Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Protocol
2.2. Antioxidants
2.2.1. Photosynthetic Pigments
Ch-b = 27.43A649 − 8.12A664;
C c = (1000A470 − 2.13Ch-a − 97.632C-b)/209
2.2.2. Ascorbic Acid
2.2.3. Flavonoids
2.3. Nitrates
2.4. Total Soluble Solids (TSS)
2.5. Element Composition
2.6. Statistical Analysis
3. Results and Discussion
3.1. Plant Growth and Yield
3.2. Antioxidants
3.2.1. Photosynthetic Pigments
3.2.2. Ascorbic Acid
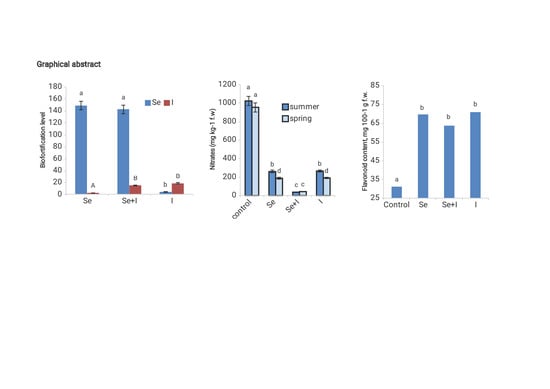
3.2.3. Flavonoids
3.3. Nitrate Accumulation
3.4. Total Soluble Solids (TSS)
3.5. Biofortification Levels
3.6. Element Composition
3.6.1. Macroelements
3.6.2. Trace Elements
3.6.3. Heavy Metals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drutel, A.; Archambeand, F.; Caron, P. Selenium and the thyroid gland: More good news for clinicians. Clin. Endocrinol. 2013, 78, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Macias, J.; Leija-Martinez, P.; Gonzalez-Orales, S.; Juarez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, A.H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in higher plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Çakır, Ö.; Turgut-Kara, N.; Arı, S. Selenium metabolism in plants: Molecular approaches. Advances in Selected Plant Physiology Aspects. Montanaro, G., Ed.; 2012. Available online: http://www.intechopen.com/books/advances-in-selected-plant-physiologyaspects/selenium-metabolism-in-plants-molecular-approaches (accessed on 25 April 2012).
- Golubkina, N.A.; Papazyan, T.T. Selenium in Nutrition. Plants, Animals, Human Beings; Pechatny Gorod: Мoscow, Russia, 2006. (In Russian) [Google Scholar]
- Zimmermann, M.B.; Köhrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium in higher plants: Physiological role, antioxidant metabolism and abiotic stress tolerance. J. Plant Sci. 2010, 5, 354–375. [Google Scholar] [CrossRef]
- Smolen, S.; Sady, W. Influence of soil application of iodine and sucrose on mineral composition of spinach plants. Acta Sci. Pol. Hortorum Cultus 2011, 10, 3–13. [Google Scholar]
- Shekari, L.; Kamelmanesh, M.M.; Mozafariyan, M.; Hasanuzzaman, M.; Sadeghi, F. Role of selenium in mitigation of cadmium toxicity in pepper grown in hydroponic condition. J. Plant Nutr. 2017, 40, 761–772. [Google Scholar] [CrossRef]
- Van Huysen, T.; Abdel-Ghany, S.; Hale, K.L.; La Duc, D.; Terry, N.; Pilon-Smits, E.A.H. Overexpression of cystathionine-γ-synthase enhances selenium volatilization in Brassica juncea. Planta 2003, 218, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Koronowicz, A.; Kapusta-Duch, J. Biofortification of carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front. Plant Sci. 2016, 7, 730. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Kacjan Maršić, N.; Turk, J.; Pirc, M.; Golob, A.; Jerše, A.; Kroflič, A.; Šircelj, H.; Stibilj, V. The effect of different compounds of selenium and iodine on selected biochemical and physiological characteristics in common buckwheat and pumpkin sprouts. Acta Biol. Slovenica (Ljubljana) 2015, 58, 35–44. [Google Scholar]
