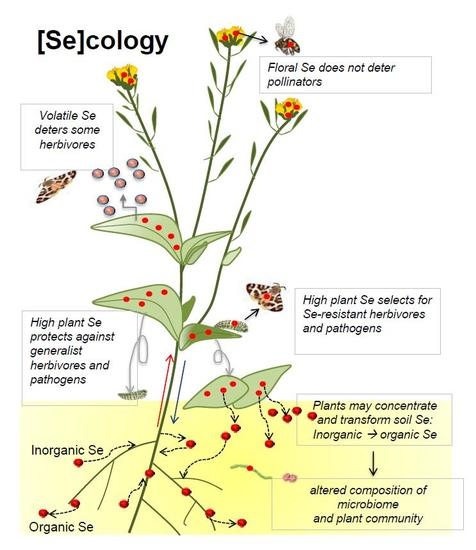
On the Ecology of Selenium Accumulation in Plants
Abstract
:1. Two Faces of Se in Biology—An Introduction to Se Benefits and Toxicity
2. What Can Plants Do with Se, at the Cellular and Whole-Plant Level?
3. Ecological Effects of Plant-Accumulated Se on Ecological Partners
3.1. Plant–Herbivore Interactions
3.2. Plant–Pollinator Interactions
3.3. Plant–Microbe Interactions
3.4. Plant–Plant Interactions
4. Integrative Discussion on the Ecological Implications of Plant Se Accumulation
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Gladyshev, V.N. Comparative genomics of trace elements: Emerging dynamic view of trace element utilization and function. Chem. Rev. 2009, 109, 4828–4861. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Anderson, J.W. Selenium interactions in sulfur metabolism. In Sulfur Nutrition and Assimilation in Higher Plants—Regulatory, Agricultural and Environmental Aspects; De Kok, L.J., Ed.; SPB Academic Publishing: The Hague, The Netherlands, 1993; pp. 49–60. [Google Scholar]
- Stadtman, T.C. Selenium biochemistry. Annu. Rev. Biochem. 1990, 59, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Wilber, C.G. Toxicology of selenium: A review. Clin. Toxicol. 1980, 17, 171–230. [Google Scholar] [CrossRef]
- Lyons, G.; Stangoulis, J.; Graham, R. High-selenium wheat: Biofortification for better health. Nutr. Res. Rev. 2003, 16, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.A.H.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Beath, O.A.; Gilbert, C.S.; Eppson, H.F. The use of indicator plants in locating seleniferous areas in Western United States. I. General. Am. J. Bot. 1939, 26, 257–269. [Google Scholar] [CrossRef]
- Cappa, J.J.; Pilon-Smits, E.A.H. Evolutionary aspects of hyperaccumulation. Planta 2014, 239, 267–275. [Google Scholar] [CrossRef] [PubMed]
- El Kassis, E.; Cathala, N.; Rouached, H.; Fourcroy, P.; Berthomieu, P.; Terry, N.; Davidian, J.-C. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007, 143, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Neuhierl, B.; Böck, A. On the mechanism of selenium tolerance in selenium accumulating plants. Purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus bisulcatus. Eur. J. Biochem. 1996, 239, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H.; Hwang, S.; Lytle, C.M.; Zhu, Y.; Tai, J.C.; Bravo, R.C.; Chen, Y.; Leustek, T.; Terry, N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999, 119, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Alford, E.R.; Pilon-Smits, E.A.H.; Marcus, M.A.; Fakra, S.C.; Paschke, M.W. No evidence for a cost of tolerance: Selenium hyperaccumulation by Astragalus does not inhibit root nodule symbiosis. Am. J. Bot. 2012, 99, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Cappa, J.J.; Yetter, C.; Fakra, S.F.; Cappa, P.J.; DeTar, R.; Landes, C.; Pilon-Smits, E.A.H.; Simmons, M.P. Evolution of selenium hyperaccumulation in Stanleya (Brassicaceae) as inferred from phylogeny, physiology and x-ray microprobe analysis. New Phytol. 2015, 205, 583–595. [Google Scholar] [CrossRef]
- Freeman, J.L.; Zhang, L.H.; Marcus, M.A.; Fakra, S.; McGrath, S.P.; Pilon-Smits, E.A.H. Spatial imaging, speciation and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol. 2006, 142, 124–134. [Google Scholar] [CrossRef]
- Quinn, C.F.; Prins, C.N.; Freeman, J.L.; Gross, A.M.; Hantzis, L.J.; Reynolds, R.J.B.; Yang, S.; Covey, P.A.; Bañuelos, G.S.; Pickering, I.J.; et al. Selenium accumulation in flowers and its effects on pollination. New Phytol. 2011, 192, 727–737. [Google Scholar] [CrossRef]
