Phytotoxicity of Essential Oils on Selected Weeds: Potential Hazard on Food Crops
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Winter Savory, Peppermint, and Anise Essential Oils
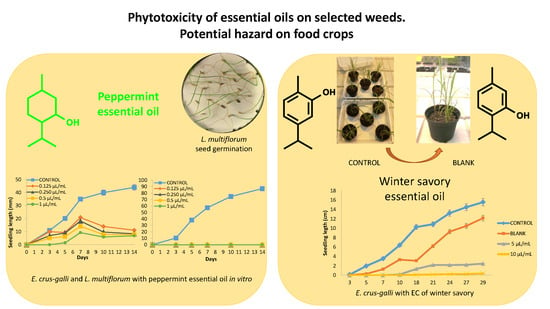
2.2. Seed Germination and Seedling Growth Inhibition of P. oleracea, L. multiflorum, and E. crus-galli, and Maize, Rice, and Tomato with Essential Oils
2.3. Seed Germination and Seedling Growth Inhibition of P. oleracea, L. multiflorum, and E. crus-galli, and Maize, Rice, and Tomato with an Emulsifiable Concentrate Including Winter Savory or Peppermint Essential Oils
3. Discussion
4. Materials and Methods
4.1. Essential Oil
4.2. Seeds
4.3. Gas Chromatography- Mass Spectrometry
4.4. Identification
4.5. In Vitro Assays: P. oleracea, L. multiflorum, E. crus-galli, Maize, Rice, and Tomato Seed Germination and Seedling Growth with Essential Oils
4.6. In Vivo Assays: P. oleracea, L. multiflorum, E. crus-galli, Maize, Rice, and Tomato with an Emulsifiable Concentrate of Winter Savory or Peppermint
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.; Lorraine-Colwill, D.; Dellow, J.; Preston, C. Evolved resistance to glyphosate in rigid ryegrass (Lolium rigidum) in Australia. Weed Sci. 1998, 46, 604–607. [Google Scholar]
- Bagavathiannan, M.V.; Norsworthy, J.K.; Smith, K.L.; Neve, P. Modeling the evolution of glyphosate resistance in barnyardgrass (Echinochloa crus-galli) in cotton-based production systems of the midsouthern United States. Weed Technol. 2013, 27, 475–487. [Google Scholar] [CrossRef]
- Shaner, D.L.; Lindenmeyer, R.B.; Ostlie, M.H. What have the mechanisms of resistance to glyphosate taught us? Pest Manag. Sci. 2012, 68, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nandula, V.K.; Tehranchian, P.; Bond, J.A.; Norsworthy, J.K.; Eubank, T.W. Glyphosate resistance in common ragweed (Ambrosia artemisiifolia L.) from Mississippi, USA. Weed Biol. Manag. 2017, 17, 45–53. [Google Scholar] [CrossRef]
- Mariager, T.P.; Madsen, P.V.; Ebbehoj, N.E.; Schmidt, B.; Juhl, A. Severe adverse effects related to dermal exposure to a glyphosate-surfactant herbicide. Clin. Toxicol. 2013, 51, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef] [PubMed]
- Dumas, E.; Giraudo, M.; Goujon, E.; Halma, M.; Knhili, E.; Stauffert, M.; Batisson, I.; Besse-Hoggan, P.; Bohatier, J.; Bouchard, P.; et al. Fate and ecotoxicological impact of new generation herbicides from the triketone family: An overview to assess the environmental risks. Hazard. Mater. 2017, 325, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Collavo, A.; Sattin, M. First glyphosate-resistant Lolium spp. biotypes found in a European annual arable cropping system also affected by ACCase and ALS resistance. Weed Res. 2014, 54, 325–334. [Google Scholar] [CrossRef]
- Masabni, J.G.; Zandstra, B.H.; Yerkes, C.N.; Weller, S.C. Linuron resistance in Portulaca oleracea. In Proceedings of the Second International Weed Control Congress, Copenhagen, Denmark, 25–28 June 1996; pp. 571–575. [Google Scholar]
- International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org/Summary/MOA.aspx?MOAID=12 (accessed on 23 April 2017).
