Recent Advances in the Phytochemistry of Bryophytes: Distribution, Structures and Biological Activity of Bibenzyl and Bisbibenzyl Compounds
Abstract
:1. Introduction
2. Extraction and Purification of Major Bioactive Compounds
| Type of Chemical | Name of the Chemical | Name of the Taxa | Content (mg/100 g dw) | λmax (nm) | Mass Spectra (m/z) | References |
|---|---|---|---|---|---|---|
| Prebibenzyl | Dumhirone A (20) | Dumortiera hirsuta | 0.4 | 274 in MeOH | 230.0963 [M]+ | [51] |
| Prelunularin(22) | Ricciocarpos natans | 1.2 | 272 | na | [52] | |
| Bibenzyl | Dihydroresveratol (65) | Blasia pusilla | 2.82 | na | na | [53] |
| Brittonin A (39) | Frullaniabrittoniae subsp. truncatifolia | 50 | 217 | 362 [M]+ | [54] | |
| Brittonin A (39) | Frullania inouei | 2.55 | na | na | [55] | |
| Brittonin B (40) | Frullania brittoniae subsp. truncatifolia | 25 | 276 | 346 [M]+ | [54] | |
| Brittonin B (40) | Frullania inouei | 0.83 | na | na | [55] | |
| 3,4′-Dimethoxyl-4-hydroxylbibenzyl (141) | Radula complanata | 11.76–14.11 | na | 258 [M]+, | [56] | |
| 2-(3-Methyl-2-butenyl)-3,5-dihydroxybibenzyl (32) | Radula voluta | 1034.38 | 284 in MeOH | 282 [M]+ | [57] | |
| 2-Geranyl-3,5-dihydroxybienzyl (33) | Radula voluta | 381.25 | 284 in MeOH | 350 [M]+ | [57] | |
| 3,5-Dihydroxy-4-(3-hydroxy-3-methylbutyl) bibenzyl (34) | Radula laxiramea | 117.30 | 300 [M]+ | [45] | ||
| Radulanolide (44) | Radula complanata | 42.82 | 216 | 322 [M]+ | [40] | |
| Radulanin H (48) | Radula complanata | 999.14 | 220 | 324 [M]− | [40] | |
| Radulanin L (51) | Radula complanata | 147.54 | 282 | 296.1446 [M]+ | [56] | |
| Lunularin (21) | Blasia pusilla | 3.77 | na | na | [53] | |
| Lunularin (21) | Dumortiera hirsuta | 0.85 | na | na | [51] | |
| Lunularic acid (23) | Blasia pusilla | 0.48 | na | na | [53] | |
| Prenyl bibenzyl | Perrottetin A (52) | Radula perrottetii | 11,162.08 | 211 | 298 [M-91]− | [40] |
| Perrottetin D (10) | Radula perrottetii | 6116.20 | 216 | 296 [M]− | [40] | |
| Perrottetin D (10) | Radula perrottetii | 149.85 | na | 296.1425 [M]+ | [56] | |
