Evaluation of Ion-Exchange Characteristics of Cesium in Natural Japanese Rocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Japanese Rocks
2.2. Cesium Adsorption Experiments
2.3. Cesium Desorption Experiments
2.4. Heat Treatment Experiments
3. Results and Discussion
3.1. Adsorption of Cs+ onto Natural Japanese Rocks
3.2. Adsorption Behavior of Cs+ with Seawater
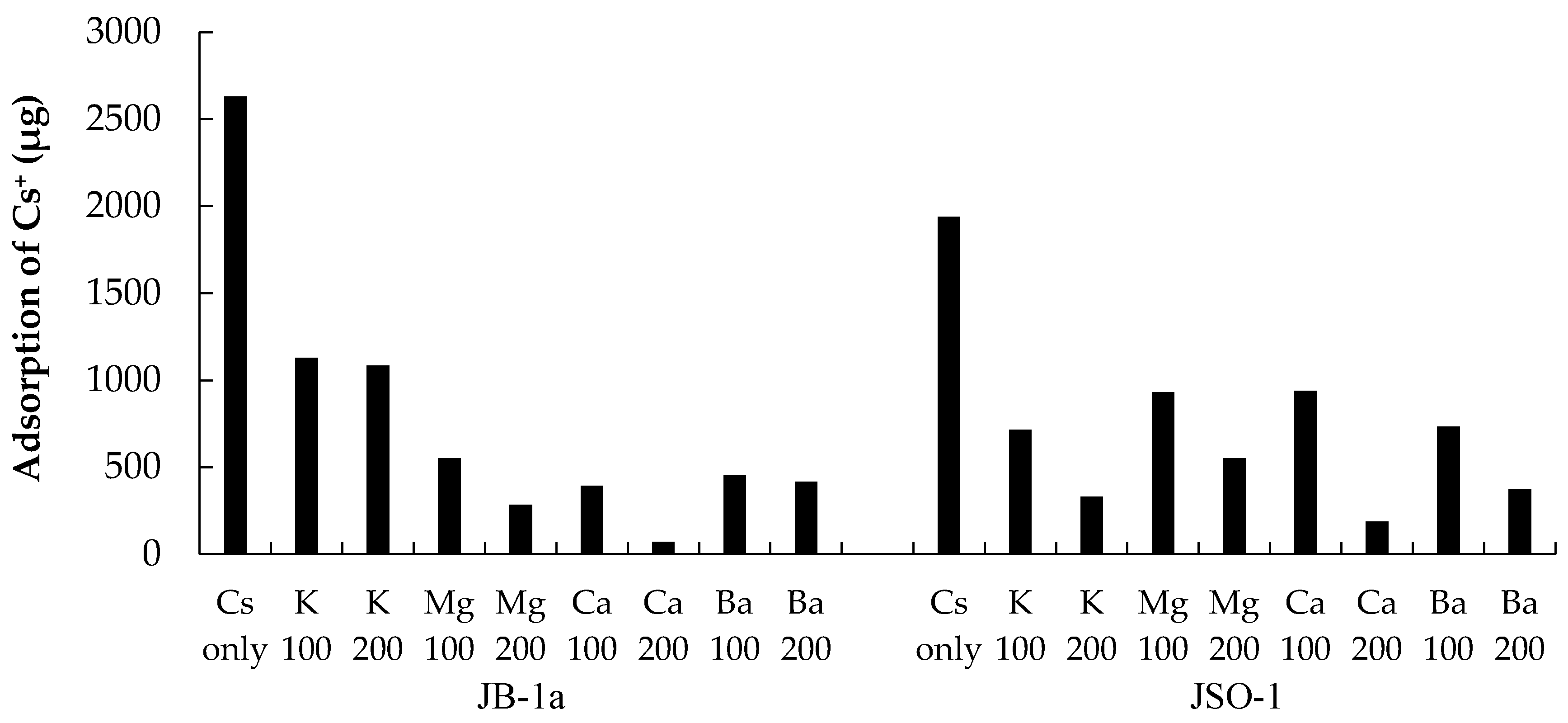
3.3. Adsorption of Cs+ with Coexisting Ions
3.4. Adsorption Quantity of Cs+ for pH Change
3.5. Desorption of Cs+ from Natural Japanese Rocks
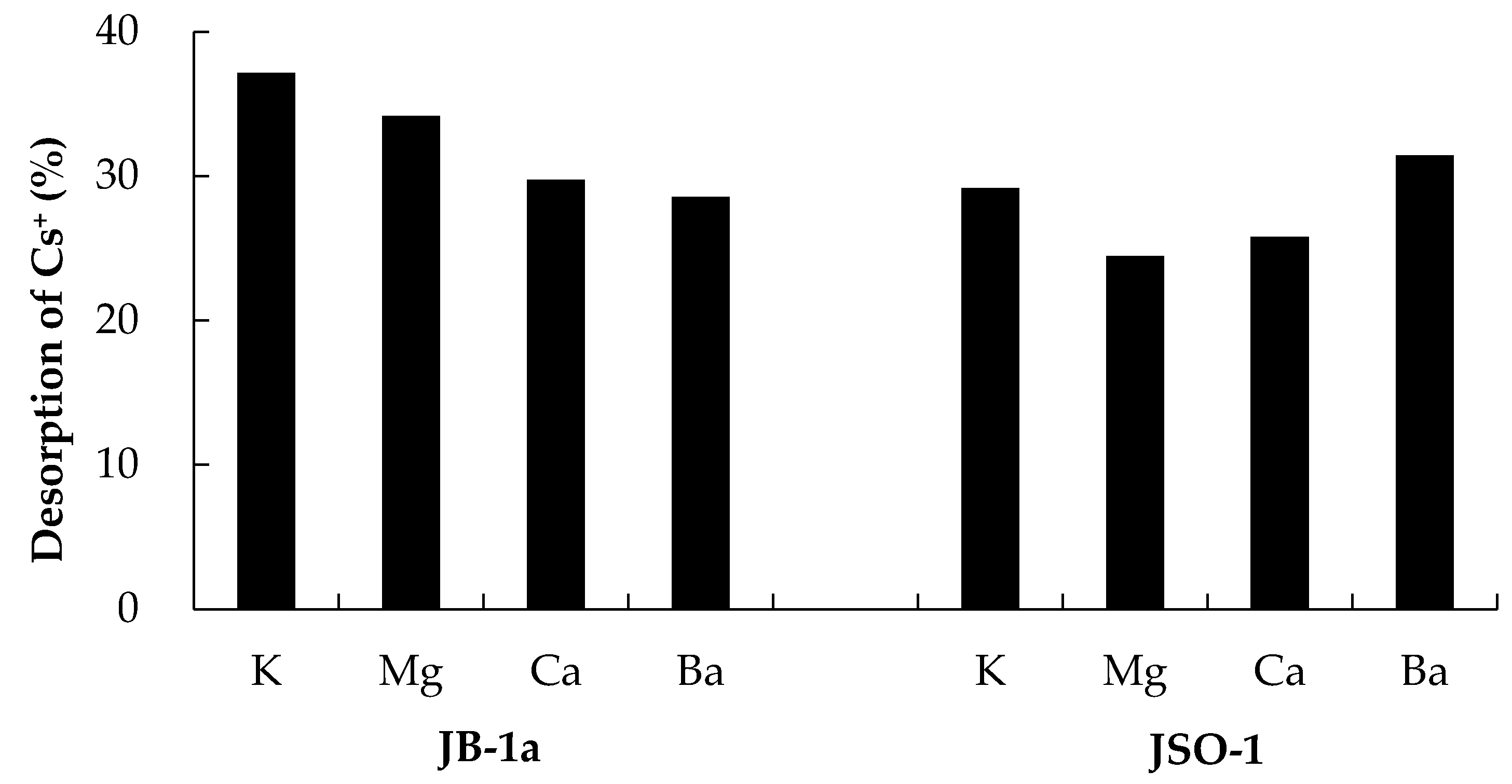
3.6. Desorption of Cs+ with Coexisting Ions
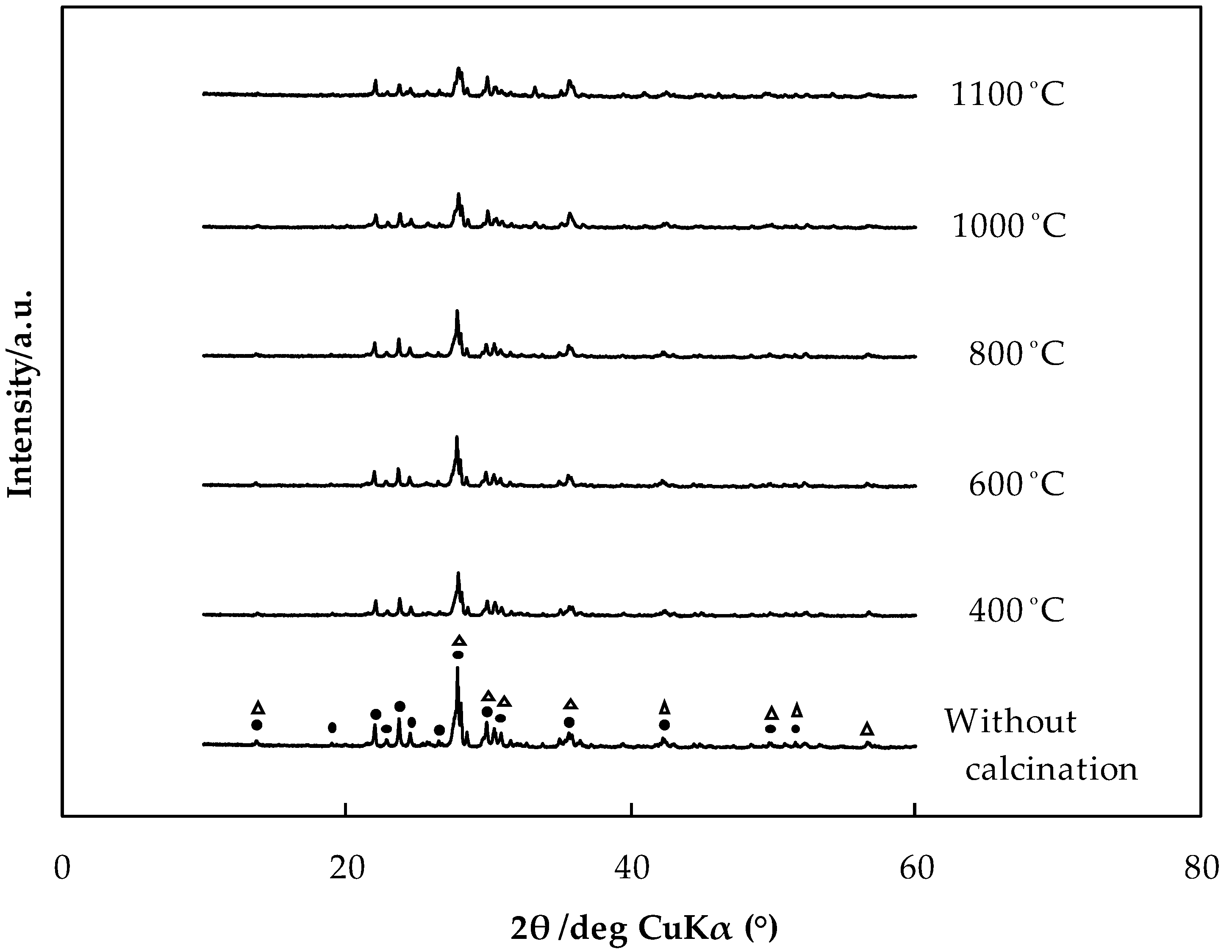
3.7. Effect of the Heat Treatment of Rocks
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujii, K.; Ikeda, S.; Akama, A.; Komatsu, M.; Takahashi, M.; Kaneko, S. Vertical migration of radiocesium and clay mineral composition in five forest soils contaminated by the Fukushima nuclear accident. Soil Sci. Plant Nutr. 2014, 60, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, D.; Takahashi, A.; Tanaka, H.; Sato, M.; Fukuda, S.; Kamimura, R.; Kawamoto, T. Variation in available cesium concentration with parameters during temperature induced extraction of cesium from soil. J. Environ. Radioact. 2015, 140, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Hirose, A.; Motai, S.; Kikuchi, R.; Tanoi, K.; Nakanishi, T.; Yaita, T.; Kogure, T. Cesium adsorption/desorption behavior of clay minerals considering actual contamination conditions in Fukushima. Sci. Rep. 2016, 6, 21543. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T.; Morimoto, K.; Tamura, K.; Sato, H.; Yamagishi, A. XRD and HRTEM Evidence for Fixation of Cesium Ions in Vermiculite Clay. Chem. Lett. 2012, 41, 380–382. [Google Scholar] [CrossRef] [Green Version]
