Inheritance and Genetic Mapping of the Reduced Height (Rht18) Gene in Wheat
Abstract
:1. Introduction
2. Results and Discussion
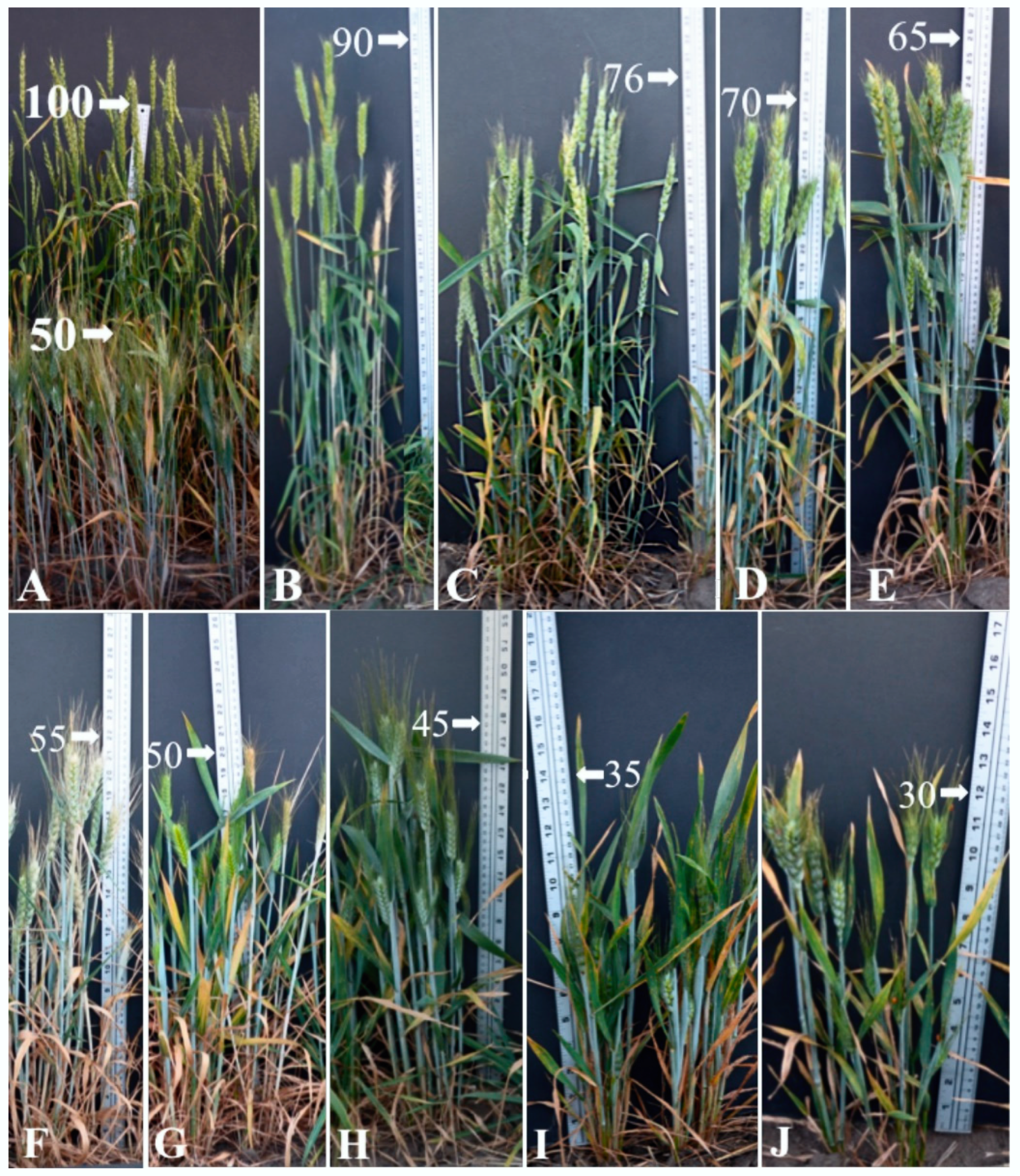
2.1. Plant Height of F2 and F2:3 Progenies
2.2. Spike Morphology
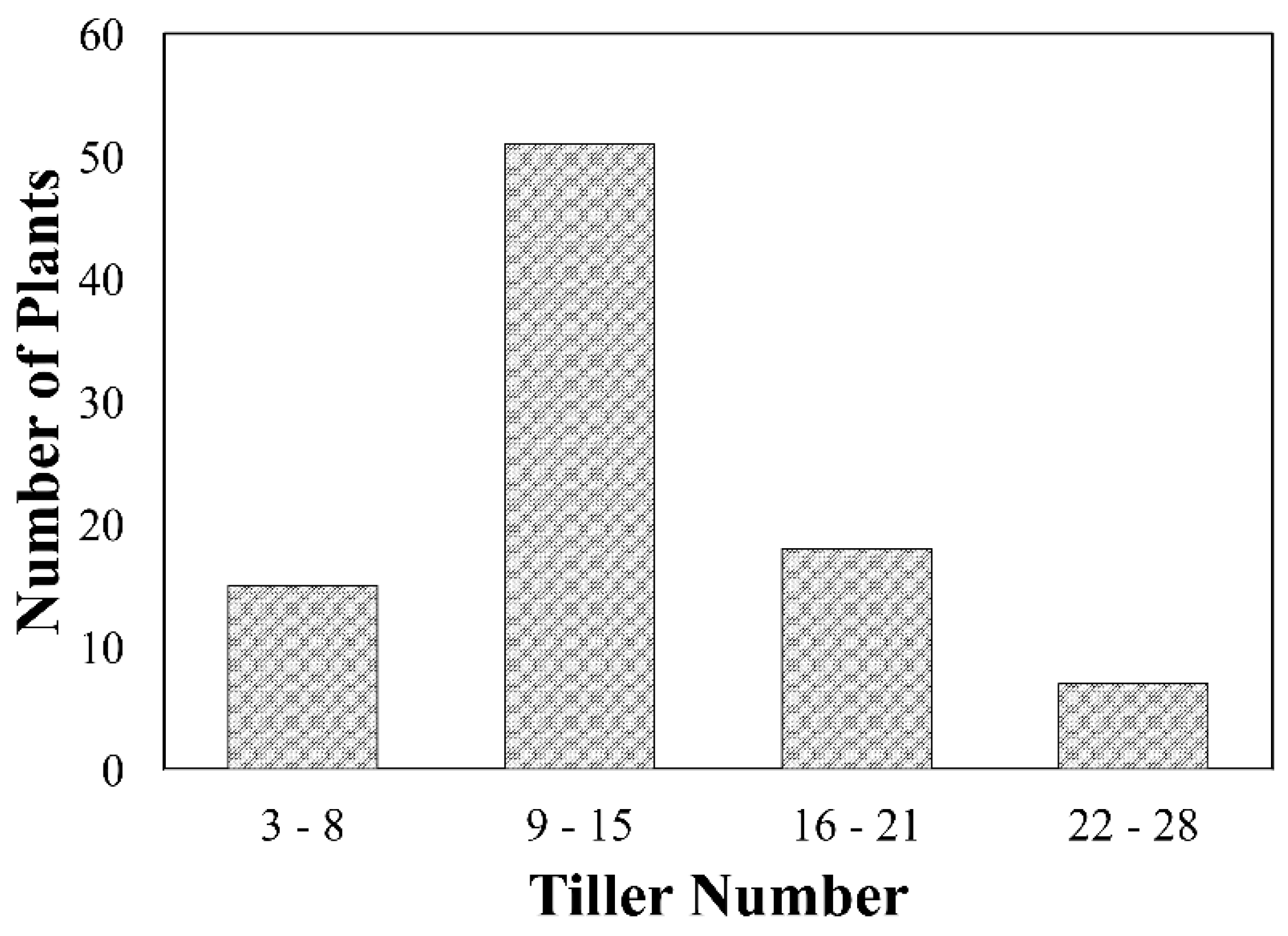
2.3. Tiller Number
2.4. Genetic Mapping of Rht18
3. Materials and Methods
3.1. Plant Materials
3.2. F1 and Plant Growing Conditions
3.3. Phenotypic Screening
3.4. DNA Isolation and Genotyping
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Germplasm Resources Information Network. Available online: https://www.ars-grin.gov/ (accessed on 12 July 2018).
