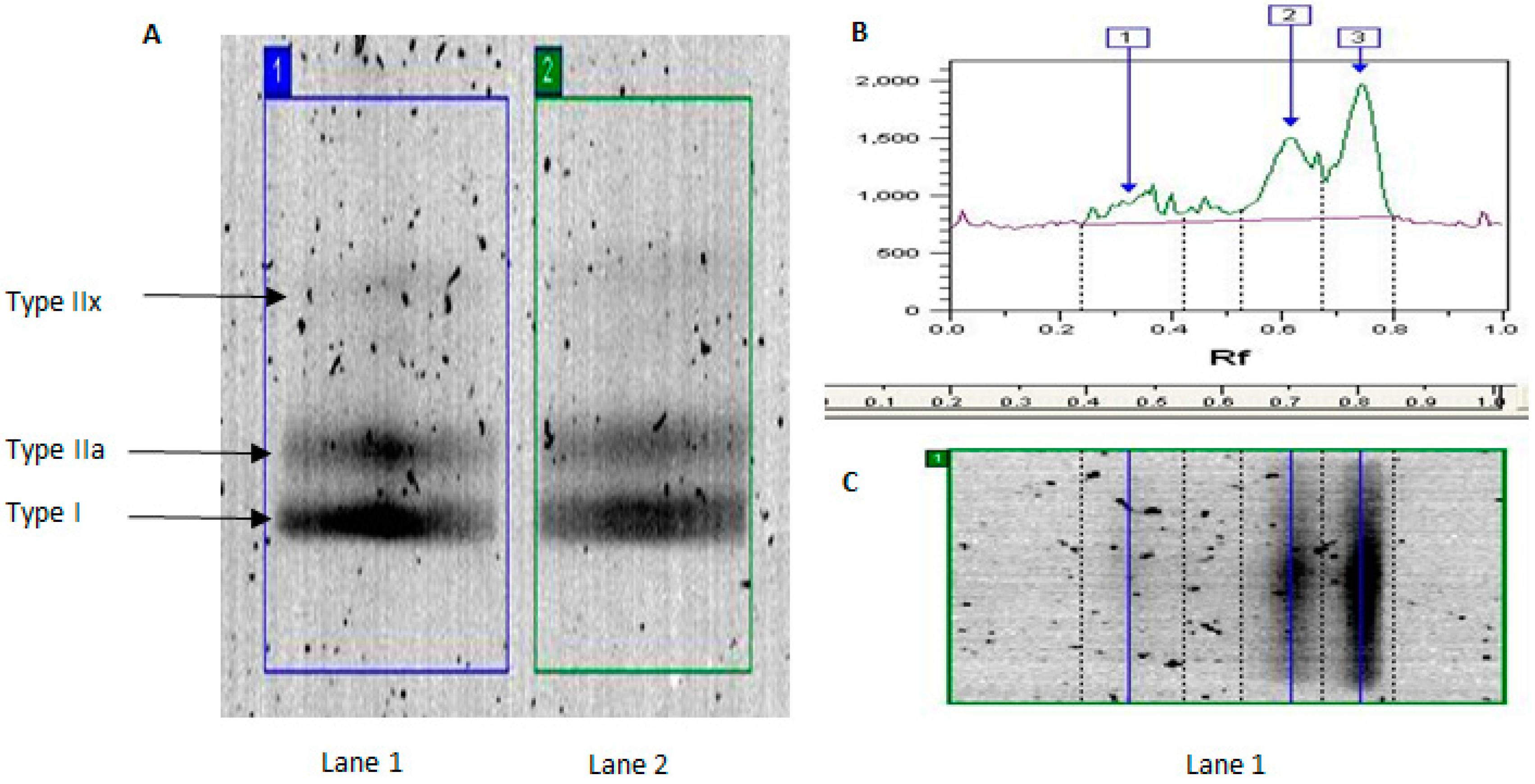
Traditional biochemical and histochemical approaches to characterizing skeletal muscle phenotype involve assessment of metabolic enzyme activity (e.g., citrate synthase activity) and myosin heavy chain (MyHC) isoform content and/or ATPase activity [
8,
9]. Such approaches have revealed that musculoskeletal ageing is associated with reduced citric acid cycle and electron transport chain subunit activity and content [
10,
11], although this is modifiable by physical activity levels [
1]. Ageing is also associated with reduced MyHCIIa and MyHCIIx expression, consistent with type II MU loss [
7]. Unfortunately, the techniques used to collect the aforementioned data require relatively large amounts of sample and are ill-suited to multiplex analysis so typically just a few proteins are studied in isolation [
9,
12,
13,
14] and in a serial rather than parallel manner. The latter is a particular concern because metabolic and contractile differences can be uncoupled [
12,
15]. Resultantly, metabolic differences can exist despite similar MyHC isoform content [
12]. The age-related appearance of hybrid skeletal muscle fibers that co-express multiple MyHC isoforms further complicates this issue [
16]. The collective limitations of traditional biochemical and histochemical approaches preclude complete characterization of age and physical activity level phenotypes in skeletal muscle.
