Identification of a Bioactive Compound against Adult T-cell Leukaemia from Bitter Gourd Seeds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Active Compounds in BGSE
2.2. The Inhibitory Effects of Octadecanoic Acid Analogs on ATL Cell Lines
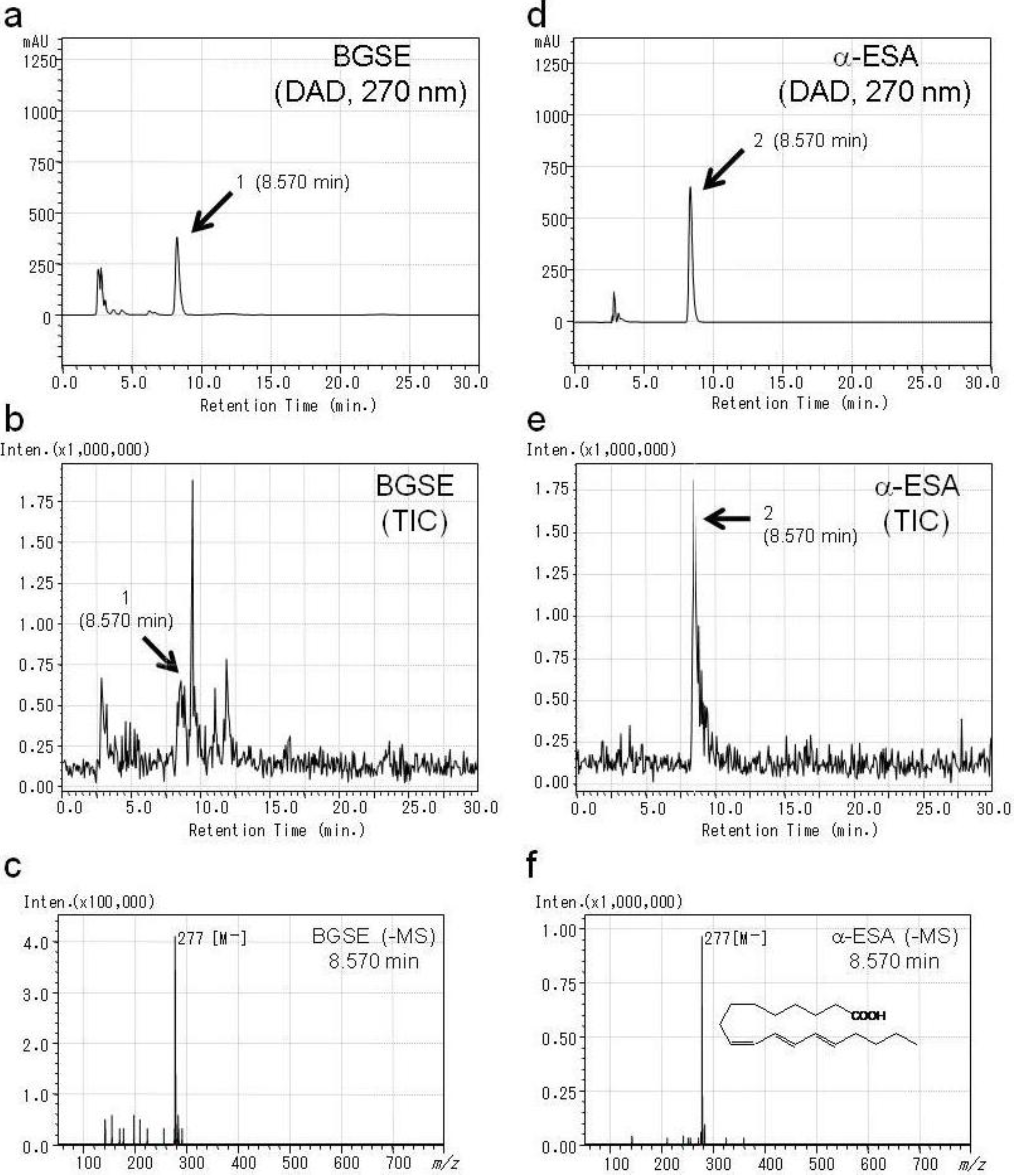
| Compounds | IC50 (µM) | ||
|---|---|---|---|
| ED | Su9T01 | ||
| α-ESA | (C18:3, n-5) | 8.9 | 29.3 |
| γ-linolenic acid | (C18:3, n-6) | 61.3 | 174.3 |
| α-linolenic acid | (C18:3, n-3) | 129.9 | 167.4 |
| linoleic acid | (C18:2, n-6) | 100.7 | 180.1 |
| oleic acid | (C18:1, n-9) | 500.0–166.7 | 500.0–166.7 |
| elaidic acid | (C18:1, n-9) | >500.0 | >500.0 |
| stearic acid | (C18:0) | 500.0–166.7 | >500.0 |
| EGCG | (Positive Control) | 152.7 | 166.0 |
2.3. The Effect of α-ESA on ATL Cell Line and Phytohemagglutinin-Activated Human Peripheral Blood Mononuclear Cell (PBMC) Proliferation
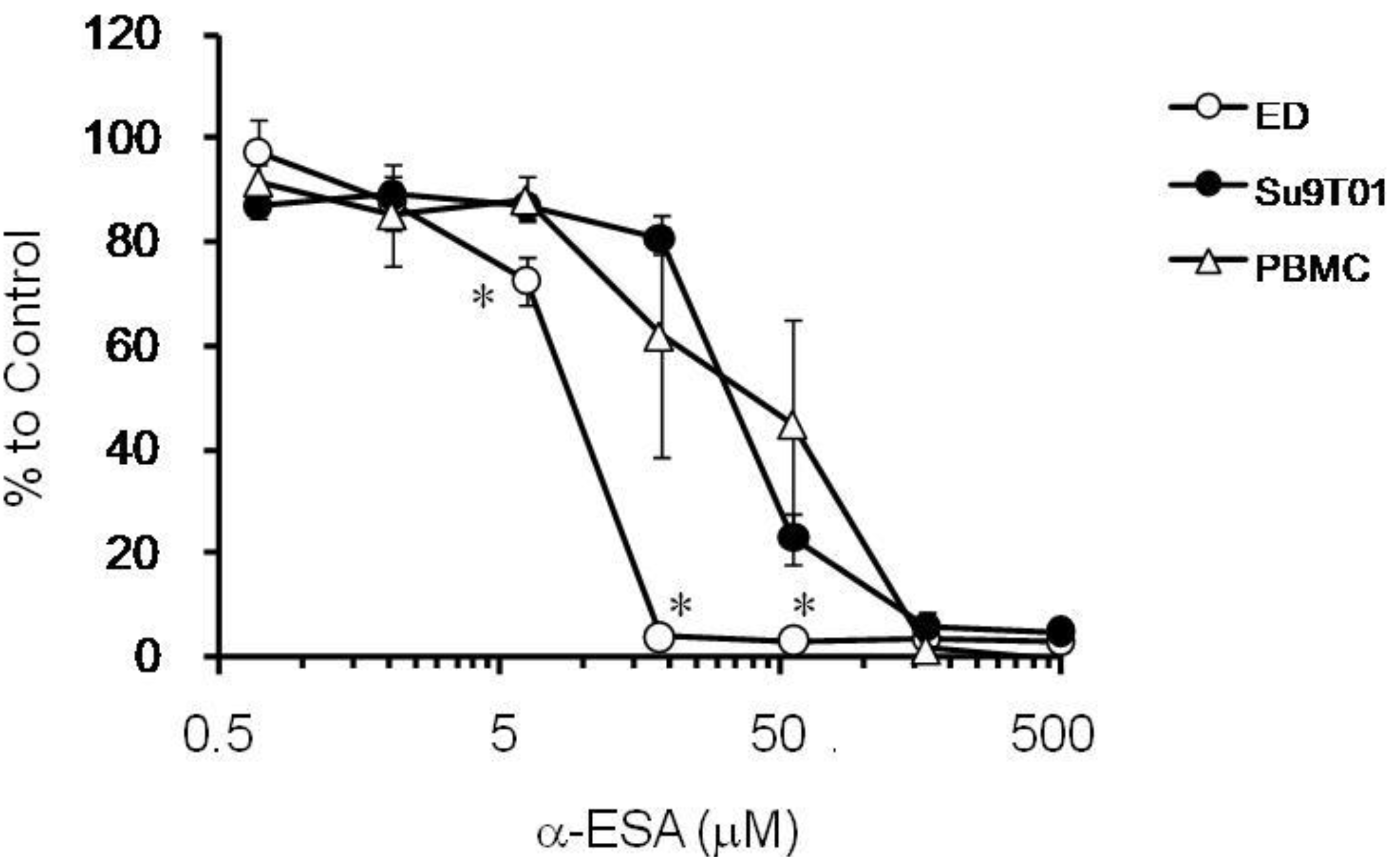
3. Experimental
3.1. Chemicals
3.2. Identification of Compounds in BGSE
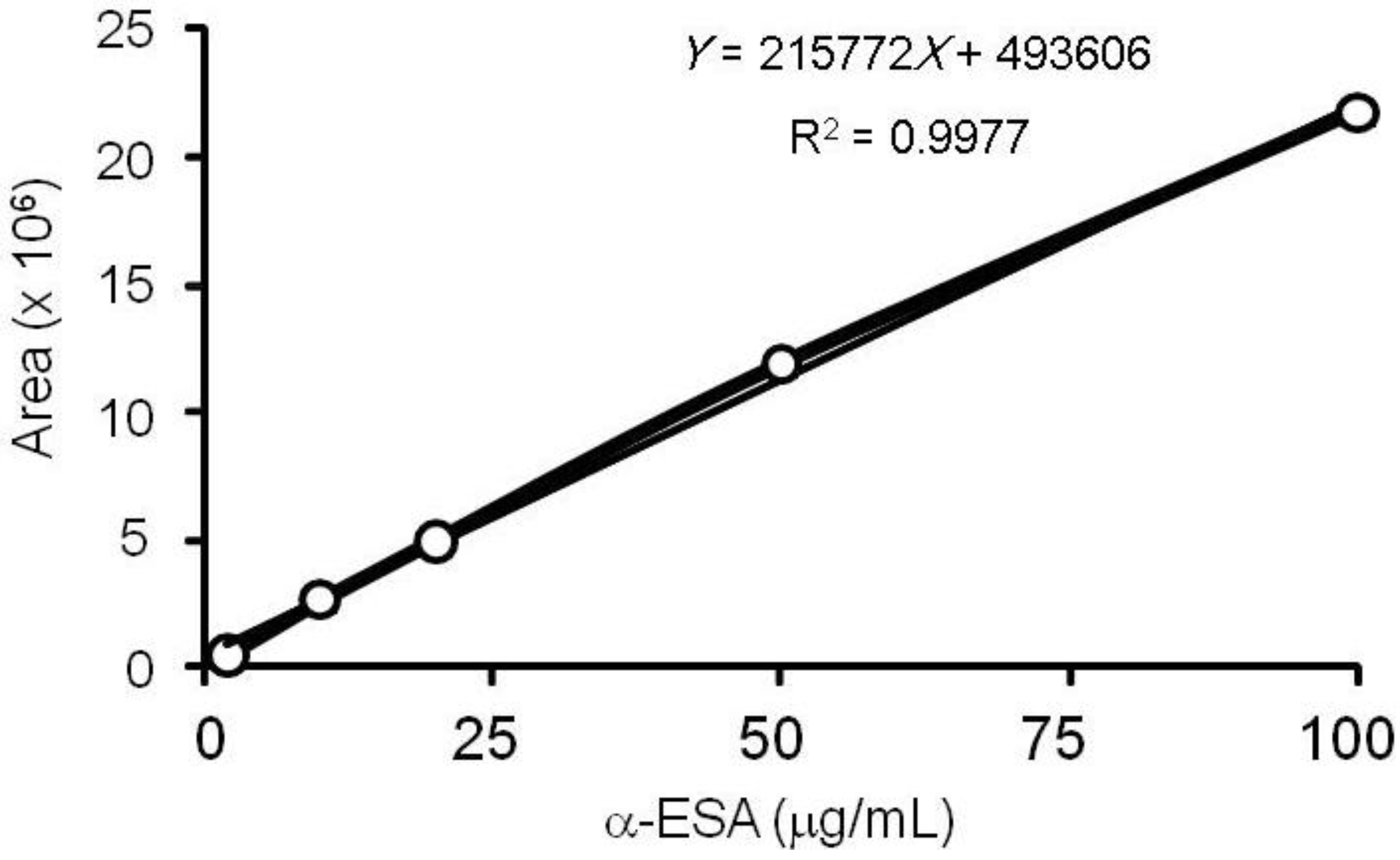
3.3. α-ESA Calibration Curve
3.4. ATL Cell Proliferation Assay
3.5. Isolation and Culture of PBMCs
3.6. Statistics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Arisawa, K.; Soda, M.; Endo, S.; Kurokawa, K.; Katamine, S.; Shimokawa, I.; Koba, T.; Takahashi, T.; Saito, H.; Doi, H.; et al. Evaluation of adult T-cell leukemia/lymphoma incidence and its impact on non-Hodgkin lymphoma incidence in southwestern Japan. Int. J. Cancer 2000, 85, 319–324. [Google Scholar] [CrossRef]