- Cappa, J.J.; Cappa, P.J.; El Mehdawi, A.F.; McAleer, J.M.; Simmons, M.P.; Pilon-Smits, E.A.H. Characterization of selenium and sulfur accumulation in Stanleya (Brassicaceae). A field survey and common-garden experiment. Am. J. Bot. 2014, 101, 830–839. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Marshall, B.; Broadley, M.R. Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann. Bot. 2007, 100, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Schneberg, K.A.; Pilon-Smits, E.A.H. Sulfur–selenium–molybdenum interactions distinguish selenium hyperaccumulator Stanleya pinnata from non-hyperaccumulator Brassica juncea (Brassicaceae). Planta 2014, 239, 479–491. [Google Scholar] [CrossRef] [PubMed]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and non-hyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef] [PubMed]
- El-Mehdawi, A.F.; Pilon-Smits, E.A.H. Ecological aspects of plant selenium hyperaccumulation. Plant Biol. 2012, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vickerman, D.B.; Shannon, M.C.; Bañuelos, G.S.; Grieve, C.M.; Trumble, J.T. Evaluation of Atriplex lines for selenium accumulation, salt tolerance and suitability for a key agricultural insect pest. Environ. Pollut. 2002, 120, 463–473. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Vickerman, D.B.; Trumble, J.T.; Shannon, M.C.; Davis, C.D.; Finley, J.W.; Mayland, H.F. Biotransfer possibilities of selenium from plants used in phytoremediation. Int. J. Phytoremed. 2002, 4, 315–331. [Google Scholar]
- Hanson, B.R.; Garifullina, G.F.; Lindblom, S.D.; Wangeline, A.; Ackley, A.; Kramer, K.; Norton, A.P.; Lawrence, C.B.; Pilon-Smits, E.A.H. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol. 2003, 159, 461–469. [Google Scholar] [CrossRef]
- Hanson, B.R.; Lindblom, S.D.; Loeffler, M.L.; Pilon-Smits, E.A.H. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol. 2004, 162, 655–662. [Google Scholar] [CrossRef]
- Freeman, J.L.; Lindblom, S.D.; Quinn, C.F.; Fakra, S.; Marcus, M.A.; Pilon-Smits, E.A.H. Selenium accumulation protects plants from herbivory by orthoptera due to toxicity and deterrence. New Phytol. 2007, 175, 490–500. [Google Scholar] [CrossRef]
- Quinn, C.F.; Freeman, J.L.; Galeas, M.L.; Klamper, E.M.; Pilon-Smits, E.A.H. The role of selenium in protecting plants against prairie dog herbivory: Implications for the evolution of selenium hyperaccumulation. Oecologia 2008, 155, 267–275. [Google Scholar] [CrossRef]
- Quinn, C.F.; Freeman, J.L.; Reynolds, R.J.B.; Cappa, J.J.; Fakra, S.C.; Marcus, M.A.; Lindblom, S.D.; Quinn, E.K.; Bennett, L.E.; Pilon-Smits, E.A.H. Selenium hyperaccumulation offers protection from cell disruptor herbivores Plant Physiol. Plant Physiol. 2010, 153, 1630–1652. [Google Scholar] [CrossRef]
- Freeman, J.L.; Quinn, C.F.; Lindblom, S.D.; Klamper, E.M.; Pilon-Smits, E.A.H. Selenium protects the hyperaccumulator Stanleya pinnata against black-tailed prairie dog herbivory in native seleniferous habitats. Am. J. Bot. 2009, 96, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of selenium on plant metabolism and implications for crops and consumers. In Selenium in Plants. Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.Q., Eds.; Springer: Cham, Switzerland, 2017; pp. 257–275. [Google Scholar]