| 2-Carbomethoxy-3,5,4″-trihydroxy- 4,6-(3-methyl-2-butenyl)-Bibenzyl (27) | Lethocolea glossophyla | 72 | 313 in MeOH | 424 [M]+ | [57] | |
| 2,4-di-(3-methyl-2-butenyl)-3,5,4′-Trihydroxy-bibenzyl (28) | Lethocolea glossophyla | 44 | 313 in MeOH | 366 [M]+ | [57] | |
| 2,2-Dimethyl-5-hydroxy-6-(3-methyl-2-butenyl)-7-[2-(4′-hydroxyphenyl)-ethyl]-chromene (29) | Lethocolea glossophyla | 52 | 315 in MeOH | 364 [M]+ | [57] | |
| 2,2-Dimethyl-5-hydroxyl-7-[2-(4′-hydroxyphenyl)-ethyl]-8-(3-methyl-2-butenyl)-chromene (30) | Lethocolea glossophyla | 232 | 320 in MeOH | 364 [M]+ | [57] | |
| Bibenzyl cannabinoid | Perrottetinene (14) | Radula marginata | 562.5 | na | na | [46] |
| Perrottetinene (14) | Radula laxiramea | 18.57 | na | na | [45] | |
| Perrottetinenic acid (58) | Radula marginata | 87.5 | 301.4 in EtOH | 392.1985, 392 [M]+ | [46] | |
| Cinnamoyl bibenzyl | Pallidisetin A (35) | Polytrichum pallidisetum | 0.50 | 318 | 342.1277 [M]+ | [58] |
| Pallidisetin B (36) | Polytrichum pallidisetum | 0.21 | 294 | 342.1236 [M]+ | [58] | |
| Bibenzyl derivative | Pellepiphyllin (37) | Pellia epiphylla | 6.8 | na | 258 [M]+ | [44] |
| 7-Hydroxy-pellepiphyllin (38) | Pellia epiphylla | 0.24 | na | 273 [M-H]− | [44] | |
| 2,5,4′-Trihydroxy–bibenzyl (24) | Ricciocarpus natans | 0.8 | 284 | na | [52] | |
| 3-Methoxybibenzyl (26) | Radula complanata | 11.76 | 212 | na | [59] | |
| 3,3′,4,4′-Tetramethoxy-bibenzyl (41) | Frullania inouei | 0.34 | na | na | [55] | |
| Chrysotobibenzyl (42) | Frullania inouei | 9.02 | na | na | [55] | |
| Bisbibenzyl and its derivative | Riccardin A (11) | Riccardia multifida | 130.64 | 213 | 438.1831 | [42] |
| Riccardin A (11) | Riccardia multifida | 4458.33 | 283 | na | [43] | |
| Riccardin B (12) | Riccardia multifida | 115.64 | 233 | 424.1674 | [42] | |
| Riccardin B (12) | Riccardia multifida | 4250 | 280 | 424.1700 | [43] | |
| Riccardin C (15) | Plagiochasma intermedium | 0.22 a | na | na | [60] | |
| Riccardin C (15) | Marchantia palmata | 9 | na | na | [61] | |