- Proietti, F.A.; Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Murphy, E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005, 24, 6058–6068. [Google Scholar] [CrossRef]
- Kai, H.; Akamatsu, E.; Torii, E.; Kodama, H.; Yukizaki, C.; Sakakibara, Y.; Suiko, M.; Morishita, K.; Kataoka, H.; Matsuno, K. Inhibition of proliferation by agricultural plant extracts in seven human adult T-cell leukaemia (ATL)-related cell lines. J. Nat. Med. 2011, 65, 651–655. [Google Scholar] [CrossRef]
- Fang, E.F.; Ng, T.B. Bitter gourd (Momordica charantia) is a cornucopia of health: A review of its credited antidiabetic, anti-HIV, and antitumor properties. Curr. Mol. Med. 2011, 11, 417–436. [Google Scholar] [CrossRef]
- Soundararajan, R.; Prabha, P.; Rai, U.; Dixit, A. Antileukemic Potential of Momordica charantia Seed Extracts on Human Myeloid Leukemic HL60 Cells. J. Evid.-Based Complement. Alternat. Med. 2012. [Google Scholar] [CrossRef]
- Amakura, Y.; Kondo, K.; Akiyama, H.; Ito, H.; Hatano, T.; Yoshida, T.; Maitani, T. Characteristic long-chain fatty acid of Pleurocybella porrigens. Shokuhin Eiseigaku Zasshi 2006, 47, 178–181. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Tokuyama, Y.; Igarashi, M.; Miyazawa, T. Tumor growth suppression by alpha-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation. Carcinogenesis 2004, 25, 1417–1425. [Google Scholar] [CrossRef]