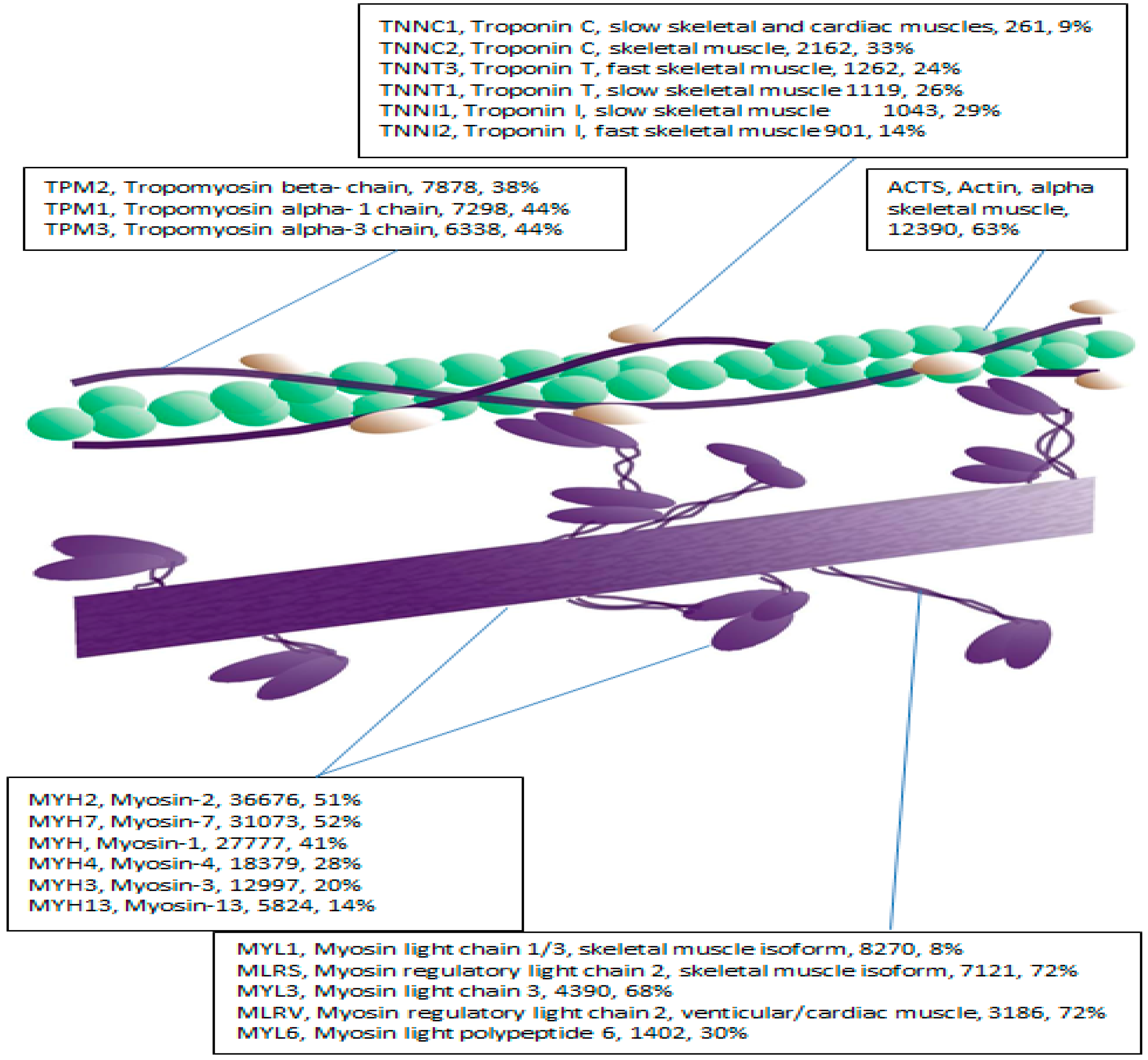
Proteomic approaches to characterizing skeletal muscle phenotype confer several important advantages, notably the capacity to analyze multiple proteins in a parallel and un-biased manner [
17,
18,
19,
20]. Consequently, proteomic research has advanced understanding of the phenotypical changes associated with musculoskeletal ageing [
7,
10,
21,
22,
23,
24,
25]. For example, Gelfi
et al. [
7] reported myosin regulatory light chain (MLRS) content was lower in older compared with younger individuals, which may contribute to age-related contractile dysfunction [
26]. Nevertheless, existing literature is broadly limited by two major caveats. First, the confounding influence of activity levels has seldom been standardized or controlled in human studies. For instance, lower MLRS isoform content of elderly individuals [
7] could reflect reduced physical activity rather than a direct effect of ageing
per se. Second, inconsistencies exist between proteomic studies in model species (e.g., rodents) compared with humans. For example, in contrast to humans, commonly used rodent models are inbred and rodents express a fast type MyHCII isoform (Type II b [
9,
15]), which is not present in human locomotive muscles.
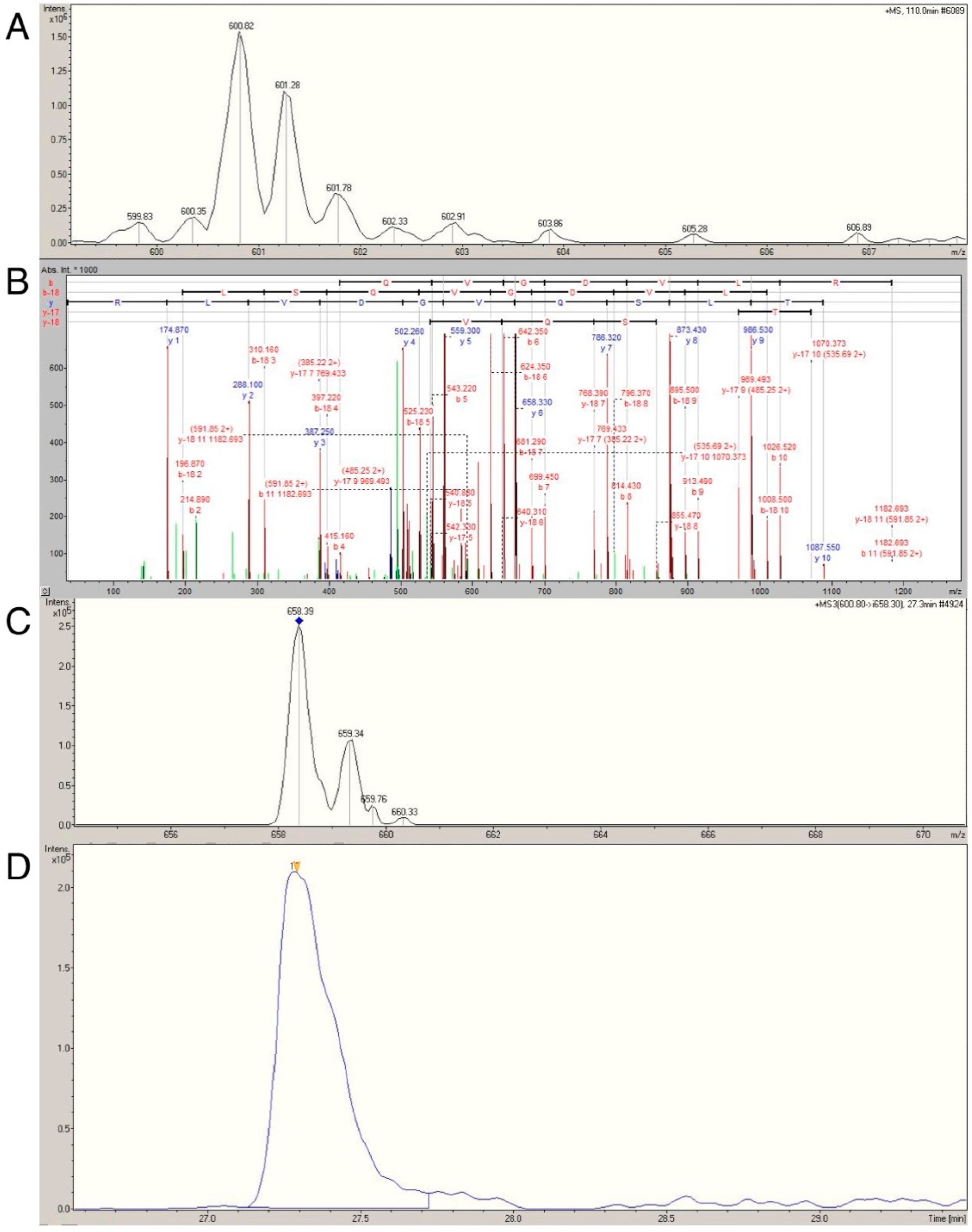
Two-dimensional gel electrophoresis (2DGE) has been commonly employed in muscle proteomic investigations but this technique has widely acknowledged [
12,
19,
21,
27,
28,
29] technical limitations. In an effort to overcome some of the technical issues associated with 2DGE, we have used a high-performance liquid chromatography coupled mass spectrometry (LC-MS) that affords time-efficient (180 min per sample), automated and quantitative analysis of skeletal muscle samples [
