Plant Cellular and Molecular Biotechnology: Following Mariotti’s Steps
Abstract
:1. Introduction
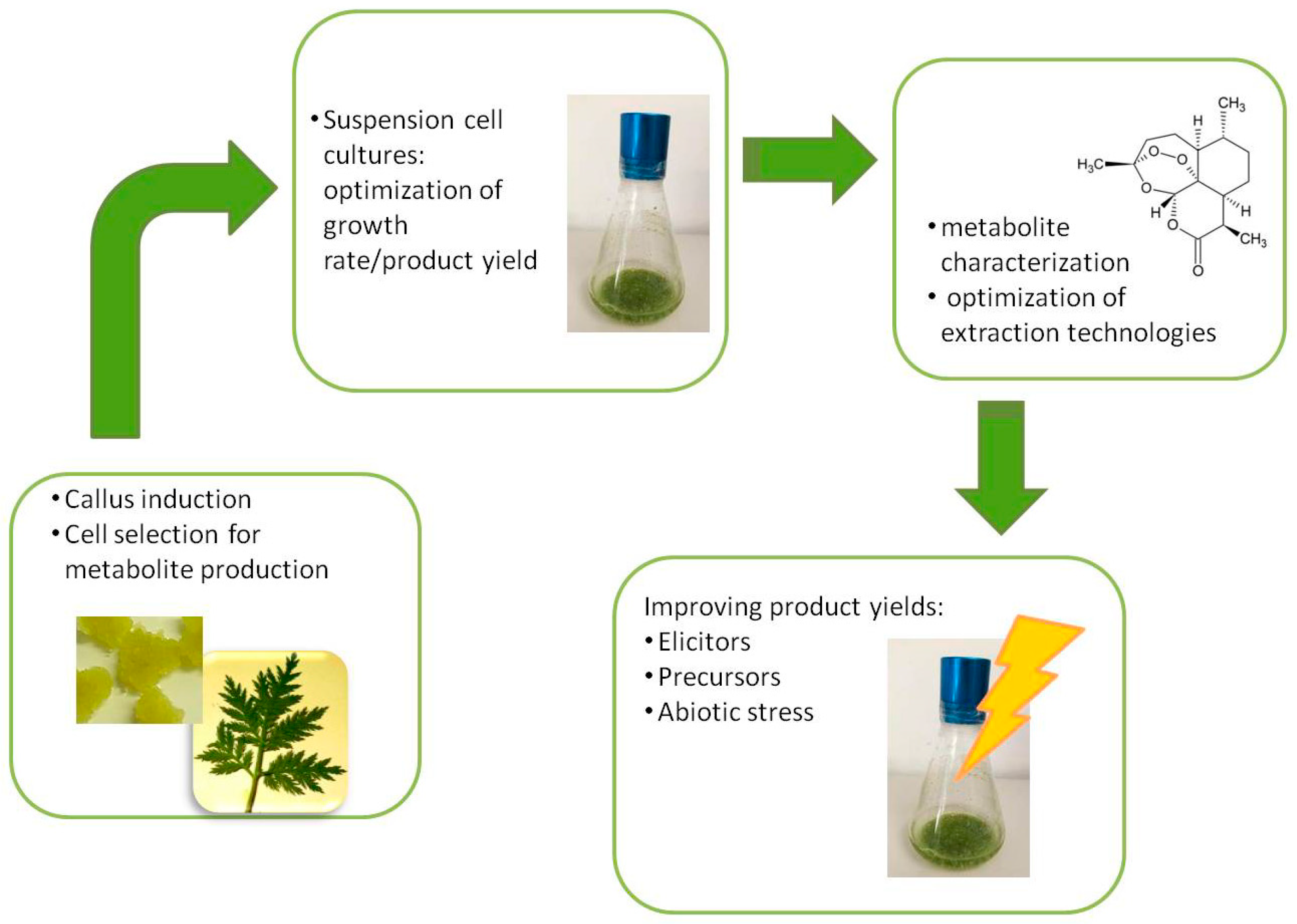
2. In Vitro Plant Cell and Tissue Cultures for Applied Biotechnology
3. The “Hairy Root” Syndrome Induced by Agrobacterium rhizogenes
4. Application of A. rhizogenes rol Genes to Fruit Tree Transformation
5. Genetic Transformation of Legumes
6. KNOX Transcription Factors as Key Regulators of Hormonal Homeostasis in Plant Morphogenesis
7. Phase Change in Fruit Trees: Advances and Perspectives in Peach and Prunus Species
8. Conclusions
Author contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Caretto, S.; Giardina, M.C.; Nicolodi, C.; Mariotti, D. Chlorsulfuron resistance in Daucus carota cell lines and plants: Involvement of gene amplification. Theor. Appl. Genet. 1994, 88, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Chaleff, R.; Ray, T. Herbicide-resistant mutants from tobacco cell cultures. Science 1994, 223, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Caretto, S.; Giardina, M.; Nicolodi, C.; Mariotti, D. In-vitro cell selection: Production and characterization of tobacco cell lines and plants resistant to the herbicide chlorsulfuron. J. Genet. Breed. 1993, 47, 115–120. [Google Scholar]
- Terry, R.W.; Newell, F.B.; Stephen, F.S.; Donald, P. Biochemical mechanism and molecular basis for ALS-inhibiting herbicide resistance in sugarbeet (Beta vulgaris) somatic cell selections. Weed Sci. 1998, 46, 13–23. [Google Scholar]
- Caretto, S.; Giardina, M.C.; Nicolodi, C.; Mariotti, D. Acetohydroxyacid synthase gene amplification induces clorsulfuron resistance in Daucus Carota L. In Current Issues in Plant Molecular and Cellular Biology, Proceedings of the VIIIth International Congress on Plant Tissue and Cell Culture, Florence, Italy, 12–17 June 1994; Terzi, M., Cella, R., Falavigna, A., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 235–240. [Google Scholar]
- Duke, S.O. Perspectives on transgenic, herbicide-resistant crops in the United States almost 20 years after introduction. Pest Manag. Sci. 2015, 71, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Barrell, P.J.; Latimer, J.M.; Baldwin, S.J.; Thompson, M.L.; Jacobs, J.M.E.; Conner, A.J. Somatic cell selection for chlorsulfuron-resistant mutants in potato: Identification of point mutations in the acetohydroxyacid synthase gene. BMC Biotechnol. 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Eng. Biotechnol. 2008, 111, 187–228. [Google Scholar] [PubMed]
- Mene-Saffrane, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef]
- Caretto, S.; Nisi, R.; Paradiso, A.; De Gara, L. Tocopherol production in plant cell cultures. Mol. Nutr. Food Res. 2010, 54, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Yoshikawa, T.; Kimura, T.; Kaneko, H. Production of tocopherols by cell culture of safflower. Phytochemistry 1987, 26, 2741–2747. [Google Scholar] [CrossRef]
- Caretto, S.; Bray Speth, E.; Fachechi, C.; Gala, R.; Zacheo, G.; Giovinazzo, G. Enhancement of vitamin E production in sunflower cell cultures. Plant Cell Rep. 2004, 23, 174–179. [Google Scholar] [CrossRef]
- Caretto, S.; Paradiso, A.; D’Amico, L.; De Gara, L. Ascorbate and glutathione metabolism in two sunflower cell lines of differing α-tocopherol biosynthetic capability. Plant Physiol. Biochem. 2002, 40, 509–513. [Google Scholar] [CrossRef]
- Almagro, L.; Raquel Tudela, L.; Belén Sabater-Jara, A.; Miras-Moreno, B.; Pedreño, M.A. Cyclodextrins increase phytosterol and tocopherol levels in suspension cultured cells obtained from mung beans and safflower. Biotechnol. Prog. 2017, 33, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef] [PubMed]
- José Bagur, M.; Alonso Salinas, G.; Jiménez-Monreal, A.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2018, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, P.; Straubinger, M. Saffron—Renewed interest in an ancient spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Moradi, A.; Zarinkamar, F.; Caretto, S.; Azadi, P. Influence of thidiazuron on callus induction and crocin production in corm and style explants of Crocus sativus L. Acta Physiol. Plant 2018, 40, 185. [Google Scholar] [CrossRef]
- Malik, S.; Cusidó, R.M.; Mirjalili, M.H.; Moyano, E.; Palazón, J.; Bonfill, M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process Biochem. 2011, 46, 23–34. [Google Scholar] [CrossRef]
- Graham, I.A.; Besser, K.; Blumer, S.; Branigan, C.A.; Czechowski, T.; Elias, L.; Guterman, I.; Harvey, D.; Isaac, P.G.; Khan, A.M.; et al. The genetic map of Artemisia annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science 2010, 327, 328–331. [Google Scholar] [CrossRef]
- Townsend, T.; Segura, V.; Chigeza, G.; Penfield, T.; Rae, A.; Harvey, D.; Bowles, D.; Graham, I.A. The use of combining ability analysis to identify elite parents for Artemisia annua F1 hybrid production. PLoS ONE 2013, 8, e61989. [Google Scholar] [CrossRef]
- Tang, K.; Shen, Q.; Yan, T.; Fu, X. Transgenic approach to increase artemisinin content in Artemisia annua L. Plant Cell Rep. 2014, 33, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nowak, G.; Reed, D.W.; Covello, P.S. The production of artemisinin precursors in tobacco. Plant Biotechnol. J. 2011, 9, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef]
- Weathers, P.J.; Jordan, N.J.; Lasin, P.; Towler, M.J. Simulated digestion of dried leaves of Artemisia annua consumed as a treatment (pACT) for malaria. J. Ethnopharmacol. 2014, 151, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Caretto, S.; Quarta, A.; Durante, M.; Nisi, R.; De Paolis, A.; Blando, F.; Mita, G. Methyl jasmonate and miconazole differently affect arteminisin production and gene expression in Artemisia annua suspension cultures. Plant Biol. 2011, 13, 51–58. [Google Scholar] [CrossRef]
- Ahlawat, S.; Saxena, P.; Alam, P.; Wajid, S.; Abdin, M.Z. Modulation of artemisinin biosynthesis by elicitors, inhibitor, and precursor in hairy root cultures of Artemisia annua L. J. Plant Interact. 2014, 9, 811–824. [Google Scholar] [CrossRef]