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricci, D.; Epifano, F.; Genovese, S.; Curini, M. Chemical composition and antifungal activity of the essential oil of Satureja montana from central Italy. Chem. Nat. Comp. 2007, 43, 622–624. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Hadian, J. Allelopathic potential of essential oils from four Satureja spp. Biol. Agric. Hortic. 2013, 29, 244–257. [Google Scholar] [CrossRef]
- Yadegarinia, D.; Gachkar, L.; Bagher Rezaei, M.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Guerra, I.C.D.; de Oliveira, P.D.L.; Santos, M.M.F.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souza, E.L. The effects of composite coatings containing chitosan and Mentha (piperita L. or x villosa Huds) essential oil on postharvest mold occurrence and quality of table grape cv. Isabella. Innov. Food Sci. Emerg. Technol. 2016, 34, 112–121. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Mahdavikia, F.; Saharkhiz, J.M. Phytotoxic activity of essential oil and water extract of peppermint (Mentha x piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Tavalli, V.; Rahmati, S.; Bahmanzadegan, A. Antioxidant activity, polyphenolic contents and essential oil composition of Pimpinella anisum L. as affected by zinc fertilizer. J. Sci. Food Agric. 2017, 97, 4883–4889. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; Catalan, C.A.N. Chemical composition and antioxidant potential of essential oil and oleoresins from anise seeds (Pimpinella anisum L.). Int. J. Essent. Oil Ther. 2008, 2, 122–130. [Google Scholar]
- Evrendilek, G.A. Empirical prediction and validation of antibacterial inhibitory effects of various plant essential oils on common pathogenic bacteria. Int. J. Food Microbiol. 2015, 202, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Tiptiri-Koupeti, A.; Vamvakias, M.; Bardouki, H.; Panayiotidis, M.I.; Galanis, A.; Kourkoutas, Y.; Chlichlia, K.; et al. Phytochemical profile and evaluation of the biological activities of essential oils derived from the Greek aromatic plant species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 2016, 21, 1069. [Google Scholar] [CrossRef] [PubMed]
- Starovic, M.; Ristic, D.; Pavlovic, S.; Ristic, M.; Stevanovic, M.; AlJuhaimi, F.; Svetlana, N.; Özcan, M.M.J. Antifungal activities of different essential oils against anise seeds mycopopulations. Food Saf. Food Qual. 2016, 67, 61–92. [Google Scholar]
- Kubo, I.; Fujita, K.; Nihei, K. Antimicrobial activity of anethole and related compounds from aniseed. J. Sci. Food Agric. 2008, 88, 242–247. [Google Scholar] [CrossRef]