| Riccardin C (15) | Blasia pusilla | 53.35 3.74 | na | na | [53] | |
| Riccardin C (15) | Dumortiera hirsuta | 0.23 | na | 425.30 [M−] | [51] | |
| Riccardin D (9) | Monoclea forsteri | 141 | na | 424.1674 | [62] | |
| Isoriccardin C (83) | Plagiochasma intermedium | 0.05 a | na | na | [60] | |
| Isoriccardin C (83) | Marchantia palmata | 12.5 | na | na | [61] | |
| Isoriccardin C (83) | Marchantia paleacea | 4.31 | na | na | [63] | |
| Isoriccardin D (87) | Marchantia polymorpha | 0.45 | na | 424.16 [M]+ | [64] | |
| Riccardin F (79) | Plagiochasma intermedium | 0.07 a | na | na | [60] | |
| Riccardin F (79) | Blasia pusilla | 14.64 | 215 | 438 [M]+ | [53] | |
| Riccardin H (8) | Marchantia polymorpha | 0.06 | na | 456 [M]+ | [33] | |
| Riccardin I (82) | Asterella angusta | 0.32 | na | 454.1440 [M]+ | [65] | |
| Paleatin A (95) | Marchantia paleacea subsp. diptera | 5.75 | na | na | [21] | |
| Paleatin B (96) | Marchantia paleacea subsp. diptera | 16.51 | na | na | [21] | |
| Polymorphatin A (136) | Marchantia polymorpha | 0.40 | na | 424 [M]+ | [64] | |
| Marchantin A (1) | Marchantia polymorpha | 0.13 | na | 439 [M]+ | [33] | |
| Marchantin A (1) | Marchantia polymorpha | 377.5 | na | na | [21] | |
| Marchantin A (1) | Marchantia paleacea subsp. diptera | 1204.54 | na | na | [21] | |
| Marchantin A (1) | Marchantia paleacea subsp. diptera | 5000–6000 | na | na | [47] | |
| Marchantin A (1) | Marchantia paleacea subsp. diptera | 1191.90 | na | na | [48] | |
| Marchantin A (1) | Marchantia tosana | na | na | 440.1632 | [32] | |
| Marchantin B (2) | Marchantia polymorpha | 0.13 | na | 455 [M]+ | [33] | |
| Marchantin B (2) | Marchantia polymorpha | 12.87 | na | na | [21] | |
| Marchantin B (2) | Marchantia paleacea subsp. diptera | 13.48 | na | na | [21] | |
| Marchantin C (3) | Marchantia paleacea subsp. diptera | 16.79 | na | na | [21] | |
| Marchantin C (3) | Marchantia polymorpha | 6.5 | na | na | [21] | |
| Marchantin C (3) | Marchantia polymorpha | 40 | 208.5 | na | [61] | |
| Marchantin C (3) | Marchantia palmata | 10 | na | na | [61] | |