- Zamboni, A.; Vrhovsek, U.; Kassemeyer, H.H.; Mattivi, F.; Velasco, R. Elicitor-induced resveratrol production in cell cultures of different grape genotypes (Vitis spp.). Vitis 2006, 45, 63–68. [Google Scholar]
- Durante, M.; Caretto, S.; Quarta, A.; De Paolis, A.; Nisi, R.; Mita, G. beta-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl. Microbiol. Biotechnol. 2011, 90, 1905–1913. [Google Scholar] [CrossRef]
- Chilton, M.D.; Tepfer, D.A.; Petit, A.; David, C.; Cassedelbart, F.; Tempe, J. Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant-root cells. Nature 1982, 295, 432–434. [Google Scholar] [CrossRef]
- Cardarelli, M.; Mariotti, D.; Pomponi, M.; Spano, L.; Capone, I.; Costantino, P. Agrobacterium rhizogenes T-DNA genes capable of inducing hairy root phenotype. Mol. Gen. Genet. 1987, 209, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zambryski, P.; Tempe, J.; Schell, J. Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell 1989, 56, 193–201. [Google Scholar] [CrossRef]
- Zambryski, P.; Joos, H.; Genetello, C.; Leemans, J.; Montagu, M.V.; Schell, J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983, 2, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Tepfer, D. Transformation of several species of higher plants by Agrobacterium rhizogenes: Sexual transmission of the transformed genotype and phenotype. Cell 1984, 37, 959–967. [Google Scholar] [CrossRef]
- White, F.F.; Taylor, B.H.; Huffman, G.A.; Gordon, M.P.; Nester, E.W. Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J. Bacteriol. 1985, 164, 33–44. [Google Scholar] [PubMed]
- Costantino, P.; Capone, I.; Cardarelli, M.; De Paolis, A.; Mauro, M.L.; Trovato, M. Bacterial plant oncogenes: The rol genes’ saga. Genetica 1994, 94, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Spano, L.; Mariotti, D.; Cardarelli, M.; Branca, C.; Costantino, P. Morphogenesis and auxin sensitivity of transgenic tobacco with different complements of Ri T-DNA. Plant Physiol. Biochem. 1988, 87, 479–483. [Google Scholar] [CrossRef]
- Capone, I.; Cardarelli, M.; Mariotti, D.; Pomponi, M.; De Paolis, A.; Costantino, P. Different promoter regions control level and tissue specificity of expression of Agrobacterium rhizogenes rolB gene in plants. Plant Mol. Biol. 1991, 16, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; Spanò, L.; Mariotti, D.; Mauro, M.L.; Van Sluys, M.A.; Costantino, P. The role of auxin in hairy root induction. Mol. Genet. Genom. 1987, 208, 457–463. [Google Scholar] [CrossRef]
- Spano, L.; Mariotti, D.; Pezzotti, M.; Damiani, F.; Arcioni, S. Hairy root transformation in alfalfa (Medicago sativa L.). Theor. Appl. Genet. 1987, 73, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Frugis, G.; Caretto, S.; Santini, L.; Mariotti, D. Agrobacterium rhizogenes rol genes induce productivity-related phenotypical modifications in “creeping-rooted” alfalfa types. Plant Cell Rep. 1995, 14, 488–492. [Google Scholar] [CrossRef]
- Rugini, E.; Mariotti, D. Agrobacterium rhizogenes T-DNA genes and rooting in woody species. Acta Hortic. 1992, 300, 301–308. [Google Scholar] [CrossRef]
- Rugini, E.; Pellegrineschi, A.; Mencuccini, M.; Mariotti, D. Increase of rooting ability in the woody species kiwi (Actinidia deliciosa A. Chev.) by transformation with Agrobacterium rhizogenes rol genes. Plant Cell Rep. 1991, 10, 291–295. [Google Scholar] [CrossRef]
- Rinallo, C.; Mariotti, D. Rooting of Castanea sativa Mill, shoots: Effect of Agrobacterium rhizogenes T-DNA genes. J. Hortic. Sci. 1993, 68, 399–407. [Google Scholar] [CrossRef]
- Mariotti, D.; Fontana, G.; Santini, L.; Costantino, P. Evaluation under field conditions of the morphological alterations (“hairy root phenotype”) induced on Nicotiana tabacum by different Ri plasmid T-DNA genes. J. Genet. Breed. 1989, 43, 157–164. [Google Scholar]
- Mauro, M.L.; Costantino, P.; Bettini, P.P. The never ending story of rol genes: A century after. Plant Cell Tissue Organ Cult. 2017, 131, 201–212. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Gorpenchenko, T.Y.; Veremeichik, G.N.; Shkryl, Y.N.; Tchernoded, G.K.; Bulgakov, D.V.; Aminin, D.L.; Zhuravlev, Y.N. The rolB gene suppresses reactive oxygen species in transformed plant cells through the sustained activation of antioxidant defense. Plant Physiol. 2012, 158, 1371–1381. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, W.; Zhou, X.; Huang, F.; Shao, L.; Luo, M. The putative Agrobacterium transcriptional activator-like virulence protein VirD5 may target T-complex to prevent the degradation of coat proteins in the plant cell nucleus. New Phytol. 2014, 203, 1266–1281. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Aminin, D.L.; Shkryl, Y.N.; Gorpenchenko, T.Y.; Veremeichik, G.N.; Dmitrenok, P.S.; Zhuravlev, Y.N. Suppression of reactive oxygen species and enhanced stress tolerance in Rubia cordifolia cells expressing the rolC oncogene. Mol. Plant Microbe Interact. 2008, 21, 1561–1570. [Google Scholar] [CrossRef]
- Trovato, M.; Maras, B.; Linhares, F.; Costantino, P. The plant oncogene rolD encodes a functional ornithine cyclodeaminase. Proc. Natl. Acad. Sci. USA 2001, 98, 13449–13453. [Google Scholar] [CrossRef]
- Levesque, H.; Delepelaire, P.; Rouze, P.; Slightom, J.; Tepfer, D. Common evolutionary origin of the central portions of the Ri TL-DNA of Agrobacterium rhizogenes and the Ti T-DNAs of Agrobacterium tumefaciens. Plant Mol. Biol. 1988, 11, 731–744. [Google Scholar] [CrossRef]
- Otten, L. The Agrobacterium phenotypic plasticity (Plast) genes. Curr. Top. Microbiol. Immunol. 2018. [Google Scholar]
- Mohajjel-Shoja, H.; Clement, B.; Perot, J.; Alioua, M.; Otten, L. Biological activity of the Agrobacterium rhizogenes-derived trolC gene of Nicotiana tabacum and its functional relation to other plast genes. Mol. Plant Microbe Interact. 2011, 24, 44–53. [Google Scholar] [CrossRef]
- Chen, K.; de Borne, F.D.; Sierro, N.; Ivanov, N.V.; Alouia, M.; Koechler, S.; Otten, L. Organization of the TC and TE cellular T-DNA regions in Nicotiana otophora and functional analysis of three diverged TE-6b genes. Plant J. 2018, 94, 274–287. [Google Scholar] [CrossRef]