- Ruiz, R.A.; Reglero, G.; Ibañez, E. Recent trends in the advanced analysis of bioactive fatty acids. J. Pharm. Biomed. Anal. 2012, 51, 305–326. [Google Scholar]
- Li, H.C.; Yashiki, S.; Sonoda, J.; Lou, H.; Ghosh, S.K.; Byrnes, J.J.; Lema, C.; Fujiyoshi, T.; Karasuyama, M.; Sonoda, S. Green tea polyphenols induce apotosis in vitro in peripheral blood T lymphocytes of adult T-cell leukemia patients. Jpn. J. Cancer Res. 2000, 91, 34–40. [Google Scholar] [CrossRef]
- Kobori, M.; Ohnishi-Kameyama, M.; Akimoto, Y.; Yukizaki, C.; Yoshida, M. α-eleostearic acid and its dihydroxy derivative are major apoptosis-inducing components of bitter gourd. J. Agric. Food Chem. 2008, 56, 10515–10520. [Google Scholar] [CrossRef]
- Wendel, M.; Heller, A.R. Anticancer actions of omega-3 fatty acids—Current state and future perspectives. Anticancer Agents Med. Chem. 2009, 9, 457–470. [Google Scholar] [CrossRef]
- Sasaki, H.; Nishikata, I.; Shiraga, T.; Akamatsu, E.; Fukami, T.; Hidaka, T.; Kubuki, Y.; Okayama, A.; Hamada, K.; Okabe, H.; et al. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood 2005, 105, 1204–1213. [Google Scholar]
- Watanabe, M.; Nakahata, S.; Hamasaki, M.; Saito, Y.; Kawano, Y.; Hidaka, T.; Yamashita, K.; Umeki, K.; Taki, T.; Taniwaki, M.; et al. Downregulation of CDKN1A in adult T-cell leukemia/lymphoma despite overexpression of CDKN1A in human T-lymphotropic virus 1-infected cell lines. J. Virol. 2010, 84, 6966–6977. [Google Scholar] [CrossRef]