- Skuhrovec, J.; Douda, O.; Pavela, R.; Klouček, P.; Bozik, M.; Zouhar, M. The effects of Pimpinella anisum essential oils on young larvae Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Am. J. Potato Res. 2017, 94, 64–69. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed]
- Dhima, K.; Vasilakoglou, I.; Garane, V.; Ritzoulis, C.; Lianopoulou, V.; Panou-Philotheou, E. Competitiveness and essential oil phytotoxicity of seven annual aromatic plants. Weed Sci. 2010, 58, 457–465. [Google Scholar] [CrossRef]
- Young, D.G. Composition and Method for Treating an Herbicide. U.S. Patent 20170056941 A1, 2 March 2017. [Google Scholar]
- Fernández-Moreno, P.T.; Bastida, F.; De Prado, R. Evidence, mechanism and alternative chemical seedbank-level control of glyphosate resistance of a rigid ryegrass (Lolium rigidum) biotype from Southern Spain. Front. Plant Sci. 2017, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.; Ruchel, Q.; Agostinetto, D.; Lamego, F.P.; Langaro, A.C.; Piesanti, S.R. Verification of the mechanism of glyphosate resistance in Italian ryegrass biotype. Planta Daninha 2016, 34, 565–573. [Google Scholar] [CrossRef]
- Yanniccari, M.; Vila-Aiub, M.; Istilart, C.; Acciaresi, H.; Castro, A.M. Glyphosate resistance in perennial ryegrass (Lolium perenne L.) is associate with a fitness penalty. Weed Sci. 2016, 64, 71–79. [Google Scholar] [CrossRef]
- Trifan, A.; Aprotosoaie, A.C.; Brebu, M.; Cioanca, O.; Gille, E.; Hancianu, M.; Miron, A. Chemical composition and antioxidant activity of essential oil from Romanian Satureja montana L. Farmacia 2015, 63, 413–416. [Google Scholar]
- Mihajilov-Krestev, T.; Radnovic, D.; Kitic, D.; Jovanovic, V.S.; Mitic, V.; Stojanovic-Radic, Z.; Zlatkovic, B. Chemical composition, antimicrobial, antioxidative and anticholinesterase activity of Satureja montana L. ssp montana essential oil. Cent. Eur. J. Biol. 2014, 9, 668–677. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Gholijani, N.; Gharagozloo, M.; Farjadian, S.; Amirghofran, Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol. 2016, 13, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.Z.; Gazioglu, I.; Dag, A.; Salmas, R.E.; Kayik, G.; Durdagi, S.; Sonmez, F. Synthesis, anticholinesterase activity and molecular modelling study of novel carbamate-substituted thymol/carvacrol derivatives. Bioorg. Med. Chem. Lett. 2017, 25, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.D.; Blázquez, M.A. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immunol. 2017, 28, 1168–1180. [Google Scholar] [CrossRef] [Green Version]