- Matveeva, T.V. Agrobacterium-mediated transformation in the evolution of plants. In Agrobacterium Biology; Gelvin, S., Ed.; Springer: Cham, Switzerland, 2018; Volume 418, pp. 421–441. [Google Scholar]
- Doran, P.M. Biotechnology of hairy root systems. Adv. Biochem. Eng. Biotechnol. 2013, 134, V–Vi. [Google Scholar]
- Bulgakov, V.P. Functions of rol genes in plant secondary metabolism. Biotechnol. Adv. 2008, 26, 318–324. [Google Scholar] [CrossRef]
- Chandra, S. Natural plant genetic engineer Agrobacterium rhizogenes: Role of T-DNA in plant secondary metabolism. Biotechnol. Lett. 2012, 34, 407–415. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Dubrovina, A.S.; Veselova, M.V.; Bulgakov, V.P.; Fedoreyev, S.A.; Zhuravlev, Y.N. The rolB gene-induced overproduction of resveratrol in Vitis amurensis transformed cells. J. Biotechnol. 2007, 128, 681–692. [Google Scholar] [CrossRef]
- Arshad, W.; Haq, I.U.; Waheed, M.T.; Mysore, K.S.; Mirza, B. Agrobacterium-mediated transformation of tomato with rolB gene results in enhancement of fruit quality and foliar resistance against fungal pathogens. PLoS ONE 2014, 9, e96979. [Google Scholar] [CrossRef]
- Bettini, P.P.; Marvasi, M.; Fani, F.; Lazzara, L.; Cosi, E.; Melani, L.; Mauro, M.L. Agrobacterium rhizogenes rolB gene affects photosynthesis and chlorophyll content in transgenic tomato (Solanum lycopersicum L.) plants. J. Plant Physiol. 2016, 204, 27–35. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Veremeichik, G.N.; Grigorchuk, V.P.; Rybin, V.G.; Shkryl, Y.N. The rolB gene activates secondary metabolism in Arabidopsis calli via selective activation of genes encoding MYB and bHLH transcription factors. Plant Physiol. Biochem. PPB 2016, 102, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.G.; Fuchs, B.; Collier, R.; Lutke, W.K. Generation of composite plants using Agrobacterium rhizogenes. Methods Mol. Biol. 2006, 343, 155–167. [Google Scholar]
- Boisson-Dernier, A.; Chabaud, M.; Garcia, F.; Becard, G.; Rosenberg, C.; Barker, D.G. Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol. Plant Microbe Interact. 2001, 14, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ho-Plagaro, T.; Huertas, R.; Tamayo-Navarrete, M.I.; Ocampo, J.A.; Garcia-Garrido, J.M. An improved method for Agrobacterium rhizogenes-mediated transformation of tomato suitable for the study of arbuscular mycorrhizal symbiosis. Plant Methods 2018, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.; Santala, J.; Nielsen, S.L.; Huhns, M.; Broer, I.; Valkonen, J.P. Composite potato plants with transgenic roots on non-transgenic shoots: A model system for studying gene silencing in roots. Plant Cell Rep. 2014, 33, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Neb, D.; Das, A.; Hintelmann, A.; Nehls, U. Composite poplars: A novel tool for ectomycorrhizal research. Plant Cell Rep. 2017, 36, 1959–1970. [Google Scholar] [CrossRef]
- Pacifici, E.; Polverari, L.; Sabatini, S. Plant hormone cross-talk: The pivot of root growth. J. Exp. Bot. 2015, 66, 1113–1121. [Google Scholar] [CrossRef]
- Benfey, P.N.; Bennett, M.; Schiefelbein, J. Getting to the root of plant biology: Impact of the Arabidopsis genome sequence on root research. Plant J. 2010, 61, 992–1000. [Google Scholar] [CrossRef]
- Moriguchi, K.; Maeda, Y.; Satou, M.; Hardayani, N.S.; Kataoka, M.; Tanaka, N.; Yoshida, K. The complete nucleotide sequence of a plant root-inducing (Ri) plasmid indicates its chimeric structure and evolutionary relationship between tumor-inducing (Ti) and symbiotic (Sym) plasmids in Rhizobiaceae. J. Mol. Biol. 2001, 307, 771–784. [Google Scholar] [CrossRef]
- Suzuki, K.; Hattori, Y.; Uraji, M.; Ohta, N.; Iwata, K.; Murata, K.; Kato, A.; Yoshida, K. Complete nucleotide sequence of a plant tumor-inducing Ti plasmid. Gene 2000, 242, 331–336. [Google Scholar] [CrossRef]
- Rugini, E. Progress in studies on in vitro culture of Almonds. In Proceedings of the 41° Conference on Plant Tissue Culture and Its Agricultural Applications, Nothingam, UK, 17–21 September 1984; p. 73. [Google Scholar]
- Rugini, E.; Fedeli, E. Olive (Olea europaea L.) as an oilseed crop. In Legumes and Oilseed Crops I; Bajaj, Y.P.S., Ed.; Springer: New York, NY, USA, 1990; pp. 593–641. [Google Scholar]
- Rugini, E. Involvement of polyamines in auxin and Agrobacterium rhizogenes-induced rooting of fruit trees in vitro. Am. J. Hortic. Sci. 1992, 117, 532–536. [Google Scholar]
- Rugini, E. Piante da frutto transgeniche e considerazioni sulle conseguenze dei divieti impost alla ricerca in Italia. Italus Hortus 2015, 12, 79–92. [Google Scholar]
- Rugini, E.; Silvestri, C.; Cristofori, V.; Brunori, E.; Biasi, R. Ten years field trial observations of ri-TDNA cherry Colt rootstocks and their effect on grafted sweet cherry cv Lapins. Plant Cell Tissue Organ Cult. 2015, 123, 557–568. [Google Scholar] [CrossRef]
- Damiano, C.; Archilletti, T.; Caboni, E.; Lauri, P.; Falasca, G.; Mariotti, D.; Ferraiolo, G. Agrobacterium mediated transformation of almond: In vitro rooting through localized infection of A. rhizogenes w.t. Acta Hortic. 1995, 392, 161–170. [Google Scholar] [CrossRef]
- Rugini, E.; Gutierrez-Pesce, P. Transformation in Prunus species. In Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1999; Volume 44, pp. 245–262. [Google Scholar]
- Geier, T.; Eimert, K.; Scherer, R.; Nickel, C. Production and rooting behaviour of rolB-transgenic plants of grape rootstock ‘Richter 110’ (Vitis berlandieri × V. rupestris). Plant Cell Tissues Organ Cult. 2008, 94, 269–280. [Google Scholar] [CrossRef]
- Rugini, E. Trasformation of kiwi, cherry and papaya with rol genes. In Proceedings of the V Congress on University and Biotechnology Innovation, Brescia, IT, USA, 20–21 June 1994; University of Brescia: Brescia, IT, USA, 1994; pp. 68–69. [Google Scholar]
- La Malfa, S.; Distefano, G.; Domina, F.; Nicolosi, E.; Toscano, V.; Gentile, A. Evaluation of Citrus rootstock transgenic for rolABC gene. Acta Hortic. 2011, 892, 131–140. [Google Scholar] [CrossRef]
- Rugini, E.; Rita, B.; Muleo, R. Olive (Olea europaea var. sativa) transformation. In Molecular Biology of Woody Plants; Jain, S., Minocha, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; Volume 2, pp. 245–279. [Google Scholar]