- Benedicto, A.; Missana, T.; Fernández, A.M. Interlayer collapse affects on cesium adsorption onto illite. Environ. Sci. Technol. 2014, 48, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Zaunbrecher, L.K.; Cygan, R.T.; Elliott, W.C. Molecular models of cesium and rubidium adsorption on weathered micaceous minerals. J. Phys. Chem. A 2015, 119, 5691–5700. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.J.; Shaw, S.; Peacock, C.L.; Trivedi, D.; Small, J.S.; Abrahamsen, L.G.; Burke, I.T. Ionic strength and pH dependent multi-site sorption of Cs onto a micaceous aquifer sediment. Appl. Geochem. 2014, 40, 32–42. [Google Scholar] [CrossRef]
- Endo, M.; Yoshikawa, E.; Muramatsu, N.; Takizawa, N.; Kawai, T.; Unuma, H.; Sasaki, A.; Masano, A.; Takeyama, Y.; Kahara, T. The removal of cesium ion with natural Itaya zeolite and the ion exchange characteristics. J. Chem. Technol. Biotechnol. 2013, 88, 1597–1602. [Google Scholar] [CrossRef]
- Chiang, P.N.; Wang, M.K.; Huang, P.M.; Wang, J.J. Effects of low molecular weight organic acids on (137) Cs release from contaminated soils. Appl. Radiat. Isot. 2011, 69, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Takizawa, N.; Togashi, K.; Sasaki, A.; Endo, M. Adsorption/Desorption Characteristics of Cesium Ions on Natural and Synthetic Minerals. J. Ion Exch. 2018, 29, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Osuna, F.J.; Cota, A.; Pavón, E.; Pazos, M.C.; Alba, M.D. Cesium adsorption isotherm on swelling high-charged micas from aqueous solutions: Effect of temperature. Am. Mineral. 2018, 103, 623–628. [Google Scholar] [CrossRef]