| Marchantin C (3) | Dumortiera hirsuta | 0.3 | na | na | [60] | |
| Marchantin C (3) | Schistochila glaucescens | 61.22 | na | na | [49] | |
| Marchantin C (3) | Reboulia hemisphaerica | 120 | na | 424.1680 [M]+ | [66] | |
| Marchantin C (3) | Marchantia paleacea | 38.79 | na | na | [63] | |
| Isomarchantin C (67) | Marchantia polymorpha | 8.63 | 211.5 | 424.1707 | [61] | |
| Isomarchantin C (67) | Marchantia palmata | 6 | na | na | [21] | |
| Marchantin D (147) | Marchantia paleacea subsp. diptera | 5.30 | na | na | [21] | |
| Marchantin E (4) | Marchantia paleacea subsp. diptera | 126.36 | na | na | [21] | |
| Marchantin E (4) | Marchantia polymorpha | 0.11 | na | 469 | [33] | |
| Marchantin E (4) | Marchantia paleacea subsp. diptera | 125.03 | na | na | [48] | |
| Marchantin H (144) | Plagiochasma intermedium | 0.04 a | na | na | [60] | |
| Marchantin M (72) | Reboulia hemisphaerica | 100 | 283 | 454.1774 [M]+ | [66] | |
| Marchantin N (74) | Reboulia hemisphaerica | 120 | 276.5 | 471 [M]+ | [66] | |
| Marchantin O (75) | Reboulia hemisphaerica | 300 | 274 | 438.1817 [M]+ | [66] | |
| Marchantin J (68) | Marchantia polymorpha | 30.30 | na | 424 [M]+ | [64] | |
| Neomarchantin A (5) | Plagiochasma intermedium | 0.05 | na | na | [60] | |
| Neomarchantin A (5) | Marchantia polymorpha | 0.04 | na | 423 [M]+ | [33] | |
| Neomarchantin A (5) | Schistochila glaucescens | 51.02 | 272 in EtOH | 424 [M]+ | [49] | |
| Neomarchantin B (77) | Schistochila glaucescens | 42.85 | 272 in EtOH | 440 [M+ | [49] | |
| Pakyonol A (145) | Plagiochasma intermedium | 0.40 a | na | na | [60] | |
| Angustatin A (146) | Asterella angusta | 0.28 | na | 436.1330 [M]+ | [65] | |
| Isoplagiochin A (62) | Plagiochila sp. | 2.85 | 288–250 Sh | 423 [M+H]+ | [67] | |
| Isoplagiochin A (102a) | Plagiochila fruticosa | 33.48 a | na | na | [68] | |
| Isoplagiochin A (102a) | Heteroscyphus planus | 0.29 | 292 | 422.1542 [M]+ | [69] | |
| Isoplagiochin B (103a) | Plagiochila fruticosa | 17.79 a | na | na | [68] | |