12]. Recent application of this approach identified heart-type fatty acid binding protein as a novel biomarker of aerobic capacity in both rodent and human skeletal muscle samples [
12]; confirming the utility of this novel phenotypical approach. We reasoned that application of LC-MS to the myofibrillar fraction may advance phenotyping by enabling the measurement of isoform expression and splice variation of the myofibrillar proteome. Accordingly, the aims of the present study were to: (1) quantify differences in MyHC content using traditional denaturing gel electrophoresis methods; then (2) mine the myofibrillar proteome using LC-MS/MS; and (3) investigate differences between muscle samples of young and elderly adults using LC-MS label-free profiling. In addition, trained and untrained participants were included in each age group to further dissect the influence of physical activity levels on phenotypical biomarkers of ageing [
10,
11,
30]. Finally, we used multiple reaction monitoring to verify discoveries generated by LC-MS profiling.
3. Discussion
We used traditional biochemical analysis of MyHC isoforms to confirm age-related differences in human muscle phenotype and further demonstrate the effect of habitual activity on these data. We expand on our previous work [
12,
28], by using label-free LC-MS profiling to phenotype the human myofibrillar proteome. We resolved 47 myofibrillar proteins without prior orthogonal separation, which is consistent with the well-documented high-abundance of a small number of contractile proteins in skeletal muscle [
17,
19,
20]. Indeed, just ten contractile and metabolic proteins account for over half of the total protein content in skeletal muscle [
29,