- Durrant, C.B.; Begg, J.D.; Kersting, A.B.; Zavarin, M. Cesium sorption reversibility and kinetics on illite, montmorillonite, and kaolinite. Sci. Total Environ. 2018, 610, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Q.; Jin, X.Y.; Lu, X.Q.; Chen, Z.L. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Bostick, B.C.; Vairavamurthy, M.A.; Karthikeyan, K.G.; Chorover, J. Cesium adsorption on clay minerals: An EXAFS spectroscopic investigation. Environ. Sci. Technol. 2002, 36, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Thiry, Y.; Funakawa, S.; Kosaki, T. Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand. Soil Sci. Plant Nutr. 2008, 54, 479–489. [Google Scholar] [CrossRef] [Green Version]
- GSJ Geochemical Reference Samples Data Base. Available online: https://gbank.gsj.jp/geostandards/welcome.html (accessed on 14 June 2018).
- Johnson, W.M.; Maxwell, J.A. Rock and Mineral Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1981; ISBN 13 9780471027430. [Google Scholar]
- Miura, K. Weathering during late Pliocene of Gotsu plutonic rocks. J. Jpn. Soc. Eng. Geol. 1973, 14, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Onikata, M.; Kondo, M.; Hayashi, N.; Yamanaka, S. Complex formation of cation-exchanged montmorillo-nites with propylene carbonate: Osmotic swelling in aqueous electrolyte solutions. Clays Clay Miner. 1999, 47, 672–677. [Google Scholar] [CrossRef]