- Grosso, C.; Coelho, J.A.; Urieta, J.S.; Palabra, A.M.F.; Barroso, J.G. Herbicidal activity of volatiles from coriander, winter savory, cotton lavender, and thyme isolated by hydrodistillation and supercritical fluid extraction. J. Agric. Food Chem. 2010, 58, 11007–11013. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Sena, I.; Moiteiro, C.; Bennett, R.; Mota, M.; Figueiredo, A.C. Nematoxic and phytotoxic activity of Satureja montana and Ruta graveolens essential oils on Pinus pinaster shoot cultures and P. pinaster with Bursaphelenchus xylophilus in vitro co-cultures. Ind. Crops Prod. 2015, 7, 59–65. [Google Scholar] [CrossRef]
- Fatemi, F.; Dini, S.; Rezaei, M.B.; Dadkhah, A.; Dabbagh, R.; Naij, S. The effect of γ-irradiation on the chemical composition and antioxidant activities of peppermint essential oil and extract. J. Essent. Oil Res. 2014, 26, 97–104. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Awaad, M.H.H.; Abdel-Alim, G.A.; Sayed, K.S.S.; Ahmed, K.A.; Nada, A.A.; Metwalli, A.S.Z.; Alkhalaf, A.N. Immunostimulant effects of essential oils of peppermint and eucalyptus in chickens. Pak. Vet. J. 2010, 30, 61–66. [Google Scholar]
- Cavalieri, A.; Caporali, F. Effects of essential oils of cinnamon, lavender and peppermint on germination of Mediterranean weeds. Allelopath. J. 2010, 25, 441–452. [Google Scholar]
- Niinomi, Y.; Ikeda, M.; Yamashita, M.; Ishida, Y.; Asai, M.; Shimono, Y.; Tominaga, T.; Sawada, H. Glyphosate-resistant Italian ryegrass (Lolium multiflorum) on rice paddy levees in Japan. Weed Biol. Manag. 2013, 13, 31–38. [Google Scholar] [CrossRef]
- Grulova, D.; De Martino, L.; Mancini, E.; Salamon, I.; De Feo, V. Seasonal variability of the main components in essential oil of Mentha x piperita L. J. Sci. Food Agric. 2014, 95, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Chalchat, J.C. Chemical composition and antifungal effect of anise (Pimpinella anisum L.) fruit oil at ripening stage. Ann. Microbiol. 2006, 5, 353–358. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Shukla, H.S.; Tripathi, S.G. Antifungal substance in the essential oil of anise (Pimpinella anisum L.). Agric. Biol. Chem. 1987, 51, 1991–1993. [Google Scholar] [CrossRef]
- Sharma, P.K.; Raina, A.P.; Dureja, P. Evaluation of the antifungal and phytotoxic effects of various essential oils against Sclerotium rolfsii (Sacc) and Rhizotonia bataticola (Taub). Arch. Phytopathol. Plant Prot. 2009, 42, 65–72. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Blázquez, M.A.; Carbó, E. Control of Portulaca oleracea by boldo and lemon essential oils indifferent soils. Ind. Crops Prod. 2015, 76, 515–521. [Google Scholar] [CrossRef]





| RI | Compound | S. montana Peak Area (%) | M. piperita Peak Area (%) | P. anisum Peak Area (%) |
|---|---|---|---|---|
| Monoterpene hydrocarbons | 23.16 ± 0.33 | - | - | |
| 931 | α-Thujene | 0.88 ± 0.01 | - | - |
| 939 | α-Pinene | 0.77 ± 0.00 | - | - |