| Isoplagiochin C (104) | Herbertus sakerraii | 4.36 | na | na | [70] | |
| Isoplagiochin C (104) | Plagiochila fruticosa | 8.34 a | 287 | 422.1485 [M]+ | [68] | |
| Isoplagiochin D (105) | Herbertus sakerraii | 35.43 | na | na | [70] | |
| Isoplagiochin D (105) | Plagiochila fruticosa | 5.32 a | na | 424.1654 [M]+ | [68] | |
| Isoplagiochin F (107) | Plagiochila sp. | 2.85 | 283–251 Sh | 441 [M+H]+ | [67] | |
| Dihydroisoplagiochin (108) | Plagiochila sp. | 5 | 288–282 Sh | 425 [M+H]+ | [67] | |
| Monochlorinated isoplagiochin D (109) | Plagiochila sp. | 0.71 | 288–282 | 460 | [67] | |
| 2,12-Dichloroisoplagiochin D (110) | Herbertus sakuraii | 6.48 | 288 | 492.0904 | [70] | |
| 12,7′-Dichloroisoplagiochin D (111) | Herbertus sakuraii | 3.04 | 288 | 492.0923 | [70] | |
| 12, 10′-Dichloroisoplagiochin C (112) | Herbertus sakuraii | 4.36 | 294 | 490.0714 | [70] | |
| 12-Chloroisoplagiochin D (113) | Mastigophora diclados | 0.94 | na | na | [70] | |
| 2,12-Dichloroisoplagiochin D (110) | Mastigophora diclados | 0.68 | na | na | [70] | |
| Plagiochin E (7) | Marchantia polymorpha | 13.40 | na | 424.1685 | [33] | |
| Planusin A (106) | Heteroscyphus planus | 3.23 | 297 | 422.1485 [M]+ | [69] | |
| Pusillatin A (90) | Blasia pusilla | 0.65 | 212 in EtOH | 869 [M+Na]+, 846 [M]+ | [53] | |
| Pusillatin B (91) | Blasia pusilla | 5.18 | 227 in EtOH | 869 [M+Na]+, 846 [M]+ | [53] | |
| Pusillatin C (92) | Blasia pusilla | 19.69, 3.05 | 215 in EtOH | 869 [M+Na]+, 846 [M]+ | [53] | |
| Perrottetin E (13) | Radula perrottetii | 9.62 | na | na | [71] | |
| Perrottetin E (13) | Radula perrottetii | 83.57 | na | na | [56] | |
| Perrottetin E (13) | Plagiochila sp. | 1.42 | na | na | [67] | |
| Perrottetin E (13) | Pellia epiphylla | 218.4 | na | na | [44] | |
| Perrottetin E (13) | Marchantia polymorpha | 2.02 | na | 424.16 [M]+ | [64] | |
| Perrottetin E (13) | Radula laxiramea | 36.82 | na | na | [45] | |
| Perrottetin F (55) | Radula perrottetii | 63.55 | na | na | [71] | |
| Perrottetin F (55) | Radula perrottetii | 86.45 | na | na | [56] | |