- Galeas, M.L.; Klamper, E.M.; Bennett, L.E.; Freeman, J.L.; Kondratieff, B.C.; Pilon-Smits, E.A.H. Selenium hyperaccumulation affects plant arthropod load in the field. New Phytol. 2008, 177, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Marcus, M.A.; Fakra, S.; Devonshire, J.; McGrath, S.P.; Pilon-Smits, E.A.H. Seeds of selenium hyperaccumulators Stanleya pinnata and Astragalus bisulcatus are colonized by Se-resistant, Se-excluding wasp and beetle herbivores. PLoS ONE 2012, 7, e50516. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Quinn, C.F.; Marcus, M.A.; Fakra, S.; Pilon-Smits, E.A.H. Selenium tolerant diamondback moth disarms hyperaccumulator plant defense. Curr. Biol. 2006, 16, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.R.; Quinn, C.F.; Freeman, J.L.; Lindblom, S.D.; Marcus, M.S.; Fakra, S.C.; Gilligan, T.M.; Alford, E.R.; Wangeline, A.L.; Pilon-Smits, E.A.H. Selenium distribution and speciation in hyperaccumulator Astragalus bisulcatus and associated ecological partners. Plant Physiol. 2012, 159, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Hladun, K.R.; Smith, B.H.; Mustard, J.A.; Morton, R.R.; Trumble, J.T. Selenium toxicity to honey bee (Apis mellifera L.) pollinators: Effects on behaviors and survival. PLoS ONE 2012, 7, e34137. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.N.; Gupta, G.; Jha, P.; Rajesh, M. Association of rhizospheric/endophytic bacteria with plants: A potential gateway to sustainable agriculture. Greener J. Agric. Sci. 2013, 3, 73–84. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant-endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Wangeline, A.L.; Valdez, J.R.; Lindblom, S.D.; Bowling, K.L.; Reeves, F.B.; Pilon-Smits, E.A.H. Characterization of rhizosphere fungi from selenium hyperaccumulator and nonhyperaccumulator plants along the eastern Rocky Mountain Front Range. Am. J. Bot. 2011, 98, 1139–1147. [Google Scholar] [CrossRef] [Green Version]
- Lindblom, S.D.; Fakra, S.C.; Landon, J.; Schulz, P.; Tracy, B.; Pilon-Smits, E.A.H. Inoculation of Astragalus racemosus and Astragalus convallarius with selenium-hyperaccumulator rhizosphere fungi affects growth and selenium accumulation. Planta 2013, 237, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, S.D.; Fakra, S.C.; Landon, J.; Schulz, P.; Tracy, B.; Pilon-Smits, E.A.H. Inoculation of selenium hyperaccumulator Stanleya pinnata and related non-accumulator Stanleya elata with hyperaccumulator rhizosphere fungi—Effects on Se accumulation and speciation. Physiol. Plant. 2013, 150, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wanek, P.L.; Vance, G.F.; Stahi, P.D. Selenium uptake by plants: Effects of soil steaming, root addition, and selenium augmentation. Commun. Soil Sci. Plant Anal. 1999, 30, 265–278. [Google Scholar] [CrossRef]
- Larsen, E.H.; Lobinski, R.; Burger-Meÿer, K.; Hansen, M.; Ruzik, R.; Mazurowska, L.; Rasmussen, P.H.; Sloth, J.J.; Scholten, O.; Kik, C. Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal. Bioanal. Chem. 2006, 385, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, L.; Yang, K.; Zhang, S. Influence of mycorrhizal inoculation on the accumulation and speciation of selenium in maize growing in selenite and selenate spiked soils. Pedobiologia 2011, 54, 267–272. [Google Scholar] [CrossRef]
- Lindblom, S.D.; Valdez, J.R.; Fakra, S.C.; Marcus, M.A.; Wangeline, A.L.; Pilon-Smits, E.A.H. Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Exp. Environ. Bot. 2013, 88, 33–42. [Google Scholar] [CrossRef]