| 953 | Camphene | 0.32 ± 0.00 | - | - |
| 979 | β-Pinene | 0.11 ± 0.08 | - | - |
| 993 | Myrcene | 1.39 ± 0.01 | - | - |
| 1005 | α-Phellandrene | 0.21 ± 0.00 | - | - |
| 1012 | δ-3-Carene | 0.08 ± 0.00 | - | - |
| 1019 | α-Terpinene | 1.62 ± 0.01 | - | - |
| 1029 | p-Cymene | 11.41 ± 0.01 | - | - |
| 1033 | Limonene | 0.35 ± 0.26 | - | - |
| 1053 | trans-Ocimene | 0.04 ± 0.00 | - | - |
| 1063 | γ-Terpinene | 5.78 ± 0.01 | - | - |
| 1090 | Terpinolene | 0.21 ± 0.01 | - | - |
| Oxygenated monoterpenes | 71.90 ± 0.08 | 94.77 ± 0.07 | - | |
| 1035 | 1,8-Cineole | 0.07 ± 0.00 | - | - |
| 1070 | cis-Sabinene hydrate | 0.20± 0.01 | - | - |
| 1098 | trans-Sabinene hydrate | 0.07 ± 0.01 | - | - |
| 1101 | Linalool | 2.34 ± 0.01 | - | - |
| 1146 | Camphor | 0.02 ± 0.00 | - | - |
| 1150 | Isopulegol | - | 0.80 ± 0.02 | - |
| 1155 | Menthone | - | 23.33 ± 0.59 | - |
| 1164 | iso-Menthone | - | 16.33 ± 0.03 | - |
| 1169 | Borneol | 0.71 ± 0.02 | - | |
| 1176 | Menthol | - | 48.23 ± 0.36 | - |
| 1179 | Terpinen-4-ol | 1.04 ± 0.01 | - | |
| 1184 | iso-Menthol | - | 0.52 ± 0.03 | - |
| 1186 | p-Cymen-8-ol | 0.02 ± 0.01 | - | |
| 1188 | neo-iso-Menthol | - | 0.22 ± 0.01 | - |
| 1191 | α-Terpineol | 0.41 ± 0.01 | 0.26 ± 0.01 | - |
| 1203 | trans-Dihydrocarvone | 0.03 ± 0.01 | 0.09 ± 0.01 | - |
| 1237 | Methyl ether Thymol | 0.26 ± 0.01 | - | - |
| 1242 | Pulegone | - | 0.85 ± 0.06 | - |
| 1246 | Neral | 0.06 ± 0.01 | - | - |
| 1249 | Carvone | 0.06 ± 0.01 | - | - |
| 1256 | Piperitone | - | 0.68 ± 0.06 | - |
| 1297 | Thymol | 23.20 ± 0.06 | - | - |
| 1298 | Menthyl acetate | - | 3.38 ± 0.26 | - |
| 1307 | iso-Menthyl acetate | - | 0.06 ± 0.07 | - |
| 1314 | Carvacrol | 43.34 ± 0.09 | - | - |
| 1374 | Carvacryl acetate | 0.08 ± 0.01 | - | - |
| Sesquiterpene hydrocarbons | 3.11 ± 0.02 | 2.49 ± 0.04 | 0.09 ± 0.00 | |
| 1338 | δ-Elemene | - | 0.13 ± 0.01 | - |
| 1351 | α-Cubebene | - | 0.08 ± 0.02 | - |
| 1388 | β-Bourbonene | - | 0.34 ± 0.02 | - |
| 1390 | β-Elemene | - | 0.14 ± 0.01 | - |
| 1416 | α-cis-Bergamotene | - | - | 0.01 ± 0.00 |
| 1420 | β-Caryophyllene | 2.81 ± 0.01 | 1.26 ± 0.04 | - |
| 1437 | α-trans-Bergamotene | - | - | 0.08 ± 0.00 |
| 1454 | α-Humulene | 0.11 ± 0.01 | - | - |
| 1495 | Viridiflorene | 0.05 ± 0.01 | - | - |
| 1500 | α-Muurolene | - | 0.11 ± 0.00 | - |
| 1509 | β-Bisabolene | 0.06 ± 0.00 | - | - |
| 1514 | γ-Cadinene | 0.03 ± 0.01 | 0.12 ± 0.00 | - |
| 1524 | δ-Cadinene | 0.06 ± 0.00 | 0.30 ± 0.01 | - |
| Oxygenated sesquiterpenes | 0.35 ± 0.02 | 0.26 ± 0.01 | - | |
| 1565 | E-Nerolidol | - | 0.09 ± 0.01 | - |
| 1578 | Spathulenol | 0.04±0.01 | 0.09±0.00 | - |
| 1583 | Caryophyllene oxide | 0.31±0.01 | 0.09±0.01 | - |
| Diterpene hydrocarbons | 0.06±0.01 | - | - | |