- Germ, M.; Luthar, Z.; Benkovic, E.T.; Zhou, M.; Golob, A.; Marsic, N.K. Fagopyrin and rutin concentration in seeds of common buckwheat plants treated with Se and I. Folia Biol. Geol. 2017, 58, 45–51. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Huang, Y.; Hu, Y.; Liu, Y.; Christie, P. Interactions between selenium and iodine uptake by spinach (Spinacia oleracea L.) in solution culture. Plant Soil 2004, 261, 99–105. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium and iodine concentrations on food crops grown on loess plateau in China. J. Soil Sci. Plant Nutr. 2014, 14, 459–470. [Google Scholar] [CrossRef]
- De Souza, M.P.; Pilon-Smits, E.A.H.; Lytle, C.M.; Hwang, S.; Tai, J.; Honma, T.S.U.; Yeh, L.; Terry, N. Rate-limiting steps in selenium assimilation and volatilization by Indian Mustard. Plant Physiol. 1998, 117, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Thakur, A.K.; Barothia, N.D.; Chatterjee, S.S. Therapeutic potentials of Brassica juncea: An overview. TANG Int. J. Gen. Trad. Med. 2011, 1. Available online: http://www.koreascience.or.kr/article/ArticleFullRecord.jsp?cn=TJHOBI_2011_v1n1_2.1 (accessed on 26 September 2018). [CrossRef]
- Lawson, P. Development and Evaluation of Iodine Biofortification Strategies for Vegetables; Logos Verlag Berlin GmbH: Berlin, Germany, 2014. [Google Scholar]
- Dere, Ş.; Gunes, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Botany 1998, 22, 13–17. [Google Scholar]
- Association Official Analytical Chemists. The Official Methods of Analysis of AOAC International; 22 Vitamin C; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Da Silva, L.A.L.; Pezzini, B.R.; Soares, L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L. (Lamiaceae) leaves. Pharmacogn. Mag. 2015, 11, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Lakarova, H.V.; Kuznetsov, V.V.; Skalnaya, M.G. Analytical Methods in Bioelementology; Saint Petersburg-Science: Saint Petersburg, Russia, 2009. (In Russian) [Google Scholar]
- Malagoli, M.; Schiavon, M.; dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant. Sci. 2015, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Aksu, G.; Temel, E.; Altay, H. Comparison of iodine application methods in rocket plant. Agron. Res. 2017, 15, 5–10. [Google Scholar]
- Krzepiłko, A.; Zych-Wężyk, I.; Święciło, A.; Molas, J.; Skwaryło-Bednarz, B. Effect of iodine biofortification of lettuce seedlings on their mineral composition and biological quality. J. Elem. 2016, 21, 1071–1080. [Google Scholar] [CrossRef]