- Alford, E.R.; Lindblom, S.D.; Pittarello, M.; Freeman, J.L.; Fakra, S.C.; Marcus, M.A.; Broeckling, C.; Paschke, M.W. Roles of rhizobial symbionts in Astragalus selenium hyperaccumulation. Am. J. Bot. 2014, 101, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Sura-de Jong, M.; Reynolds, R.J.; Richterova, K.; Musilova, L.; Staicu, L.C.; Chocholata, I.; Cappa, J.J.; Taghavi, S.; van der Lelie, D.; Frantik, T.; et al. Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties. Front. Plant Sci. 2015, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.T.; Bauer, J.; Metcalf, J.L.; Lovecka, P.; Sura-de Jong, M.; Warris, S.; Mooijman, P.J.W.; van der Meer, I.; Knight, R.; Pilon-Smits, E.A.H. Plant selenium hyperaccumulation affects rhizosphere: Enhanced species richness and altered species composition. Phytobiomes 2018, 2, 82–91. [Google Scholar] [CrossRef]
- de Souza, M.P.; Chu, D.; Zhao, M.; Zayed, A.M.; Ruzin, S.E.; Schichnes, D.; Terry, N. Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian mustard. Plant Physiol. 1999, 119, 565–574. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.P.; Huang, C.P.A.; Chee, N.; Terry, N. Rhizosphere bacteria enhance the accumulation of selenium and mercury in wetland plants. Planta 1999, 209, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, S.; Lampis, S.; Vallini, G. Selenite precipitation by a rhizospheric strain of Stenotrophomonas sp. isolated from the root system of Astragalus bisulcatus: A biotechnological perspective. Environ. Int. 2005, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W. Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 2009, 321, 385–408. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Paredes, C.; Rengel, Z.; de la Luz, M.M. Endophytic bacteria from selenium-supplemented wheat plants could be useful for plant-growth promotion, biofortification and Gaeumannomyces graminis biocontrol in wheat production. Biol. Fertil. Soils 2014, 50, 983–990. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Paschke, M.; Pilon-Smits, E.A.H. Symphyotrichum ericoides populations from seleniferous and non-seleniferous soil display striking variation in selenium accumulation. New Phytol. 2015, 206, 231–242. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef] [PubMed]
- El Mehdawi, A.F.; Quinn, C.F.; Pilon-Smits, E.A.H. Effects of selenium hyperaccumulation on plant–plant interactions: Evidence for elemental allelopathy. New Phytol. 2011, 191, 120–131. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Quinn, C.F.; Pilon-Smits, E.A.H. Selenium hyperaccumulators facilitate selenium-tolerant neighbors via phytoenrichment and reduced herbivory. Curr. Biol. 2011, 21, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.F.; Wyant, K.; Wangeline, A.L.; Shulman, J.; Galeas, M.L.; Valdez, J.R.; Paschke, M.W.; Pilon-Smits, E.A.H. Enhanced decomposition of selenium hyperaccumulator litter in a seleniferous habitat—Evidence for specialist decomposers. Plant Soil 2011, 341, 51–61. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Lindblom, S.D.; Cappa, J.J.; Fakra, S.C.; Pilon-Smits, E.A.H. Do selenium hyperaccumulators affect selenium speciation in neighboring plants and soil? An x-ray microprobe analysis. Int. J. Phytoremed. 2015, 17, 753–765. [Google Scholar] [CrossRef]



| Plant-herbivore interactions (Section 3.1) | ||
| Generalist herbivores (Se-sensitive) | ||
| Plant Se deters herbivores from feeding (many invertebrate taxa, prairie dogs) | HA, non-HA | L, F |
| Plant Se deters herbivores from ovipositing (moth) | HA | L |
| Plant Se is toxic, even deadly to herbivores when ingested (many invert taxa) | HA, non-HA | L |
| High-Se plants harbor fewer invertebrates in the field | HA | F |