- Rugini, E.; Gutierrez-Pesce, P.; Spampinato, P.L.; Ciarmiello, A.; D’Ambrosio, C. New perspective for biotechnologies in olive breeding: Morphogenesis, in vitro selection and gene transformation. Acta Hortic. 1999, 474, 107–110. [Google Scholar] [CrossRef]
- Rugini, E.; Cristofori, V.; Silvestri, C. Genetic improvement of olive (Olea europaea L.) by conventional and in vitro biotechnology methods. Biotechnol. Adv. 2016, 34, 687–696. [Google Scholar] [CrossRef]
- Zhu, L.; Holefors, A.; Ahlman, A.; Xue, Z.; Welander, M. Transformation of the apple rootstock M.9/29 with the rolB gene and its influence on rooting and growth. Plant Sci. 2001, 160, 433–439. [Google Scholar] [CrossRef]
- Smolka, A.; Li, X.Y.; Heikelt, C.; Welander, M.; Zhu, L.H. Effects of transgenic rootstocks on growth and development of non-transgenic scion cultivars in apple. Transgenic Res. 2010, 19, 933–948. [Google Scholar] [CrossRef]
- Zhu, L.-H.; Li, X.-Y.; Ahlman, A.; Welander, M. The rooting ability of the dwarfing pear rootstock BP10030 (Pyrus communis) was significantly increased by introduction of the rolB gene. Plant Sci. 2003, 165, 829–835. [Google Scholar] [CrossRef]
- Landi, L.; Capocasa, F.; Costantini, E.; Mezzetti, B. ROLC strawberry plant adaptability, productivity, and tolerance to soil-borne disease and mycorrhizal interactions. Transgenic Res. 2009, 18, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Silva, T.; Beyl, C.A.; Dortch, G. Agrobacterium rhizogenes mediated-transformation of Asimina triloba L. cuttings. Pak. J. Biol. Sci. 2007, 10, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.G.; Luza, J. Development anatomy of roots induced by Agrobacterium rhizogenes in Malus pumila ‘M.26’ shoots grown in vitro. Int. J. Plant Sci. 1993, 154, 59–67. [Google Scholar] [CrossRef]
- Gutierrez-Pesce, P.; Taylor, K.; Muleo, R.; Rugini, E. Somatic embryogenesis and shoot regeneration from transgenic roots of the cherry rootstock Colt (Prunus avium × P. pseudocerasus) mediated by pRi 1855 T-DNA of Agrobacterium rhizogenes. Plant Cell Rep. 1998, 17, 574–580. [Google Scholar] [CrossRef]
- Yazawa, M.; Suginuma, C.; Ichikawa, K.; Kamada, H.; Akihama, T. Regeneration of transgenic plants from hairy root of kiwi fruit (Actinidia deliciosa) induced by Agrobacterium rhizogenes. Jpn. J. Breed. 1995, 45, 241–244. [Google Scholar] [CrossRef]
- Balestra, G.M.; Rugini, E.; Varvaro, L. Increased susceptibility to Pseudomonas syringae pv. Syringae and Pseudomonas viridiflava of kiwi plants having transgenic rolABC genes and its inheritance in the T1 offspring. J. Phytopathol. 2001, 149, 189–194. [Google Scholar] [CrossRef]
- Rugini, E. Risultati preliminari sulla caratterizzazione morfo-fisiologica di cultivar di actinidia (Actinidia deliciosa A. Chev.) transgenica con geni rol per modificare l’architettura e la capacità rizogena della pianta. In Atti Giornate Scientifiche S.O.I; Istituto Sperimentale Frutticoltura: Rome, Italy, 1992; pp. 142–143. [Google Scholar]
- Druart, P.; Delporte, F.; Brazda, M.; Ugarte-Ballon, C.; da Câmara Machado, A.; Laimer da Câmara Machado, M.; Jacquemin, J.; Watillon, B. Genetic transformation of cherry trees. Acta Hortic. 1998, 468, 71–76. [Google Scholar] [CrossRef]
- Vahdati, K.; McKenna, J.R.; Dandekar, A.M.; Uratsu, S.L.; Hackett, W.P.; Negrei, P.; McGranahan, G.H. Rooting and other characteristics of a transgenic walnut hybrid (Juglans hindsii × J. regia) rootstock expressing rolABC. J. Am. Soc. Sci. 2002, 127, 724–728. [Google Scholar]
- Gentile, A.; Deng, Z.N.; La Malfa, S.; Domina, F.; Germanà, C.; Tribulato, E. Morphological and physiological effects of rolABC genes into Citrus genome. Acta Hortic. 2004, 632, 235–242. [Google Scholar] [CrossRef]
- Rugini, E. Risultati preliminari di una sperimentazione di campo di miglioramento genetico dell’Actinidia con tecniche biotecnologiche per tolleranza a stress idrico, funghi patogeni, e modifica dell’architettura della chioma. Kiwi Inf. 2012, 4–18. [Google Scholar]
- Welander, M.; Pawlicki, N.; Holefors, A.; Wilson, F. Genetic transformation of the apple rootstock M26 with the RolB gene and its influence on rooting. J. Plant Physiol. 1998, 153, 371–380. [Google Scholar] [CrossRef]
- Zhu, L.-H.; Welander, M. Growth characteristics of apple cultivar Gravenstein plants grafted onto the transformed rootstock M26 with rolA and rolB genes under non-limiting nutrient conditions. Plant Sci. 1999, 147, 75–80. [Google Scholar] [CrossRef]
- Firson, A.; Dolgov, S. Agrobacterium transformation of Actinidia kolomikta. Acta Hortic. 1997, 447, 323–327. [Google Scholar] [CrossRef]
- Mezzetti, B.; Costantini, E.; Chionchetti, F.; Landi, L.; Pandolfini, T.; Spena, A. Genetic transformation in strawberry and raspberry for improving plant productivity and fruit quality. Acta Hortic. 2004, 649, 107–110. [Google Scholar] [CrossRef]
- Bell, R.L.; Scorza, R.; Srinivasan, C.; Webb, K. Transformation of “Beurre Bosc” pear with the rolC gene. J. Am. Soc. Hortic. Sci. 1999, 124, 570–574. [Google Scholar]
- Kaneyoshi, J.; Kobayashi, S. Characteristics of transgenic trifoliate orange (Poncirus trifoliata Raf.) possessing the rolc gene of Agrobacterium rhizogenes Ri plasmid. Hortic. J. 1999, 68, 734–738. [Google Scholar] [CrossRef]
- Eapen, S. Advances in development of transgenic pulse crops. Biotechnol. Adv. 2008, 26, 162–168. [Google Scholar] [CrossRef]
- Fontana, G.S.; Santini, L.; Caretto, S.; Frugis, G.; Mariotti, D. Genetic transformation in the grain legume Cicer arietinum L. (chickpea). Plant Cell Rep. 1993, 12, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, D.; Fontana, G.S.; Santini, L. Genetic transformation of grain legumes: Phaseolus vulgaris L. and Phaseolus coccineus L. J. Genet. Breed. 1989, 43, 77–82. [Google Scholar]
- International Service for the Acquisition of Agri-Biotech Applications, I. Soybean (Glycine max L.) GM Events (40 Events). Available online: http://www.isaaa.org/gmapprovaldatabase/crop/default.asp?CropID=19&Crop=Soybean (accessed on 28 November 2018).