- Schiavon, M.; dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Caruso, G.; Conti, S.; La Rocca, G. Influence of crop cycle and nitrogen fertilizer form on yield and nitrate content in different species of vegetables. Adv. Hort. Sci. 2011, 25, 81–89. [Google Scholar]
- Rios, J.J.; Blasco, B.; Rosales, M.A.; Sanchez-Rodriguez, E.; Leyva, R.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Response of nitrogen metabolism in lettuce plants subjected to different doses and forms of selenium. J. Sci. Food Agric. 2010, 90, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Munshi, C.B.; Mindy, N.I. Glycoalkaloid and nitrate content of potatoes as affected by method of selenium application. Biol. Trace Elem. Res. 1992, 33, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M.; Rivero, R.M.; Romero, L. Comparative effect of Al, Se and Mo toxicity on NO3− assimilation in sunflower (Helianthus annuus L.) plants. J. Environ. Manag. 2007, 83, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Sadeghzade, N. Effect of selenium on CO2 and NO3− assimilation under low and adequate nitrogen supply in wheat (Triticum aestivum L.). Photosynthetica 2014, 52, 501–510. [Google Scholar] [CrossRef]
- Golubkina, N.A. Selenium biorhythms and hormonal regulation. In Selenium. Sources, Functions and Health Effects; Aomori, C., Hokkaido, M., Eds.; Novo Science Publishers: Hauppauge, NY, USA, 2012; pp. 33–75. [Google Scholar]
- Strzetelski, P.; Smoleń, S.; Rożek, S.; Sady, W. The effect of diverse iodine fertilization on nitrate accumulation and content of selected compounds in radish plants (Raphanus sativus L.). Acta Sci. Pol. Hortorum Cultus 2010, 9, 65–73. [Google Scholar]
- Smolen, S.; Sady, W. Influence of iodine form and application method on the effectiveness of iodine biofortification, nitrogen metabolism as well as the content of mineral nutrients and heavy metals in spinach plants (Spinacia oleracea L.). Sci. Hortic. 2012, 143, 176–183. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Review. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Kowalska, I.; Czernicka, M.; Halka, M.; Kęska, K.; Sady, W. Iodine and selenium biofortification with additional application of salicylic acid affects yield, selected molecular parameters and chemical composition of lettuce plants (Lactuca sativa L. var. capitata). Front. Plant Sci. 2016, 7, 1553. [Google Scholar] [CrossRef] [PubMed]
- Amagova, Z.A.; Matsadze, V.H.; Golubkina, N.A.; Seredin, T.M.; Caruso, G. Fortification of wild garlic with selenium. Veg. Crops Russ. 2018, 4, 76–80. (In Russian) [Google Scholar] [CrossRef]
- Golubkina, N.A.; Seredin, T.M.; Koshevarov, A.A.; Shilo, L.M.; Baranova, H.V.; Pavlov, L.V. Garlic powder enriched with selenium. Trace Elem. Med. 2018, 19, 43–50. (In Russian) [Google Scholar]
- Golubkina, N.A.; Kosheleva, O.V.; Krivenkov, L.V.; Dobrutskaya, H.G.; Nadezhkin, S.М.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358. [Google Scholar] [CrossRef]