30]. LC-MS profiling detected significant differences in the abundance of peptides belonging to essential and regulatory myosin light chains between young and elderly individuals that were either sedentary or undertook regular exercise training. To verify these findings, we performed MRM of selected isoform-specific peptides using a condensed LC gradient with a sample turn-around of 40 min. In addition to verifying novel information regarding age-related differences in myosin light chain abundances, future researchers are able to capitalize on these findings and also perform rapid, semi-automated and parallel profiling of muscle proteins using our reported transitions.
Consistent with previous literature [
7,
21,
31], MyHCIIa abundance, a marker of Type IIa fiber content, was less in older compared with younger individuals, regardless of training status. Indeed, MyHCIIx, a marker of Type IIx content, was almost completely absent in the OT group. MyHCIIa and MyHC IIx phenotypes are likely to be associated with the age-related preferential loss of Type II MU [
2,
34]. The molecular sequelae underpinning Type II MU loss are unclear but this phenomenon may be driven by aberrant repulsive axonal guidance (e.g., Semaphorin 3A) mediated denervation [
34,
35,
36]. Irrespective of age, trained participants displayed greater MyHCI and lesser MyHCIIa abundance compared with untrained participants. This is consistent with greater Type I and lesser Type IIa fiber content in endurance-trained individuals owing to genetic and environmental factors [
6,
15]. Whilst our cross-sectional design precludes causative analysis [
10], MyHC data are consistent with known age- and activity-related phenotypes [
7,
21,
25]. Our ability to confirm existing paradigms of age- and activity-related MyHC phenotypes using traditional analysis meant we could largely exclude the possibility that discoveries made by LC-MS profiling were entirely specific to the population of individuals and instead can be discussed alongside existing literature.