| Isoperrottetin A (56) | Radula perrottetii | 11.96 | 213 | 426 [M]+ | [71] | |
| Perrottetin G (57) | Radula perrottetii | 12.79 | na | na | [71] | |
| Perrottetin G (57) | Radula perrottetii | 4.61 | na | na | [56] | |
| 10′-Hydroxyperrottetin E (59) | Pellia epiphylla | 139.44 | na | 442 [M]+ | [44] | |
| 10′-Hydroxyperrottetin E-11-methylether (60) | Pellia epiphylla | 11.38 | na | 456 [M]+ | [44] | |
| 14′-Hydroxyperrottetin E (61) | Pellia epiphylla | 17.47 | na | 442 [M]+ | [44] | |
| 10,10′-Dihydroxy-perrottetin E (62) | Pellia epiphylla | 81.73 | na | 458 [M]+ | [44] | |
| 13′,13″-Bis(I0′-hydroxyperrottetin E (63) | Pellia epiphylla | 2.04 | na | 882 [M]+ | [44] | |
| Perrottetin E-11-methyl ether (64) | Pellia epiphylla | 27.44 | na | 440 [M]+ | [44] | |
| Marchantin G methyl ether (78) | Marchantia palmata | 1.5 | na | na | [61] | |
| 6′,6″-Bis-riccardin C (25) | Ricciocarpus natans | 4 | 847 | na | [52] | |
| Isoriccardinoquinone A (84) | Marchantia paleacea | 10.34 | na | 468 [M]+ | [63] | |
| Isoriccardinoquinone B (85) | Marchantia paleacea | 7.75 | na | 452 [M]+ | [63] | |
| 2-Hydro-3,7-dimethoxyphenanthrene (86) | Marchantia paleacea | 19.82 | na | na | [63] | |
| 13, 13′-O-Isopropylidenericcardin D (6) | Marchantia polymorpha | 0.06 | na | 464 | [33] | |
| 11-O-Demethyl-marchantin I (143) | Asterella angusta | 0.4 | 210 in MeOH | 423 [M-H]−, 424.1712 [M] | [72] | |
| Dihydroptychantol A (135a) | Asterella angusta | 0.32 | 210 in MeOH | 423 [M-H]−, 424.1720 [M] | [72] | |
| Ptychantol A (132) | Ptychanthus strictus | 39.67 | 342 | HRMS m/z (422.15) | [73] | |
| Ptychantol B (133) | Ptychanthus strictus | 21.08 | 341 | HRMS m/z (438.14) | [73] | |
| Ptychantol C (134) | Ptychanthus strictus | 1.95 | 342 | HRMS m/z (438.14) | [73] | |
| Dibenzofuran bisbibenzyl | Asterellin A (88) | Asterella angusta | 0.4 | 212 in MeOH | 421 [M-H]−, 422.1514 [M]+ | [72] |
| Asterellin B (89) | Asterella angusta | 0.6 | na | 435 [M-H]−, 436.1655 [M]+ | [72] | |
| Chlorinated bisbibenzyl | Bazzanin A (114) | Bazzania trilobata | 0.38 | 250 | 456 [M]+ | [74] |