- Morimoto, K.; Kogure, K.; Tamura, K.; Tomofuji, T.; Yamagishi, A.; Sato, H. Desorption of Cs+ Ions Intercalated in Vermiculite Clay through Cation Exchange with Mg2+ Ions. Chem. Lett. 2012, 41, 1715–1717. [Google Scholar] [CrossRef]
- Tamura, K.; Sato, H.; Yamagishi, A. Desorption of Cs+ ions from a vermiculite by exchanging with Mg2+ ions: Effects of Cs+-capturing ligand. J. Radioanal. Nucl. Chem. 2015, 303, 2205–2210. [Google Scholar] [CrossRef]
- Dzene, L.; Tertre, E.; Hubert, F.; Ferrage, E. Nature of the sites involved in the process of cesium desorption from vermiculite. J. Colloid Interface Sci. 2015, 455, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Komy, Z.R.; Shaker, A.M.; Heggy, S.E.M.; El-Sayed, M.E.A. Kinetic study for copper adsorption onto soil minerals in the absence and presence of humic acid. Chemosphere 2014, 99, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Dumat, C.; Stauton, S. Reduced adsorption of caesium on clay minerals caused by various humic substances. J. Environ. Radioact. 1999, 46, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, E.; Baeyens, B.; Maes, A.; Cremers, A. Cesium and rubidium ion equilibriums in illite clay. J. Phys. Chem. 1983, 87, 1213–1219. [Google Scholar] [CrossRef]
- Staunton, S.; Roubaud, M. Adsorption of 137Cs on montmorillonite and illite: Effect of charge compensating cation, ionic strength, concentration of Cs, K, and fluvic acid. Clays Clay Miner. 1997, 45, 251–260. [Google Scholar] [CrossRef]
- Environmental Chemistry.com. Available online: https://environmentalchemistry.com/yogi/periodic/ioni-cradius.html (accessed on 14 June 2018).