Label-free profiling overcomes many of the technical limitations associated with 1D and 2D gel electrophoresis and could significantly advance phenotyping of skeletal muscle. Nonetheless, LC-MS analysis based solely on intensities of parent ion masses can be confounded by interference from unrelated peptides that share analogous retention times and mass-to-charge ratios [
12]. With this limitation in mind, we sought to verify the age- and activity-related differences in actin, myosin, tropomyosin, MLRS, MYL1 and MYL3 using multiple reaction monitoring (MRM; reviewed in [
37]) This technique selectively monitors and quantifies the intensity of daughter ions from known parent ions (termed transitions [
12]), which greatly enhances the specificity of the analysis. We previously used MRM to confirm that heart-type fatty acid protein was more abundant in rodents artificially selected for higher aerobic capacity compared with lower aerobic capacity [
12], affirming the utility of this approach in skeletal muscle. Application of MRM to the myofibrillar fraction presented a significant analytical challenge owing to high myofibrillar protein sequence homology. For example, MYL1 and MYL3 share 72.6% sequence identify. Consequently, only isoform specific transitions in selected proteins were analyzed.
Myosin essential (MYL1, MYL3) and regulatory (MLRS) light chains were selected for MRM analysis owing to their essential role in regulating contractile velocity [
38] and prior reports of differential expression in old compared with young skeletal muscle [
7,
22]. MYL1 and MLRS are predominately, but not exclusively [
39,
40], expressed in fast twitch muscle, whereas MYL3 is predominately expressed in slow twitch muscle [
13,
24]. Using known age and activity related phenotypes as a conceptual framework [
2,
3,
6,
21,
34], it could be predicted,
a priori, that: (1) MYL1 and MLRS content should be greater; and (2) MYL3 content should be lesser in the skeletal muscle of elderly compared to young adults. The prediction for MLRS is complicated by conflicting reports of an age-related decrease in MLRS in humans [
7] against a drastic increase in MLRS that demarcates the onset of senescence in rodent skeletal muscle [
26]. Whilst this could be related to interspecies differences [
41], it could equally be attributable to a failure to control for physical activity status particularly in human studies [
1,
42]. We reasoned that the latter was more likely owing to a recent report documenting lower MRLS content in trained compared with recreational runners [
43]. In support of this, we report novel evidence that age-related changes in MLRS abundance could easily be confounded by the physical activity status of the participants. Specifically, MLRS differed as a function of training status and the trend towards differential age-related expression was owing to significantly greater levels in YU compared with OT, but not YT compared with OT. This observation warrants further mechanistic exploration. Future reports may wish to extend our observations by comparing the relative abundance and phosphorylation status of each of the fast and slow regulatory isoforms.
We also report novel data identifying that MYL1 and MYL3 content is lesser and greater, respectively, in the muscle of old compared with younger adults. As a consequence, the ratio between MYL3 and MYL1 is dramatically different between young, elderly, trained and untrained humans (
Table 4 and
Table 5). Our findings are the first of their kind rendering comparison with previous literature difficult. This notwithstanding, MYL1, MYL3 and MLRS data confirm our MyHC analysis and are consistent with the notion that ageing is accompanied by preferential Type II MU loss [
2,
34]. It will, however, be important to confirm this observation with longitudinal studies that permit greater mechanistic insight; as one cannot rule out the possibility that innate fiber type differences between our groups underpinned our observations and not age or physical activity status
per se. Such caveats should be viewed globally; that is, they are generic to the research field, being unrestricted to the present study owing to the difficulties associated with conducting longitudinal studies of human ageing.
MRM affords semi-automated, rapid and quantitative phenotyping [
12,
37] and the potential wider application of this approach has not escaped our attention. Future investigators may wish to use our data as a resource to perform isoform specific MRM analysis of myosin light chain content; thus, obviating the limitations of traditional methods of muscle phenotyping. In the future, this approach could be further extended by spiking samples with stable isotope-labeled peptides to provide quantitative analysis of human samples.