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Borojevic, K.; Borojevic, K. The transfer and history of “reduced height genes” (Rht) in wheat from Japan to Europe. J. Hered. 2005, 96, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.H.; Rebetzke, G.J.; Chandler, P.; Bonnett, D.; Spielmeyer, W.; Richards, R.A. The effect of different height reducing genes on the early growth of wheat. Funct. Plant Biol. 2004, 31, 583–589. [Google Scholar] [CrossRef]
- Chapman, S.C.; Mathews, K.L.; Trethowan, R.M.; Singh, R.P. Relationships between height and yield in near-isogenic spring wheats that contrast for major reduced height genes. Euphytica 2007, 157, 391–397. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.H.; Rebetzke, G.J.; Azanza, F.; Richards, R.A.; Spielmeyer, W. Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat. Theor. Appl. Genet. 2005, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.; Saville, R.; Vaughan, S.P.; Chandler, P.M.; Wilhelm, E.P.; Sparks, C.A.; Al-Kaff, N.; Korolev, A.; Boulton, M.I.; Phillips, A.L.; et al. Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat. Plant Physiol. 2011, 157, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Kusano, T.; Katsumi, M.; Sano, H. Rice gibberellin-insensitive gene homolog, OsGAI, encodes a nuclear-localized protein capable of gene activation at transcriptional level. Gene 2000, 245, 21–29. [Google Scholar] [CrossRef]
- Ikeda, A.; Ueguchi-Tanaka, M.; Sonoda, Y.; Kitano, H.; Koshioka, M.; Futsuhara, Y.; Matsuoka, M.; Yamaguchi, J. Slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 2001, 13, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.M.; Marion-Poll, A.; Ellis, M.; Gubler, F. Mutants at the Slender1 locus of barley cv Himalaya. Molecular and physiological characterization. Plant Physiol. 2002, 129, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Tolmay, V.L. Resistance to biotic and abiotic stress in the Triticeae. Hereditas 2001, 135, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, W.F.; Donaldson, E.; Allan, R.E.; Jones, S.S. Winter wheat seedling emergence from deep sowing depths. Agron. J. 1998, 90, 582–586. [Google Scholar] [CrossRef]
- Chen, L.; Phillips, A.L.; Condon, A.G.; Parry, M.A.; Hu, Y.G. GA-responsive dwarfing gene Rht12 affects the developmental and agronomic traits in common bread wheat. PLoS ONE 2013, 8, e62285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Du, Y.; Yang, Z.; Condon, A.G.; Hu, Y.-G. Genetic effect of dwarfing gene Rht13 compared with Rht-D1b on plant height and some agronomic traits in common wheat (Triticum aestivum L.). Field Crops Res. 2014, 162, 39–47. [Google Scholar] [CrossRef]
- Gasperini, D.; Greenland, A.; Hedden, P.; Dreos, R.; Harwood, W.; Griffiths, S. Genetic and physiological analysis of Rht8 in bread wheat: An alternative source of semi-dwarfism with a reduced sensitivity to brassinosteroids. J. Exp. Bot. 2012, 63, 4419–4436. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N. Genetic collection and development of near-isogenic lines in durum wheat. Vavilov J. Genet. Breed. 2008, 12, 636–643. [Google Scholar]
- Vikhe, P.; Patil, R.; Chavan, A.; Oak, M.; Tamhankar, S. Mapping gibberellin-sensitive dwarfing locus Rht18 in durum wheat and development of SSR and SNP markers for selection in breeding. Mol. Breed. 2017, 37, 28. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Liu, C.Y.; Du, Y.Y.; Chen, L.; Chen, Y.F.; Hu, Y.G. Dwarfing gene Rht18 from tetraploid wheat responds to exogenous GA3 in hexaploid wheat. Cereal Res. Commun. 2017, 45, 23–34. [Google Scholar] [CrossRef]
- Padmanaban, S.; Zhang, P.; Hare, R.A.; Sutherland, M.W.; Martin, A. Pentaploid wheat hybrids: Applications, characterisation, and challenges. Front. Plant Sci. 2017, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Simpfendorfer, S.; Hare, R.A.; Eberhard, F.S.; Sutherland, M.W. Retention of D genome chromosomes in pentaploid wheat crosses. Heredity 2011, 107, 315–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, C.; Yang, Y.; Mohan, A.; Grant, N.; Gill, K.S.; Sandhu, D. Microsatellites based genetic linkage map of the Rht3 locus in bread wheat. Mol. Plant Breed. 2014, 5, 43–46. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Archer, M.A.; Wayne, R.K. Transgressive segregation, adaptation and speciation. Heredity 1999, 83, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, G.M. Inheritance of heading date, plant height, and kernel weight in two spring wheat Crosses. Crop Sci. 1972, 12, 95–98. [Google Scholar] [CrossRef]