| 2067 | Abietatriene | 0.06±0.01 | - | - |
| Aromatic compounds | 0.05±0.00 | - | 99.57 ± 0.05 | |
| 1197 | Methyl Chavicol | - | - | 0.04 ± 0.00 |
| 1253 | p-Anis aldehyde | - | - | 0.04 ± 0.00 |
| 1255 | cis-Anethole | - | - | 0.03 ± 0.00 |
| 1286 | trans-Anethole | - | - | 99.46 ± 0.05 |
| 1359 | Eugenol | 0.05 ± 0.00 | - | - |
| 1406 | Methyl Eugenol | - | - | - |
| Others | 0.09 ± 0.01 | 0.15±0.02 | - | |
| 980 | 1-Octen-3-ol | 0.09 ± 0.01 | - | - |
| 1275 | n-Decanol | - | 0.15 ± 0.02 | - |
| Total | 98.73 ± 0.40 | 97.67 ± 0.08 | 99.66 ± 0.05 | |
| Seed Germination (% ± e.d.) | |||
|---|---|---|---|
| Concentration (µL/mL) | Portulaca oleracea | ||
| Winter Savory | Peppermint | Anise | |
| Control | 85.00 ± 2.74 a | 85.00 ± 2.74 a | 85.00 ± 2.74 a |
| 0.125 | 0.00 ± 0.00 b | 81.00 ± 2.45 a,b | 82.00 ± 3.74 a |
| 0.25 | 0.00 ± 0.00 b | 80.00 ± 3.54 a,b | 85.00 ± 5.24 a |
| 0.5 | 0.00 ± 0.00 b | 75.00 ± 3.87 a,b | 82.00 ± 4.34 a |
| 1 | 0.00 ± 0.00 b | 70.00 ± 3.16 b | 81.00 ± 1.87 a |
| Concentration (µL/mL) | Lolium multiflorum | ||
| Winter savory | Peppermint | Anise | |
| Control | 67.00 ± 5.15 a | 67.00 ± 5.15 a | 67.00 ± 5.15 a |
| 0.125 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 65.00 ± 6.89 a |
| 0.25 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 64.00 ± 4.30 a |
| 0.5 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 62.00 ± 4.34 a |
| 1 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 60.00 ± 3.54 a |
| Concentration (µL/mL) | Echinochloa crus-galli | ||
| Winter savory | Peppermint | Anise | |
| Control | 86.00 ± 3.32 a | 86.00 ± 3.32 a | 86.00 ± 3.32 a |
| 0.125 | 0.00 ± 0.00 b | 82.00 ± 3.74 a,b | 89.00 ± 1.87 a |
| 0.25 | 0.00 ± 0.00 b | 82.00 ± 2.55 a,b | 88.00 ± 1.23 a |
| 0.5 | 0.00 ± 0.00 b | 80.00 ± 1.58 a,b | 83.00 ± 2.55 a |
| 1 | 0.00 ± 0.00 b | 72.00 ± 2.00 b | 85.00 ± 4.47 a |
| Seedling Growth (mm ± e.d.) | ||
|---|---|---|
| Concentration (µL/mL) | Peppermint | |
| P. oleracea | ||
| Hypocotyl | Radicle | |
| Control | 10.20 ± 0.58 a | 13.80 ± 2.04 a |
| 0.125 | 6.40 ± 0.25 a,b | 11.80 ± 1.39 a,b |
| 0.25 | 6.40 ± 0.25 a,b | 10.20 ± 1.39 a,b |
| 0.5 | 5.80 ± 0.20 b | 7.80 ± 0.37 b |
| 1 | 5.00 ± 0.00 c | 7.80 ± 0.97 b |
| Concentration (µL/mL) | E. crus-galli | |
| Hypocotyl | Radicle | |
| Control | 23.66 ± 3.80 a | 20.78 ± 1.78 a |
| 0.125 | 5.82 ± 0.71 b | 6.00 ± 1.03 b |
| 0.25 | 4.56 ± 0.37 b | 3.86 ± 0.44 b |
| 0.5 | 3.56 ± 0.72 b | 4.34 ± 0.52 b |
| 1 | 3.16 ± 0.69 b | 3.72 ± 0.67 b |
| Seedling Growth (mm ± e.d.) | ||
|---|---|---|
| Concentration (µL/mL) | Anise | |
| P oleracea | ||
| Hypocotyl | Radicle | |
| Control | 10.20 ± 0.58 a | 13.80 ± 2.04 a |
| 0.125 | 10.00 ± 0.89 a | 13.40 ± 2.58 a |