| Constituent | JA-1 | JA-3 | JB-1 | JB-1a | JB-2 | JB-3 | JG-1 | JGb-1 | JSl-1 | JCh-1 | JSd-2 | JSO-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (%) | 64.0 | 62.3 | 52.4 | 52.4 | 53.3 | 51.0 | 72.3 | 43.7 | 59.5 | 97.8 | 60.8 | 38.4 |
| Al2O3 (%) | 15.2 | 15.6 | 14.5 | 14.5 | 14.6 | 17.2 | 14.2 | 17.5 | 17.6 | 0.734 | 12.3 | 18.1 |
| Fe2O3 (%) | 2.59 | 1.15 | 2.33 | 2.55 | 3.33 | 3.20 | 0.380 | 4.79 | 1.88 | 0.272 | 4.55 | 8.58 |
| FeO (%) | 3.98 | 4.83 | 5.99 | 5.78 | 9.98 | 7.85 | 1.61 | 9.43 | 4.52 | 0.087 | 5.96 | 2.52 |
| MgO (%) | 1.57 | 3.72 | 7.71 | 7.83 | 4.62 | 5.19 | 0.740 | 7.85 | 2.41 | 0.075 | 2.73 | 2.11 |
| CaO (%) | 5.70 | 6.24 | 9.25 | 9.31 | 9.82 | 9.79 | 2.20 | 11.9 | 1.48 | 0.045 | 3.66 | 2.55 |
| Na2O (%) | 3.84 | 3.19 | 2.77 | 2.73 | 2.04 | 2.73 | 3.38 | 1.20 | 2.18 | 0.031 | 2.44 | 0.670 |
| K2O (%) | 14.2 | 1.41 | 1.43 | 1.40 | 0.420 | 0.780 | 3.98 | 0.240 | 2.85 | 0.221 | 1.15 | 0.340 |
| H2O+ (%) | 0.720 | 0.200 | 1.02 | 0.920 | 0.250 | 0.180 | 0.540 | 1.28 | 3.92 | 0.360 | 2.55 | 7.88 |
| H2O− (%) | 0.300 | 0.110 | 0.950 | 0.920 | 0.130 | 0.070 | 0.070 | 0.130 | 0.654 | 0.152 | 0.451 | - |
| 400 °C | 600 °C | 800 °C | 1000 °C | 1100 °C | |
|---|---|---|---|---|---|
| JB | 68.1 | 58.3 | 28.6 | 19.8 | 0.0 |
| JSO | 98.5 | 76.7 | 45.1 | 17.5 | 0.0 |
| JA | 35.8 | 24.5 | 26.4 | 14.2 | 0.0 |
| JSd | 100 | 30.0 | 13.6 | 0.0 | 0.0 |
| JSl | 90.2 | 83.4 | 48.0 | 21.2 | 0.0 |
| Water | HCl (pH 1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | Non calcination | 400 | 600 | 800 | 1000 | 1100 | 400 | 600 | 800 | 1000 | 1100 |
| JB | 31.0 | 3.4 | 2.2 | 0.6 | 0.2 | 0.2 | 87.1 | 47.1 | 4.8 | 1.4 | 0.2 |
| JSO | 39.2 | 11.2 | 13.0 | 5.9 | 0.5 | 0.8 | 88.6 | 50.2 | 23.8 | 2.6 | 1.5 |
| JA | 31.0 | 10.2 | 8.8 | 4.2 | 2.3 | 0.9 | 58.4 | 31.1 | 16.6 | 3.8 | 0.3 |
| JSd | 90.1 | 31.6 | 15.1 | 9.9 | 3.0 | 1.3 | 95.9 | 52.0 | 16.1 | 4.3 | 0.8 |
| JSl | 65.5 | 29.3 | 16.7 | 15.5 | 1.1 | 0.5 | 100 | 100 | 35.2 | 2.2 | 0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, T.; Sasaki, A.; Endo, M. Evaluation of Ion-Exchange Characteristics of Cesium in Natural Japanese Rocks. Technologies 2018, 6, 78. https://doi.org/10.3390/technologies6030078
Miura T, Sasaki A, Endo M. Evaluation of Ion-Exchange Characteristics of Cesium in Natural Japanese Rocks. Technologies. 2018; 6(3):78. https://doi.org/10.3390/technologies6030078
Chicago/Turabian StyleMiura, Takuya, Atsushi Sasaki, and Masatoshi Endo. 2018. "Evaluation of Ion-Exchange Characteristics of Cesium in Natural Japanese Rocks" Technologies 6, no. 3: 78. https://doi.org/10.3390/technologies6030078