- International Service for the Acquisition of Agri-Biotech Applications, I. Alfalfa (Medicago sativa) GM Events (5 Events). Available online: http://www.isaaa.org/gmapprovaldatabase/crop/default.asp?CropID=1&Crop=Alfalfa (accessed on 28 November 2018).
- International Service for the Acquisition of Agri-biotech Applications, I. Bean (Phaseolus vulgaris) GM Events (1 Event). Available online: http://www.isaaa.org/gmapprovaldatabase/crop/default.asp?CropID=3&Crop=Bean (accessed on 28 November 2018).
- Food and Agriculture Organization of the United Nations, F. FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 28 November 2018).
- Atif, R.M.; Patat-Ochatt, E.M.; Svabova, L.; Ondrej, V.; Klenoticova, H.; Jacas, L.; Griga, M.; Ochatt, S.J. Gene transfer in legumes. In Progress in Botany; Luttge, U., Beyschlag, W., Francis, D., Cushman, J., Eds.; Springer: Berlin, Germany, 2013; Volume 74, pp. 37–100. [Google Scholar]
- Iantcheva, A.; Mysore, K.S.; Ratet, P. Transformation of leguminous plants to study symbiotic interactions. Int. J. Dev. Biol. 2013, 57, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Navarrete, G.; Alvarado-Affantranger, X.; Olivares, J.-E.; Guillén, G.; Díaz-Camino, C.; Campos, F.; Quinto, C.; Gresshoff, P.M.; Sanchez, F. Fast, efficient and reproducible genetic transformation of Phaseolus spp. by Agrobacterium rhizogenes. Nat. Protoc. 2007, 2, 1819. [Google Scholar] [CrossRef] [PubMed]
- Nova-Franco, B.; Íñiguez, L.P.; Valdés-López, O.; Alvarado-Affantranger, X.; Leija, A.; Fuentes, S.I.; Ramírez, M.; Paul, S.; Reyes, J.L.; Girard, L.; et al. The Micro-RNA172c-APETALA2-1 node as a key regulator of the common bean-Rhizobium etli nitrogen fixation symbiosis. Plant Physiol. 2015, 168, 273. [Google Scholar] [CrossRef] [PubMed]
- Moda-Crinò, V.; Nicolodi, C.; Chichriccò, G.; Mariotti, D. In vitro meristematic organogenesis and plant regeneration in bean (Phaseolus vulgaris L.) cultivars. J. Genet. Breed. 1995, 49, 133–138. [Google Scholar]
- Citadin, C.T.; Ibrahim, A.B.; Aragao, F.J. Genetic engineering in Cowpea (Vigna unguiculata): History, status and prospects. GM Crops 2011, 2, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Yusuf, M.A.; Nigam, M.; Kumar, M. An update on genetic modification of Chickpea for increased yield and stress tolerance. Mol. Biotechnol. 2018, 60, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Hnatuszko-Konka, K.; Kowalczyk, T.; Gerszberg, A.; Wiktorek-Smagur, A.; Kononowicz, A.K. Phaseolus vulgaris-recalcitrant potential. Biotechnol. Adv. 2014, 32, 1205–1215. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Hodgson, L.M.; Erskine, W.; Barker, S.J. An approach to overcoming regeneration recalcitrance in genetic transformation of lupins and other legumes. Plant Cell Tissues Organ Cult. 2016, 127, 623–635. [Google Scholar] [CrossRef]
- Das, A.; Parida, S.K. Advances in biotechnological applications in three important food legumes. Plant Biotechnol. Rep. 2014, 8, 83–99. [Google Scholar] [CrossRef]
- Indurker, S.; Misra, H.S.; Eapen, S. Genetic transformation of chickpea (Cicer arietinum L.) with insecticidal crystal protein gene using particle gun bombardment. Plant Cell Rep. 2007, 26, 755–763. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Kumar, S.; Mishra, S.; Kobayashi, Y.; Panda, S.K.; Sahoo, L. Enhanced salinity tolerance in transgenic mungbean overexpressing Arabidopsis antiporter (NHX1) gene. Mol. Breed. 2016, 36, 144. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, D.; Sarin, N.B. Multiple abiotic stress tolerance in Vigna mungo is altered by overexpression of ALDRXV4 gene via reactive carbonyl detoxification. Plant Mol. Biol. 2016, 91, 257–273. [Google Scholar] [CrossRef]
- Mishra, S.; Behura, R.; Awasthi, J.P.; Dey, M.; Sahoo, D.; Das Bhowmik, S.S.; Panda, S.K.; Sahoo, L. Ectopic overexpression of a mungbean vacuolar Na+/H+ antiporter gene (VrNHX1) leads to increased salinity stress tolerance in transgenic Vigna unguiculata L. Walp. Mol. Breed. 2014, 34, 1345–1359. [Google Scholar] [CrossRef]
- Citadin, C.T.; Cruz, A.R.; Aragao, F.J. Development of transgenic imazapyr-tolerant cowpea (Vigna unguiculata). Plant Cell Rep. 2013, 32, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Pigeaire, A.; Abernethy, D.; Smith, P.M.; Simpson, K.; Fletcher, N.; Lu, C.-Y.; Atkins, C.A.; Cornish, E. Transformation of a grain legume (Lupinus angustifolius L.) via Agrobacterium tumefaciens-mediated gene transfer to shoot apices. Mol. Breed. 1997, 3, 341–349. [Google Scholar] [CrossRef]
- Atkins, C.A.; Emery, R.J.; Smith, P.M. Consequences of transforming narrow leafed lupin (Lupinus angustifolius [L.]) with an ipt gene under control of a flower-specific promoter. Transgenic Res. 2011, 20, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.J.; Si, P.; Hodgson, L.; Ferguson-Hunt, M.; Khentry, Y.; Krishnamurthy, P.; Averis, S.; Mebus, K.; O’Lone, C.; Dalugoda, D.; et al. Regeneration selection improves transformation efficiency in narrow-leaf lupin. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 126, 219–228. [Google Scholar] [CrossRef]
- Wijayanto, T.; Barker, S.J.; Wylie, S.J.; Gilchrist, D.G.; Cowling, W.A. Significant reduction of fungal disease symptoms in transgenic lupin (Lupinus angustifolius) expressing the anti-apoptotic baculovirus gene p35. Plant Biotechnol. J. 2009, 7, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Tabe, L.; Wirtz, M.; Molvig, L.; Droux, M.; Hell, R. Overexpression of serine acetlytransferase produced large increases in O-acetylserine and free cysteine in developing seeds of a grain legume. J. Exp. Bot. 2010, 61, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Aragão, F.J.L.; Vianna, G.R.; Albino, M.M.C.; Rech, E.L. Transgenic dry bean tolerant to the herbicide glufosinate ammonium. Crop Sci. 2002, 42, 1298–1302. [Google Scholar] [CrossRef]
- Rech, E.L.; Vianna, G.R.; Aragao, F.J. High-efficiency transformation by biolistics of soybean, common bean and cotton transgenic plants. Nat. Protoc. 2008, 3, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Collado, R.; Bermúdez-Caraballoso, I.; García, L.R.; Veitía, N.; Torres, D.; Romero, C.; Angenon, G. Epicotyl sections as targets for plant regeneration and transient transformation of common bean using Agrobacterium tumefaciens. In Vitro Cell. Dev. Biol. Plant 2016, 52, 500–511. [Google Scholar] [CrossRef]
- Tiwari, S.; Mishra, D.K.; Singh, A.; Singh, P.K.; Tuli, R. Expression of a synthetic cry1EC gene for resistance against Spodoptera litura in transgenic peanut (Arachis hypogaea L.). Plant Cell Rep. 2008, 27, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Mishra, D.K.; Chandrasekhar, K.; Singh, P.K.; Tuli, R. Expression of delta-endotoxin Cry1EC from an inducible promoter confers insect protection in peanut (Arachis hypogaea L.) plants. Pest Manag. Sci. 2011, 67, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Deng, X.Y.; Faustinelli, P.; Ozias-Akins, P. Bcl-xL transformed peanut (Arachis hypogaea L.) exhibits paraquat tolerance. Plant Cell Rep. 2008, 27, 85–92. [Google Scholar] [CrossRef]