| 0.25 | 9.60 ± 0.68 a | 13.60 ± 2.36 a |
| 0.5 | 8.20 ± 0.37 a | 14.60 ± 1.72 a |
| 1 | 7.60 ± 1.60 a | 13.40 ± 1.60 a |
| Concentration (µL/mL) | L. multiflorum | |
| Hypocotyl | Radicle | |
| Control | 48.50 ± 3.35 a | 39.14 ± 2.14 a |
| 0.125 | 26.21 ± 0.94 b | 27.65 ± 1.25 b |
| 0.25 | 23.07 ± 1.17 b,c | 21.29 ± 2.05 b,c |
| 0.50 | 19.71 ± 2.45 c | 18.72 ± 1.11 c |
| 1 | 12.66 ± 0.61 d | 16.66 ± 1.11 c |
| Concentration (µL/mL) | E. crus-galli | |
| Hypocotyl | Radicle | |
| Control | 23.66 ± 3.80 a | 20.78 ± 1.46 a |
| 0.125 | 19.82 ± 0.95 a | 13.24 ± 0.30 b |
| 0.25 | 18.64 ± 1.17 a | 12.90 ± 0.27 b |
| 0.5 | 14.44 ± 0.30 a,b | 12.70 ± 0.27 b |
| 1 | 8.68 ± 2.24 b | 7.19 ± 1.35 b |
| Peppermint | Seed Germination (%) ± e.d. | Seedling Growth (mm ± e.d.) | |
|---|---|---|---|
| Concentration (µL/mL) | Maize | ||
| Germination | Hypocotyl | Radicle | |
| Control | 29.00 ± 4.20 a | 5.10 ± 1.49 a | 17.65 ± 3.24 a |
| 0.125 | 15.00 ± 3.25 b | 2.29 ± 0.83 a | 4.85 ± 1.38 b |
| 0.25 | 13.50 ± 3.08 b | 2.31 ± 0.36 a | 3.95 ± 0.89 b |
| 0.5 | 7.50 ± 2.27 b | 1.54 ± 0.74 a | 2.06 ± 0.86 b |
| 1 | 6.00 ± 2.21 b | 1.72 ± 0.64 a | 2.15 ± 0.74 b |
| Concentration (µL/mL) | Rice | ||
| Germination | Hypocotyl | Radicle | |
| Control | 92.00 ± 2.55 a | 22.29 ± 5.72 a | 33.52 ± 5.90 a |
| 0.125 | 75.00 ± 3.16 b | 5.64 ± 1.43 b | 19.33 ± 2.30 b |
| 0.25 | 75.00 ± 3.16 b | 5.47 ± 1.74 b | 16.48 ± 1.69 b |
| 0.5 | 77.00 ± 6.44 b | 6.85 ± 1.68 b | 12.46 ± 1.75 b |
| 1 | 58.00 ± 2.00 c | 2.86 ± 0.23 b | 7.25 ± 0.47 b |
| Concentration (µL/mL) | Tomato | ||
| Germination | Hypocotyl | Radicle | |
| Control | 95.00 ± 1.58 a | 21.84 ± 2.00 a | 33.14 ± 3.71 a |
| 0.125 | 39.00 ± 12.59 b | 4.30 ± 3.32 b | 9.61 ± 5.23 b |
| 0.25 | 31.00 ± 16.08 b | 4.23 ± 1.68 b | 7.70 ± 2.67 b |
| 0.5 | 14.00 ± 4.30 c | 1.48 ± 0.63 b | 3.92 ± 1.34 b |
| 1 | 3.00 ± 3.00 c | 0.20 ± 0.20 b | 1.12 ± 1.12 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, M.D.; Blázquez, M.A. Phytotoxicity of Essential Oils on Selected Weeds: Potential Hazard on Food Crops. Plants 2018, 7, 79. https://doi.org/10.3390/plants7040079
Ibáñez MD, Blázquez MA. Phytotoxicity of Essential Oils on Selected Weeds: Potential Hazard on Food Crops. Plants. 2018; 7(4):79. https://doi.org/10.3390/plants7040079
Chicago/Turabian StyleIbáñez, María Dolores, and María Amparo Blázquez. 2018. "Phytotoxicity of Essential Oils on Selected Weeds: Potential Hazard on Food Crops" Plants 7, no. 4: 79. https://doi.org/10.3390/plants7040079
APA StyleIbáñez, M. D., & Blázquez, M. A. (2018). Phytotoxicity of Essential Oils on Selected Weeds: Potential Hazard on Food Crops. Plants, 7(4), 79. https://doi.org/10.3390/plants7040079