- El-Hennawy, M.A.; Abdalla, A.F.; Shafey, S.A.; Al-Ashkar, I.M. Production of doubled haploid wheat lines (Triticum aestivum L.) using anther culture technique. Ann. Agric. Sci. 2011, 56, 63–72. [Google Scholar] [CrossRef]
- Shindo, C.; Tsujimoto, H.; Sasakuma, T. Segregation analysis of heading traits in hexaploid wheat utilizing recombinant inbred lines. Heredity 2003, 90, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Zheng, J.; Liu, C.; Wang, Y.; Condon, A.G.; Chen, Y.; Hu, Y.-G. Effects of the GA-responsive dwarfing gene Rht18 from tetraploid wheat on agronomic traits of common wheat. Field Crops Res. 2015, 183, 92–101. [Google Scholar] [CrossRef]
- Bhagyalakshmi, K.; Vinod, K.K.; Kumar, M.; Arumugachamy, S.; Prabhakaran, A.; Raveendran, T.S. Interspecific hybrids from wild x cultivated Triticum crosses-a study on the cytological behavior and molecular relations. J. Crop Sci. Biotechnol. 2008, 11, 257–262. [Google Scholar]
- Casebow, R.; Hadley, C.; Uppal, R.; Addisu, M.; Loddo, S.; Kowalski, A.; Griffiths, S.; Gooding, M. Reduced height (Rht) alleles affect wheat grain quality. PLoS ONE 2016, 11, e0156056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T. Physiological and Genetic Studies of an Alternative Semi-Dwarfing Gene Rht18 in Wheat; University of Tasmania: Tasmania, Australia, 2015. [Google Scholar]
- Somers, D.J.; Isaac, P.; Edwards, K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Martinek, P.; Watanabe, N.; Kuboyama, T. Genetic mapping of gibberellic acid-sensitive genes for semi-dwarfism in durum wheat. Cereal Res. Commun. 2011, 39, 171–178. [Google Scholar] [CrossRef]
- Ford, B.; Foo, E.; Sharwood, R.E.; Karafiatova, M.; Vrána, J.; MacMillan, C.; Nichols, D.S.; Steuernagel, B.; Uauy, C.; Doležel, J.; et al. Rht18 semi-dwarfism in wheat is due to increased expression of GA 2-oxidaseA9 and lower GA Content. Plant Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, V.B. All things dwarfed and beautiful. Available online: https://web.expasy.org/spotlight/pdf/sptlt070.pdf (accessed on 12 July 2018).
- Sandhu, D.; Champoux, J.A.; Bondareva, S.N.; Gill, K.S. Identification and physical localization of useful genes and markers to a major gene-rich region on wheat group 1S chromosomes. Genetics 2001, 157, 1735–1747. [Google Scholar] [PubMed]
- GrainGenes. Available online: https://wheat.pw.usda.gov/cgi-bin/GG3/browse.cgi?class=marker;begin=8251 (accessed on 12 July 2018).
- Lander, E.S.; Green, P.; Abrahamson, J.; Barlow, A.; Daly, M.J.; Lincoln, S.E.; Newburg, L. MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1987, 1, 174–181. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distance from recombination values. Ann. Eugen. 1944, 12, 172–175. [Google Scholar] [CrossRef]



| Source | DF | SS | MS | F Value | Pr > F |
|---|---|---|---|---|---|
| Model | 76 | 155,138.4 | 2041.29 | 14.49 | <0.0001 |
| Error | 648 | 9131.879 | 140.91 | ||
| Corrected total | 724 | 246,452.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, N.P.; Mohan, A.; Sandhu, D.; Gill, K.S. Inheritance and Genetic Mapping of the Reduced Height (Rht18) Gene in Wheat. Plants 2018, 7, 58. https://doi.org/10.3390/plants7030058
Grant NP, Mohan A, Sandhu D, Gill KS. Inheritance and Genetic Mapping of the Reduced Height (Rht18) Gene in Wheat. Plants. 2018; 7(3):58. https://doi.org/10.3390/plants7030058
Chicago/Turabian StyleGrant, Nathan P., Amita Mohan, Devinder Sandhu, and Kulvinder S. Gill. 2018. "Inheritance and Genetic Mapping of the Reduced Height (Rht18) Gene in Wheat" Plants 7, no. 3: 58. https://doi.org/10.3390/plants7030058
APA StyleGrant, N. P., Mohan, A., Sandhu, D., & Gill, K. S. (2018). Inheritance and Genetic Mapping of the Reduced Height (Rht18) Gene in Wheat. Plants, 7(3), 58. https://doi.org/10.3390/plants7030058