- Řezanka, T.; Votruba, J. Analysis of Fatty Acids by APCI-MS. In Modern Methods for Lipid Analysis by Liquid Chromatography: Mass Spectrometry and Related Techniques; William, C.B., Ed.; AOCS Press: Urbana, IL, USA, 2005; pp. 242–275. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kai, H.; Akamatsu, E.; Torii, E.; Kodama, H.; Yukizaki, C.; Akagi, I.; Ino, H.; Sakakibara, Y.; Suiko, M.; Yamamoto, I.; et al. Identification of a Bioactive Compound against Adult T-cell Leukaemia from Bitter Gourd Seeds. Plants 2014, 3, 18-26. https://doi.org/10.3390/plants3010018
Kai H, Akamatsu E, Torii E, Kodama H, Yukizaki C, Akagi I, Ino H, Sakakibara Y, Suiko M, Yamamoto I, et al. Identification of a Bioactive Compound against Adult T-cell Leukaemia from Bitter Gourd Seeds. Plants. 2014; 3(1):18-26. https://doi.org/10.3390/plants3010018
Chicago/Turabian StyleKai, Hisahiro, Ena Akamatsu, Eri Torii, Hiroko Kodama, Chizuko Yukizaki, Isao Akagi, Hisatoshi Ino, Yoichi Sakakibara, Masahito Suiko, Ikuo Yamamoto, and et al. 2014. "Identification of a Bioactive Compound against Adult T-cell Leukaemia from Bitter Gourd Seeds" Plants 3, no. 1: 18-26. https://doi.org/10.3390/plants3010018
APA StyleKai, H., Akamatsu, E., Torii, E., Kodama, H., Yukizaki, C., Akagi, I., Ino, H., Sakakibara, Y., Suiko, M., Yamamoto, I., Okayama, A., Morishita, K., Kataoka, H., & Matsuno, K. (2014). Identification of a Bioactive Compound against Adult T-cell Leukaemia from Bitter Gourd Seeds. Plants, 3(1), 18-26. https://doi.org/10.3390/plants3010018