- Krishna, G.; Singh, B.K.; Kim, E.K.; Morya, V.K.; Ramteke, P.W. Progress in genetic engineering of peanut (Arachis hypogaea L.)—A review. Plant Biotechnol. J. 2015, 13, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Shethi, K.J.; Hoque, M.I.; Sarker, R.H. Agrobacterium-mediated genetic transformation in lentil (Lens culinaris Medik.) followed by in vitro flowering and seed formation. Plant Tissue Cult. Biotechnol. 2012, 22, 13–26. [Google Scholar] [CrossRef]
- O’Sullivan, D.M.; Angra, D. Advances in faba bean genetics and genomics. Front. Genet. 2016, 7, 150. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Burglin, T.R. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997, 25, 4173–4180. [Google Scholar] [CrossRef] [Green Version]
- Magnani, E.; Hake, S. KNOX lost the OX: The Arabidopsis KNATM gene defines a novel class of KNOX transcriptional regulators missing the homeodomain. Plant Cell 2008, 20, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Endrizzi, K.; Moussian, B.; Haecker, A.; Levin, J.Z.; Laux, T. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 1996, 10, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Chuck, G.; Lincoln, C.; Hake, S. KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell 1996, 8, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, E.; Veit, B.; Sinha, N.; Hake, S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 1991, 350, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, M.; Kusaba, S.; Kano-Murakami, Y.; Matsuoka, M. Ectopic expression of a tobacco homeobox gene, NTH15, dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol. 1997, 38, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Frugis, G.; Giannino, D.; Mele, G.; Nicolodi, C.; Innocenti, A.M.; Chiappetta, A.; Bitonti, M.B.; Dewitte, W.; Van Onckelen, H.; Mariotti, D. Are homeobox knotted-like genes and cytokinins the leaf architects? Plant Physiol. 1999, 119, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Frugis, G.; Giannino, D.; Mele, G.; Nicolodi, C.; Chiappetta, A.; Bitonti, M.B.; Innocenti, A.M.; Dewitte, W.; Van Onckelen, H.; Mariotti, D. Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot-like indeterminate growth associated with an accumulation of isopentenyl-type cytokinins. Plant Physiol. 2001, 126, 1370–1380. [Google Scholar] [CrossRef]
- Kakimoto, T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001, 42, 677–685. [Google Scholar] [CrossRef]
- Takei, K.; Sakakibara, H.; Sugiyama, T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 26405–26410. [Google Scholar] [CrossRef]
- Yanai, O.; Shani, E.; Dolezal, K.; Tarkowski, P.; Sablowski, R.; Sandberg, G.; Samach, A.; Ori, N. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr. Biol. 2005, 15, 1566–1571. [Google Scholar] [CrossRef]
- Jasinski, S.; Piazza, P.; Craft, J.; Hay, A.; Woolley, L.; Rieu, I.; Phillips, A.; Hedden, P.; Tsiantis, M. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 2005, 15, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Sakakibara, H.; Kojima, M.; Yamamoto, Y.; Nagasaki, H.; Inukai, Y.; Sato, Y.; Matsuoka, M. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol. 2006, 142, 54–62. [Google Scholar] [CrossRef]
- Shani, E.; Burko, Y.; Ben-Yaakov, L.; Berger, Y.; Amsellem, Z.; Goldshmidt, A.; Sharon, E.; Ori, N. Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell 2009, 21, 3078–3092. [Google Scholar] [CrossRef] [PubMed]
- Shani, E.; Ben-Gera, H.; Shleizer-Burko, S.; Burko, Y.; Weiss, D.; Ori, N. Cytokinin regulates compound leaf development in tomato. Plant Cell 2010, 22, 3206–3217. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Kamiya, N.; Ueguchi-Tanaka, M.; Iwahori, S.; Matsuoka, M. KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev. 2001, 15, 581–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka-Ueguchi, M.; Itoh, H.; Oyama, N.; Koshioka, M.; Matsuoka, M. Over-expression of a tobacco homeobox gene, NTH15, decreases the expression of a gibberellin biosynthetic gene encoding GA 20-oxidase. Plant J. 1998, 15, 391–400. [Google Scholar] [CrossRef]
- Bolduc, N.; Hake, S. The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell 2009, 21, 1647–1658. [Google Scholar] [CrossRef]
- Di Giacomo, E.; Iannelli, M.A.; Frugis, G. TALE and Shape: How to Make a Leaf Different. Plants 2013, 2, 317–342. [Google Scholar] [CrossRef]
- Bolduc, N.; Yilmaz, A.; Mejia-Guerra, M.K.; Morohashi, K.; O’Connor, D.; Grotewold, E.; Hake, S. Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 2012, 26, 1685–1690. [Google Scholar] [CrossRef] [Green Version]
- Scofield, S.; Murison, A.; Jones, A.; Fozard, J.; Aida, M.; Band, L.R.; Bennett, M.; Murray, J.A.H. Coordination of meristem and boundary functions by transcription factors in the SHOOT MERISTEMLESS regulatory network. Development 2018, 145, 157081. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. BRASSINOSTEROIDS: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, X.Y.; Cao, D.M.; Tang, W.; He, K.; Zhu, J.Y.; He, J.X.; Bai, M.Y.; Zhu, S.; Oh, E.; et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 2010, 19, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Kurata, N.; Ohyanagi, H.; Hake, S. Genome-wide study of KNOX regulatory network reveals brassinosteroid catabolic genes important for shoot meristem function in rice. Plant Cell 2014, 26, 3488–3500. [Google Scholar] [CrossRef] [PubMed]
- Gendron, J.M.; Liu, J.S.; Fan, M.; Bai, M.Y.; Wenkel, S.; Springer, P.S.; Barton, M.K.; Wang, Z.Y. Brassinosteroids regulate organ boundary formation in the shoot apical meristem of Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 21152–21157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-Garcia, S.; Cheng, J.C.; Nam, K.H.; Li, J.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, G.; Ori, N.; Sato, Y.; Hake, S. The knotted1-like homeobox gene BREVIPEDICELLUS regulates cell differentiation by modulating metabolic pathways. Genes Dev. 2003, 17, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Testone, G.; Condello, E.; Verde, I.; Nicolodi, C.; Caboni, E.; Dettori, M.T.; Vendramin, E.; Bruno, L.; Bitonti, M.B.; Mele, G.; et al. The peach (Prunus persica L. Batsch) genome harbours 10 KNOX genes, which are differentially expressed in stem development, and the class 1 KNOPE1 regulates elongation and lignification during primary growth. J. Exp. Bot. 2012, 63, 5417–5435. [Google Scholar] [CrossRef]
- Hake, S.; Smith, H.M.; Holtan, H.; Magnani, E.; Mele, G.; Ramirez, J. The role of knox genes in plant development. Annu. Rev. Cell Dev. Biol. 2004, 20, 125–151. [Google Scholar] [CrossRef]
- Kerstetter, R.; Vollbrecht, E.; Lowe, B.; Veit, B.; Yamaguchi, J.; Hake, S. Sequence analysis and expression patterns divide the maize knotted1-like homeobox genes into two classes. Plant Cell 1994, 6, 1877–1887. [Google Scholar] [CrossRef]
- Furumizu, C.; Alvarez, J.P.; Sakakibara, K.; Bowman, J.L. Antagonistic roles for KNOX1 and KNOX2 genes in patterning the land plant body plan following an ancient gene duplication. PLoS Genet. 2015, 11, e1004980. [Google Scholar] [CrossRef]