- Landini, M.; Gonzali, S.; Kiferle, C.; Tonacchera, M.; Agretti, P.; Dimida, A.; Vitti, P.; Alpi, A.; Pinchera, A.; Perata, P. Metabolic engineering of the iodine content in Arabidopsis. Sci. Rep. 2012, 2, 338. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.; Ford, R.; Norton, R.M.; Nicolas, M.E. Sodium and boron exclusion in two Brassica juncea cultivars exposed to the combined treatments of salinity and boron at moderate alkalinity. Biologia 2014, 69, 1157–1163. [Google Scholar] [CrossRef]
- Shekhawat, K.; Rathore, S.S.; Premi, O.P.; Kandpal, B.K.; Chauhan, J.S. Advances in agronomic management of Indian mustard (Brassica juncea L.) Czernj. Cosson: An Overview. Int. J. Agron. 2012, 408284. [Google Scholar] [CrossRef]
- Smoleń, S.; Sady, W.; Wierzbińska, J. The effect of KI and KIO3 fertilization on iodine uptake efficiency and content of mineral elements in leaves and fruits of tomato cultivated in hydroponics (NFT system). Ochr Śr Zasobów Nat. 2011, 48, 31–39. (In Polish) [Google Scholar]
- Jagtap, M.N.; Kulkarniand, M.V.; Puranik, P.R. Phytoremediation of metal contaminated soils with special reference to Brassica juncea (L.) czern, Macrotyloma uniflorum lam verdc. (Dolichos biflorus) and Medicago sativa L. Trends Biotechnol. Res. 2013, 2, 7–19. [Google Scholar]


| Parameter | Control | Se | Se + I | I | |
|---|---|---|---|---|---|
| Spring | |||||
| Aerial biomass yield (g·plant−1) | 33.4 ± 3.8 b | 51.5 ± 4.7 a | 30.6 ± 5.0 b | 45.8 ± 5.6 a | |
| Ratio of leaves to the aerial biomass (%) | 55.8 | 57.5 | 48.2 | 51.1 | |
| Root biomass (g·plant−1) | 1.48 ± 0.47 ab | 2.29 ± 0.48 a | 1.31 ± 0.40 b | 2.25 ± 0.37 a | |
| Plant height (cm) | 54.9 ± 8.4 b | 61.3 ± 10.0 ab | 71.9 ± 7.2 a | 60.9 ± 7.1 ab | |
| Dry matter (%) | Leaves | 8.2 ± 0.3 b | 9.2 ± 0.2 a | 8.3 ± 0.1 b | 9.1 ± 0.1 a |
| Stems | 4.5 ± 0.1 c | 4.8 ± 0.1 b | 5.0 ± 0.1 ab | 5.2 ± 0.1 a | |
| Summer | |||||
| Aerial biomass yield (g·plant−1) | 30.5 ± 3.5 b | 45.0 ± 4.7 a | 25.5 ± 4.0 b | 40.2 ± 4.4 ab | |
| Ratio of leaves to the aerial biomass (%) | 56.1 | 57.0 | 49.2 | 50.1 | |
| Root biomass (g·plant−1) | 1.40 ± 0.41 bc | 2.40 ± 0.54 a | 1.20 ± 0.40 c | 2.20 ± 0.55 ab | |
| Plant height (cm) | 55.0 ± 8.2 b | 60.4 ± 11.0 ab | 72.2 ± 8.1 a | 57.5 ± 6.7 ab | |
| Dry matter (%) | Leaves | 9.1 ± 0.1 a | 8.2 ± 0.1 b | 9.2 [g23]± 0.2 a | 9.1 ± 0.1 a |
| Stems | 4.8 ± 0.1 b | 5.1 ± 0.1 a | 5.1 ± 0.1 a | 5.2 ± 0.1 a | |
| Parameter | Control | Se | Se + I | I |
|---|---|---|---|---|
| Chlorophyll a (mg·100 g−1 f. w.) | 6.97 ± 0.6 a | 7.30 ± 0.7 a | 5.73 ± 0.5 b | 8.22 ± 0.7 a |
| Chlorophyll b (mg·100 g−1 f. w.) | 2.92 ± 0.3 b | 3.98 ± 0.4 a | 2.65 ± 0.3 b | 2.92 ± 0.3 b |
| Carotene (mg·100 g−1 f. w.) | 1.24 ± 0.1 ab | 1.38 ± 0.1 a | 1.03 ± 0.1 c | 1.13 ± 0.1 bc |