| Potential specialist herbivores (Se-resistant) | ||
| High-Se plants harbor Se-excluding seed herbivores (bruchid, chalcid inverts) | HA | F |
| High-Se plants harbor leaf herbivores, some proven Se-tolerant (invert taxa) | HA | L, F |
| High-Se litter harbors higher levels of micro-arthropod decomposers than low-Se litter, and decomposes faster | HA, non-HA | F |
| Selenium movement from herbivores to higher trophic levels | ||
| Se-tolerant herbivores are parasitized by Se-tolerant wasps (2 moths, 2 wasps) | HA | F |
| High-Se plants harbor predator inverts with elevated Se levels (many taxa) | HA | F |
| Plant-pollinator interactions (Section 3.2) | ||
| Generalist pollinators (Se-sensitive) | ||
| High-Se flowers or feeding solution do not deter foraging by invert pollinators | HA, non-HA | L, F |
| High-Se pollen and nectar is collected and ingested by honey bees | HA | F |
| High-Se food sources (chemical) are toxic to honey bees | non-plant | L |
| Potential specialist pollinators (Se-tolerant) | ||
| Native pollinators accumulate Se to high levels from high-Se flowers | HA, non-HA | F |
| Plant-microbe interactions (Section 3.3) | ||
| Generalist microbes (default for fungi: Se-sensitive; for prokaryotes: Se resistant) | ||
| High-Se plants are protected from pathogenic fungi (2 species) | non-HA | L |
| Fungi from seleniferous/non-seleniferous soils differ in Se resistance | HA, non-HA | F, L |
| Se level in host plant is not correlated with Se resistance in fungal symbionts | HA, non-HA | F, L |
| Se level in host plant is not correlated with Se resistance in bacterial symbionts | HA, non-HA | F, L |
| Se level in host plant affects rhizosphere microbiome composition, (+) sp. richness | HA, non-HA | F |
| Microbes (isolated strains or consortia) can affect plant Se accumulation, volatilization, metabolism, tolerance, and general plant growth | HA, non-HA | L |
| Potential specialist microbes | ||
| High-Se plants are observed to harbor leaf fungus (1 species) | HA | F |
| High-Se litter harbors higher levels of cultivable microbes than low-Se litter, and decomposes faster | HA, non-HA | F |
| Rhizobial endosymbiont in root nodules may affect Se accumulation and Se metabolism in host plant | HA | F, L |
| Fungal endosymbionts may affect Se accumulation and Se metabolism in host | HA | F, L |
| Certain bacterial taxa are over-abundant in rhizosphere of Se HA | HA, non-HA | F |
| Plant-plant interactions (Section 3.4) | ||
| General effects | ||
| Soil around HA plants in field is ~10 fold elevated in Se, mostly in organic form | HA, non-HA | F |
| Soil around high-Se plants in field inhibits germination, growth of Arabidopsis | HA | F, L |
| Vegetative cover is reduced around HA plants in the field, and species composition is different | HA, non-HA | F |
| Potential specialist plant species | ||
| Some Se-tolerant plant species co-occur with HAs in field; they accumulate more Se, have less herbivory and grow better because of this association | HA | F |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilon-Smits, E.A.H. On the Ecology of Selenium Accumulation in Plants. Plants 2019, 8, 197. https://doi.org/10.3390/plants8070197
Pilon-Smits EAH. On the Ecology of Selenium Accumulation in Plants. Plants. 2019; 8(7):197. https://doi.org/10.3390/plants8070197
Chicago/Turabian StylePilon-Smits, Elizabeth A. H. 2019. "On the Ecology of Selenium Accumulation in Plants" Plants 8, no. 7: 197. https://doi.org/10.3390/plants8070197