- Di Giacomo, E.; Sestili, F.; Iannelli, M.A.; Testone, G.; Mariotti, D.; Frugis, G. Characterization of KNOX genes in Medicago truncatula. Plant Mol. Biol. 2008, 67, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, E.; Laffont, C.; Sciarra, F.; Iannelli, M.A.; Frugier, F.; Frugis, G. KNAT3/4/5-like class 2 KNOX transcription factors are involved in Medicago truncatula symbiotic nodule organ development. New Phytol. 2017, 213, 822–837. [Google Scholar] [CrossRef]
- Poethig, R.S. Vegetative phase change and shoot maturation in plants. Curr. Top. Dev. Biol. 2013, 105, 125–152. [Google Scholar] [PubMed]
- Hanke, M.; Flachowsky, H.; Peil, A.; Hättasch, C. No Flower no Fruit—Genetic Potentials to Trigger Flowering in Fruit Trees. In Genes, Genomes and Genomics; Books, G.S., Ed.; Global Science Books Ltd.: Ikenobe, Japan, 2007; Volume 1, pp. 1–20. [Google Scholar]
- Layne, D.; Bassi, D. The Peach: Botany, Production and Uses; CABI: Oxfordshire, UK, 2008; pp. 1–615. [Google Scholar]
- Bitonti, M.B.; Cozza, R.; Chiappetta, A.; Giannino, D.; Ruffini Castiglione, M.; Dewitte, W.; Mariotti, D.; Van Onckelen, H.; Innocenti, A.M. Distinct nuclear organization, DNA methylation pattern and cytokinin distribution mark juvenile, juvenile-like and adult vegetative apical meristems in peach (Prunus persica (L.) Batsch). J. Exp. Bot. 2002, 53, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Giannino, D.; Mele, G.; Cozza, R.; Bruno, L.; Testone, G.; Ticconi, C.; Frugis, G.; Bitonti, M.B.; Innocenti, A.M.; Mariotti, D. Isolation and characterization of a maintenance DNA-methyltransferase gene from peach (Prunus persica [L.] Batsch): Transcript localization in vegetative and reproductive meristems of triple buds. J. Exp. Bot. 2003, 54, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Giannino, D.; Frugis, G.; Ticconi, C.; Florio, S.; Mele, G.; Santini, L.; Cozza, R.; Bitonti, M.B.; Innocenti, A.; Mariotti, D. Isolation and molecular characterisation of the gene encoding the cytoplasmic ribosomal protein S28 in Prunus persica [L.] Batsch. Mol. Gen. Genet. 2000, 263, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Van Nocker, S.; Gardiner, S.E. Breeding better cultivars, faster: Applications of new technologies for the rapid deployment of superior horticultural tree crops. Hortic. Res. 2014, 1, 14022. [Google Scholar] [CrossRef]
- Gordon, D.; Damiano, C.; DeJong, T.M. Preformation in vegetative buds of Prunus persica: Factors influencing number of leaf primordia in overwintering buds. Tree Physiol. 2006, 26, 537–544. [Google Scholar] [CrossRef]
- Reinoso, H.; Luna, V.; Pharis, R.; Bottini, R. Dormancy in peach (Prunus persica) flower buds. V. Anatomy of bud development in relation to phenological stage. Can. J. Bot. 2002, 80, 656–663. [Google Scholar] [CrossRef]
- Yamane, H. Regulation of bud dormancy and bud break in japanese apricot (Prunus mume Siebold & Zucc.) and peach [Prunus persica (L.) Batsch]: A summary of recent studies. Hortic. J. 2014, 83, 187–202. [Google Scholar]
- Hyun, Y.; Richter, R.; Coupland, G. Competence to flower: Age-controlled sensitivity to environmental cues. Plant Physiol. 2017, 173, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Wang, H.; Feng, S.; Zhang, Y. Involvement of miR156 in the regulation of vegetative phase change in plants. J. Am. Soc. Hortic. Sci. 2015, 140, 387–395. [Google Scholar]
- Wang, J.W.; Park, M.Y.; Wang, L.J.; Koo, Y.; Chen, X.Y.; Weigel, D.; Poethig, R.S. miRNA control of vegetative phase change in trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Hu, X.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. High miR156 expression is required for auxin-induced adventitious root formation via MxSPL26 independent of PINs and ARFs in Malus xiaojinensis. Front. Plant Sci. 2017, 8, 1059. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.L.; Chen, Y.K.; Xu, X.Z.; Shen, F.; Zheng, Q.B.; Du, Z.; Wang, Y.; Wu, T.; Xu, X.F.; Han, Z.H.; et al. miR156 switches on vegetative phase change under the regulation of redox signals in apple seedlings. Sci. Rep. 2017, 7, 14223. [Google Scholar] [CrossRef] [PubMed]
- Bastías, A.; Almada, R.; Rojas, P.; Donoso, J.M.; Hinrichsen, P.; Sagredo, B. Aging gene pathway of microRNAs 156/157 and 172 is altered in juvenile and adult plants from in vitro propagated Prunus sp. Cienc. Investig. Agrar. 2016, 43, 429–441. [Google Scholar] [CrossRef]
- Sgamma, T.; Cirilli, M.; Caboni, E.; Maurizio, M.; Thomas, B.; Muleo, R. In vitro plant culture system induces phase transition in fruit-bearing plants. Acta Hortic. 2016, 1110, 13–20. [Google Scholar] [CrossRef]
- Albani, M.C.; Coupland, G. Comparative analysis of flowering in annual and perennial plants. Curr. Top. Dev. Biol. 2010, 91, 323–348. [Google Scholar]
- Wells, C.E.; Vendramin, E.; Jimenez Tarodo, S.; Verde, I.; Bielenberg, D.G. A genome-wide analysis of MADS-box genes in peach [Prunus persica (L.) Batsch]. BMC Plant Biol. 2015, 15, 41. [Google Scholar] [CrossRef]
- Hong, Y.; Jackson, S. Floral induction and flower formation—The role and potential applications of miRNAs. Plant Biotechnol. J. 2015, 13, 282–292. [Google Scholar] [CrossRef]
- Li, S.; Shao, Z.; Fu, X.; Xiao, W.; Li, L.; Chen, M.; Sun, M.; Li, D.; Gao, D. Identification and characterization of Prunus persica miRNAs in response to UVB radiation in greenhouse through high-throughput sequencing. BMC Genom. 2017, 18, 938. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Yan, X.; Cheng, T.; Ma, K.; Yang, W.; Pan, H.; Zheng, C.; Zhu, X.; Wang, J.; et al. Genetic control of juvenile growth and botanical architecture in an ornamental woody plant, Prunus mume Sieb. et Zucc. as revealed by a high-density linkage map. BMC Genet. 2014, 15, S1. [Google Scholar] [CrossRef] [PubMed]
- Romeu, J.F.; Monforte, A.J.; Sanchez, G.; Granell, A.; Garcia-Brunton, J.; Badenes, M.L.; Rios, G. Quantitative trait loci affecting reproductive phenology in peach. BMC Plant Biol. 2014, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Bielenberg, D.G.; Zhebentyayeva, T.N.; Reighard, G.L.; Okie, W.R.; Holland, D.; Abbott, A.G. Mapping quantitative trait loci associated with chilling requirement, heat requirement and bloom date in peach (Prunus persica). New Phytol. 2010, 185, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Mora, J.R.; Micheletti, D.; Bink, M.; Van de Weg, E.; Cantin, C.; Nazzicari, N.; Caprera, A.; Dettori, M.T.; Micali, S.; Banchi, E.; et al. Integrated QTL detection for key breeding traits in multiple peach progenies. BMC Genom. 2017, 18, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbadini, S.; Pandolfini, T.; Girolomini, L.; Molesini, B.; Navacchi, O. Peach (Prunus persica L.). In Agrobacterium Protocols; Wang, K., Ed.; Springer: New York, NY, USA, 2015; Volume 2, pp. 205–215. [Google Scholar]