| Leaf ascorbic acid (mg·100 g−1 f. w.) | 88 ± 2 b | 97 ± 2 a | 92 ± 3 ab | 93 ± 3 ab |
| Stem ascorbic acid (mg·100 g−1 f. w.) | 26 ± 1 b | 32 ± 1 a | 24 ± 1 b | 31 ± 1 a |
| Flavonoids (mg·100 g−1 f. w.) | 31.2 ± 2.5 b | 69.7 ± 7.3 a | 63.6 ± 7.1 a | 70.9 ± 5.1 a |
| Parameter | Control | Se | Se + I | I |
|---|---|---|---|---|
| Se content (µg·kg−1 d.w.) | 60 ± 6 c | 8922 ± 870 a | 8577 ± 624 a | 258 ± 21 b |
| Se biofortification level | - | 149 | 143 | 4.3 |
| I content (µg·kg−1 d.w.) | 183 ± 18 c | 483 ± 48 b | 2790 ± 279 a | 3412 ± 341 a |
| I biofortification level | - | 2.64 | 15.2 | 18.6 |
| Element | Control | Se | Se + I | I |
|---|---|---|---|---|
| Ca | 21.0 ± 2.1 a | 16.7 ± 1.7 b | 14.5 ± 1.5 b | 17.4 ± 1.7 ab |
| K | 43.3 ± 4.3 a | 37.6 ± 3.8 ab | 30.5 ± 3.0 b | 34.0 ± 3.4 b |
| Mg | 2.82 ± 0.28 a | 2.54 ± 0.25 a | 2.55 ± 0.25 a | 3.04 ± 0.30 a |
| Na | 0.72 ± 0.07 b | 0.80 ± 0.08 b | 2.21 ± 0.22 a | 1.86 ± 0.19 a |
| P | 6.22 ± 0.62 a | 6.33 ± 0.63 a | 7.35 ± 0.74 a | 6.04 ± 0.60 a |
| K:Na | 60 | 47 | 14 | 18 |
| Element | Control | Se | Se + I | I |
|---|---|---|---|---|
| B | 16.29 ± 1.63 c | 20.34 ± 2.03 b | 25.70 ± 2.57 a | 21.20 ± 2.12 ab |
| Co | 0.13 ± 0.02 b | 0.18 ± 0.02 a | 0.16 ± 0.02 ab | 0.16 ± 0.02 ab |
| Fe | 182 ± 18 a | 199 ± 20 a | 181 ± 18 a | 206 ± 21 a |
| Li | 0.16 ± 0.02 bc | 0.18 ± 0.02 b | 0.24 ± 0.03 a | 0.12 ± 0.03 c |
| Mn | 23.96 ± 2.40 c | 29.30 ± 2.93 bc | 33.25 ± 3.32 b | 48.29 ± 4.83 a |
| Si | 170 ± 17 a | 149 ± 15 a | 167 ± 17 a | 140 ± 14 a |
| Zn | 36.0 ± 3.6 a | 34.2 ± 3.4 a | 39.6 ± 4.0 a | 40.2 ± 4.0 a |
| Element | Control | Se | Se + I | I |
|---|---|---|---|---|
| Al | 87 ± 9 b | 125 ± 13 a | 117 ± 12 a | 123 ± 12 a |
| As | 0.10 ± 0.02 a | 0.14 ± 0.02 a | 0.13 ± 0.02 a | 0.13 ± 0.02 a |
| Cd | 0.32 ± 0.04 a | 0.15 ± 0.02 c | 0.17 ± 0.02 bc | 0.20 ± 0.02 b |
| Cr | 0.64 ± 0.08 a | 0.70 ± 0.08 a | 0.60 ± 0.07 a | 0.68 ± 0.08 a |
| Cu | 8.45 ± 0.85 a | 7.61 ± 0.76 a | 8.55 ± 0.85 a | 8.71 ± 0.87 a |
| Ni | 1.69 ± 0.17 a | 1.43 ± 0.14 a | 1.59 ± 0.16 a | 1.58 ± 0.16 a |
| Pb | 0.82 ± 0.10 a | 1.03 ± 0.10 a | 0.94 ± 0.11 a | 0.93 ± 0.11 a |
| Sr | 96.6 ± 9.7 a | 64.2 ± 6.4 b | 57.0 ± 5.7 b | 68.1 ± 6.8 b |
| V | 0.26 ± 0.03 c | 0.35 ± 0.04 a | 0.31 ± 0.04 ac | 0.34 ± 0.04 ab |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Kekina, H.; Caruso, G. Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine. Plants 2018, 7, 80. https://doi.org/10.3390/plants7040080
Golubkina N, Kekina H, Caruso G. Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine. Plants. 2018; 7(4):80. https://doi.org/10.3390/plants7040080
Chicago/Turabian StyleGolubkina, Nadezhda, Helene Kekina, and Gianluca Caruso. 2018. "Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine" Plants 7, no. 4: 80. https://doi.org/10.3390/plants7040080