- Liu, H.; Qian, M.; Song, C.; Li, J.; Zhao, C.; Li, G.; Wang, A.; Han, M. Down-regulation of PpBGAL10 and PpBGAL16 delays fruit softening in peach by reducing polygalacturonase and pectin methylesterase activity. Front Plant Sci. 2018, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, A. An efficient viral vector for functional genomic studies of Prunus fruit trees and its induced resistance to Plum pox virus via silencing of a host factor gene. Plant Biotechnol. J. 2017, 15, 344–356. [Google Scholar] [CrossRef]
- Nagle, M.; Dejardin, A.; Pilate, G.; Strauss, S.H. Opportunities for innovation in genetic transformation of forest trees. Front. Plant Sci. 2018, 9, 1443. [Google Scholar] [CrossRef]
- Srinivasan, C.; Dardick, C.; Callahan, A.; Scorza, R. Plum (Prunus domestica) trees transformed with poplar FT1 result in altered architecture, dormancy requirement, and continuous flowering. PLoS ONE 2012, 7, e40715. [Google Scholar] [CrossRef]
- Petri, C.; Alburquerque, N.; Faize, M.; Scorza, R.; Dardick, C. Current achievements and future directions in genetic engineering of European plum (Prunus domestica L.). Transgenic Res. 2018, 27, 225–240. [Google Scholar] [CrossRef]
- Yamagishi, N.; Kishigami, R.; Yoshikawa, N. Reduced generation time of apple seedlings to within a year by means of a plant virus vector: A new plant-breeding technique with no transmission of genetic modification to the next generation. Plant Biotechnol. J. 2014, 12, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Viola, R.; Jung, M.H.; Koo, O.J.; Kim, S.; Kim, J.S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef] [PubMed]
- Us-Camas, R.Y.; Rivera-Solís, G.; Duarte-Aké, F.; De-la-Peña, C. In vitro culture: An epigenetic challenge for plants. Plant Cell Tissues Organ Cult. 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Yan, G.; Zhou, Y.; Zhang, K. Over-expression of the PaAP1 gene from sweet cherry (Prunus avium L.) causes early flowering in Arabidopsis thaliana. J. Plant Physiol. 2013, 170, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; An, L.; Nguyen, T.H.; Liang, H.; Wang, R.; Liu, X.; Li, T.; Qi, Y.; Yu, F. The Cloning and Functional Characterization of Peach CONSTANS and FLOWERING LOCUS T Homologous Genes PpCO and PpFT. PLoS ONE 2015, 10, e0124108. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.; Soto, E.; Leon, G.; Almeida, A.M. The sweet cherry (Prunus avium) FLOWERING LOCUS T gene is expressed during floral bud determination and can promote flowering in a winter-annual Arabidopsis accession. Plant Reprod. 2016, 29, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Xie, H.; Zhang, Y.-Q.; Oliveira, M.M.; Ma, R.-C. Expression analysis and genetic mapping of three SEPALLATA-like genes from peach (Prunus persica (L.) Batsch). Tree Genet. Genom. 2008, 4, 693–703. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. Isolation and functional characterization of SOC1-like genes in Prunus mume. J. Am. Soc. Hortic. Sci. 2016, 141, 315–326. [Google Scholar]
- Wisniewski, M.; Norelli, J.; Bassett, C.; Artlip, T.; Macarisin, D. Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (Malus × domestica) results in short-day induced dormancy and increased cold hardiness. Planta 2011, 233, 971–983. [Google Scholar] [CrossRef]
- Sasaki, R.; Yamane, H.; Ooka, T.; Jotatsu, H.; Kitamura, Y.; Akagi, T.; Tao, R. Functional and expressional analyses of PmDAM genes associated with endodormancy in Japanese apricot. Plant Physiol. 2011, 157, 485–497. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, P.; Thammannagowd, S.; Liang, H. Characterization of Peach TFL1 and comparison with FT/TFL1 gene families of the Rosaceae. J. Am. Soc. Hortic. Sci. 2013, 138, 12–17. [Google Scholar]


| Species | Gene(s) | Results | Ref. |
|---|---|---|---|
| Olive, Almond, Walnut, F12/I, MrS/5, Colt, apple | riT-DNA | Chimeric plants (better rooting) | [73,78,79,80] |
| Papaya (Carica papaya) | riT-DNA | Reduced growth habit | [81] |
| Colt rootstock (P. avium × P. pseudocerasus) | riT-DNA | Reduced growth habit | [79] |
| Kiwifruit (Actinidia deliciosa), cv Hayward | rolB | bigger fruits, drought tolerance | [43,44,81] |
| Kiwifruit (A. deliciosa), cv Hayward and GTH | rolABC | reduced plant size, flower set, increased drought tolerance | |
| Citrange Troyer (Citru sinensis × P. trifoliata) | rolABC | drought tolerance | [82] |
| Olive (Olea europaea L.) cv Canino | rolABC | Reduced growth habit, increased drought tolerance | [83,84,85] |
| Apple rootstock | rolA | Reduced growth habit | [86] |
| Apple rootstock | rolB | Reduced growth habit | [87] |
| Pear (P. communis L.) | rolB | Increased rooting ability | [88] |
| Strawberry (Fragraria × ananassa) | rolC | Higher fruit set and resistance to Phytophtora cactorum | [89] |
| Pear rootstock | rolB | Increased rooting ability | [88] |
| Richter 110 (Vitis berlandieri × V. rupestris) | rolB | better rooting | [80] |
| Gene 1 | Donor | Receiver | Assay 2 | Phenotypic Effect | Ref. |
|---|---|---|---|---|---|
| AP1 | Prunus avium | Arabidopsis thaliana | oe | early flowering | [210] |
| CO | Prunus persica | Arabidopsis thaliana | co | flowering promotion | [211] |
| FT | Prunus avium | Arabidopsis thaliana | oe | early flowering | [212] |
| Prunus persica | Arabidopsis thaliana | co | flowering promotion | [211] | |
| Populus trichocarpa | Prunus domestica | oe | early flowering | [205] | |
| MADS5 | Prunus persica | Arabidopsis thaliana | oe | early flowering | [213] |
| MADS7 | Prunus persica | Arabidopsis thaliana | oe | early flowering | [213] |
| SOC1 | Prunus mume | Arabidopsis thaliana | oe | early flowering | [214] |
| CBF | Prunus persica | Malus domestica | oe | cold-induced dormancy | [215] |
| DAM6 | Prunus mume | Populus tremula×P. tremuloides | oe | dormancy promotion | [216] |
| SVP1 | Prunus mume | Arabidopsis thaliana | oe | flowering delay | [189] |
| TFL1 | Prunus persica | Arabidopsis thaliana | oe | flowering delay | [217] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolis, A.D.; Frugis, G.; Giannino, D.; Iannelli, M.A.; Mele, G.; Rugini, E.; Silvestri, C.; Sparvoli, F.; Testone, G.; Mauro, M.L.; et al. Plant Cellular and Molecular Biotechnology: Following Mariotti’s Steps. Plants 2019, 8, 18. https://doi.org/10.3390/plants8010018
Paolis AD, Frugis G, Giannino D, Iannelli MA, Mele G, Rugini E, Silvestri C, Sparvoli F, Testone G, Mauro ML, et al. Plant Cellular and Molecular Biotechnology: Following Mariotti’s Steps. Plants. 2019; 8(1):18. https://doi.org/10.3390/plants8010018
Chicago/Turabian StylePaolis, Angelo De, Giovanna Frugis, Donato Giannino, Maria Adelaide Iannelli, Giovanni Mele, Eddo Rugini, Cristian Silvestri, Francesca Sparvoli, Giulio Testone, Maria Luisa Mauro, and et al. 2019. "Plant Cellular and Molecular Biotechnology: Following Mariotti’s Steps" Plants 8, no. 1: 18. https://doi.org/10.3390/plants8010018
APA StylePaolis, A. D., Frugis, G., Giannino, D., Iannelli, M. A., Mele, G., Rugini, E., Silvestri, C., Sparvoli, F., Testone, G., Mauro, M. L., Nicolodi, C., & Caretto, S. (2019). Plant Cellular and Molecular Biotechnology: Following Mariotti’s Steps. Plants, 8(1), 18. https://doi.org/10.3390/plants8010018