| Bazzanin B (115) | Bazzania trilobata | 2.50 | 255 | 490.0728 [M]+ | [74] | |
| Bazzanin B (115) | Bazzania trilobata | 0.44 | na | na | [75] | |
| Bazzanin C (116) | Bazzania trilobata | 1.13 | 252 | 524.0345 [M]+ | [74] | |
| Bazzanin D (117) | Bazzania trilobata | 0.93 | 250 | 524.0344 [M]+ | [74] | |
| Bazzaninin E (118) | Bazzania trilobata | 1.07 | 252 | 557.9968 [M]+ | [74] | |
| Bazzanin F (119) | Bazzania trilobata | 0.21 | 255 | 558 [M]+ | [74] | |
| Bazzanin G (120) | Bazzania trilobata | 0.65 | 255 | 592 [M]+ | [74] | |
| Bazzanin H (121) | Bazzania trilobata | 0.21 | 252 | 592 [M]+ | [74] | |
| Bazzanin I (122) | Bazzania trilobata | 0.26 | 252 | 626 [M]+ | [74] | |
| Bazzanin J (123) | Bazzania trilobata | 0.51 | 252 | 492.0898 [M]+ | [74] | |
| Bazzanin K (124) | Bazzania trilobata | 0.67 | 246 | 502 [M]+ | [74] | |
| Bazzanin S (131) | Bazzania trilobata | 2.33 | na | na | [75] | |
| Bisprenylated bisbibenzyl | Glossophylin (31) | Lethocolea glossophyla | 20 | 320 | 729 [M+H]+ | [57] |
| Derivative of bisbibenzyl with sesquiterpene moiety | Glaucescenolide (137) | Schistochila glaucescens | 44.89 | 217 in EtOH | 250.1576 [M]+ | [49] |
| GBBA b (138) | Schistochila glaucescens | 12.24 | 273 in EtOH | 679.3044 [M]+ | [49] | |
| GBBB b (139) | Schistochila glaucescens | 10.20 | 271 in EtOH | 679.3022 [M]+ | [49] |
3. The Occurrence and Diversity of Bibenzyls and Bisbibenzyls in Marchantiophyta
3.1. Diversity of Bibenzyls and Prebibenzyls
3.2. Diversity of Macrocyclic and Acyclic Bisbibenzyls
3.2.1. Marchantins, Isomarchantins and Their Derivatives
3.2.2. Bisbibenzyls of Riccardin Family and Derivatives
3.2.3. Pusilatins-Derivatives of Riccardin
3.2.4. Paleatins
3.2.5. Plagiochin Type Bisbibenzyl
3.2.6. Halogenated Bisbibenzyls
3.2.7. Ptychantols
3.2.8. Polymorphatins
3.2.9. Glaucescenolide and Bisbibenzyl Glaucescenolides (GBB)
4. Structure–Activity Relationship of Bisbibenzyls and Bibenzyls
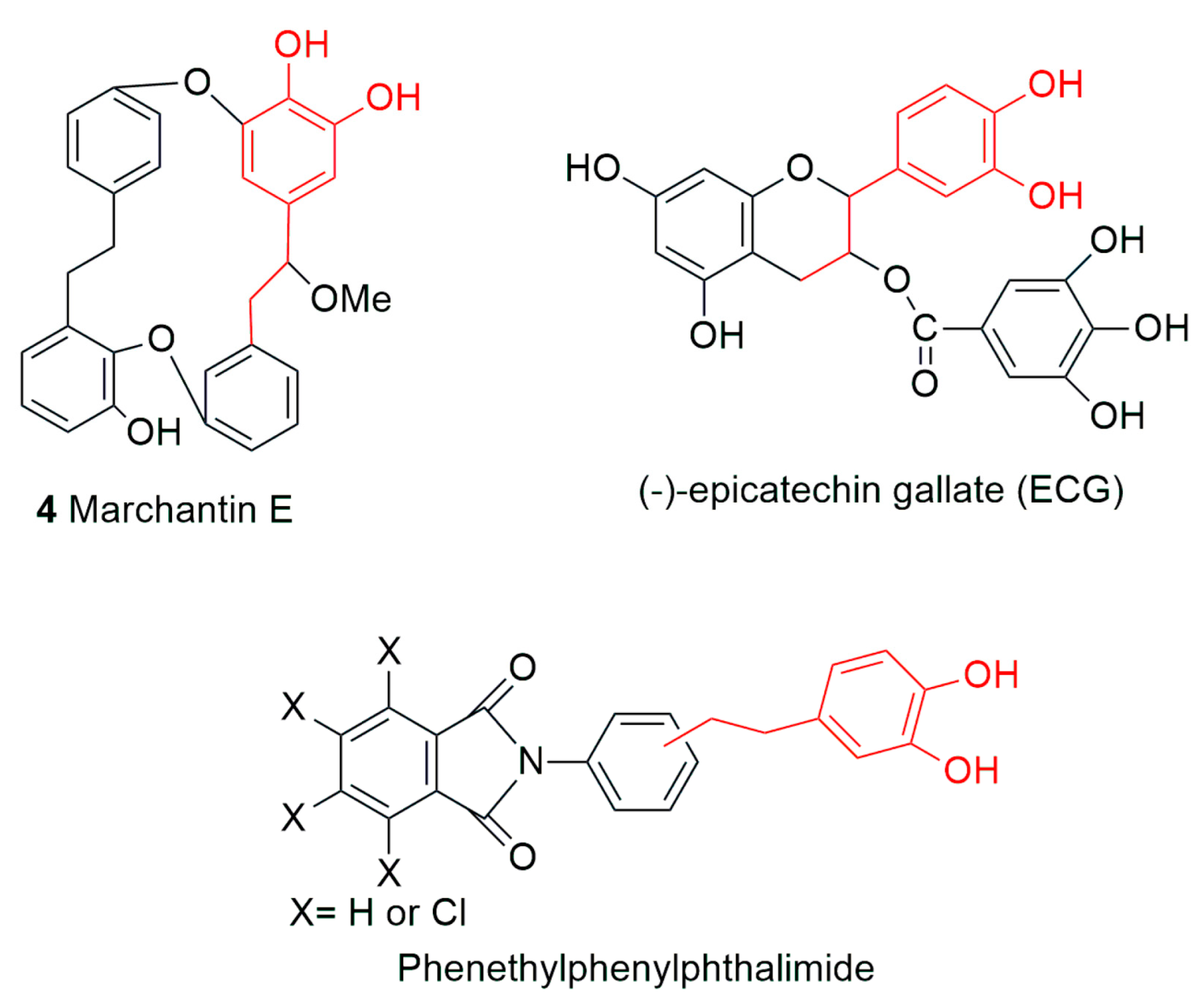



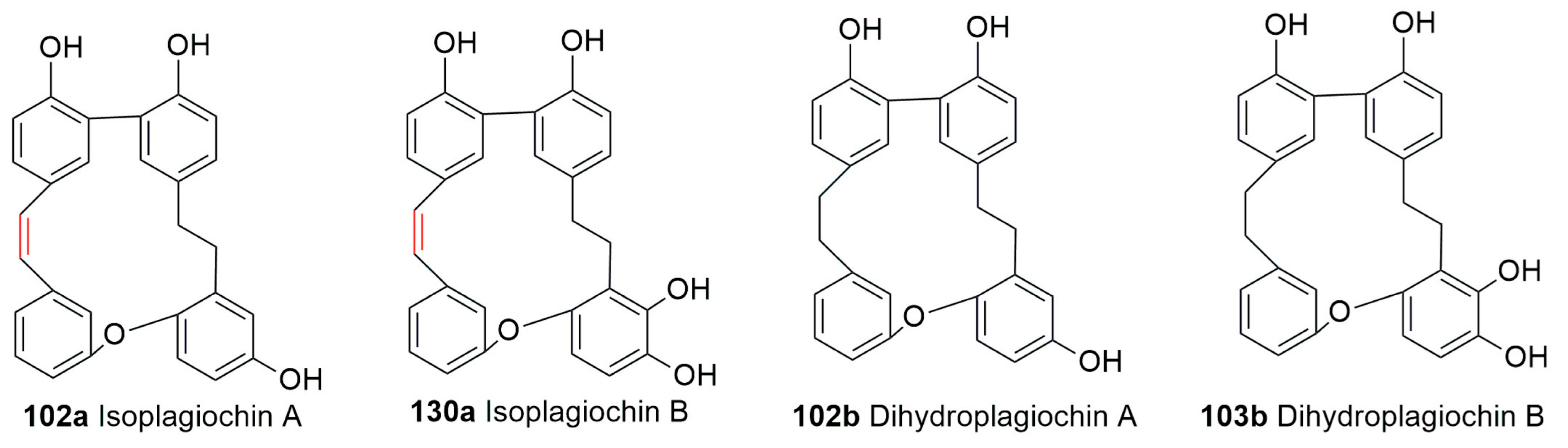
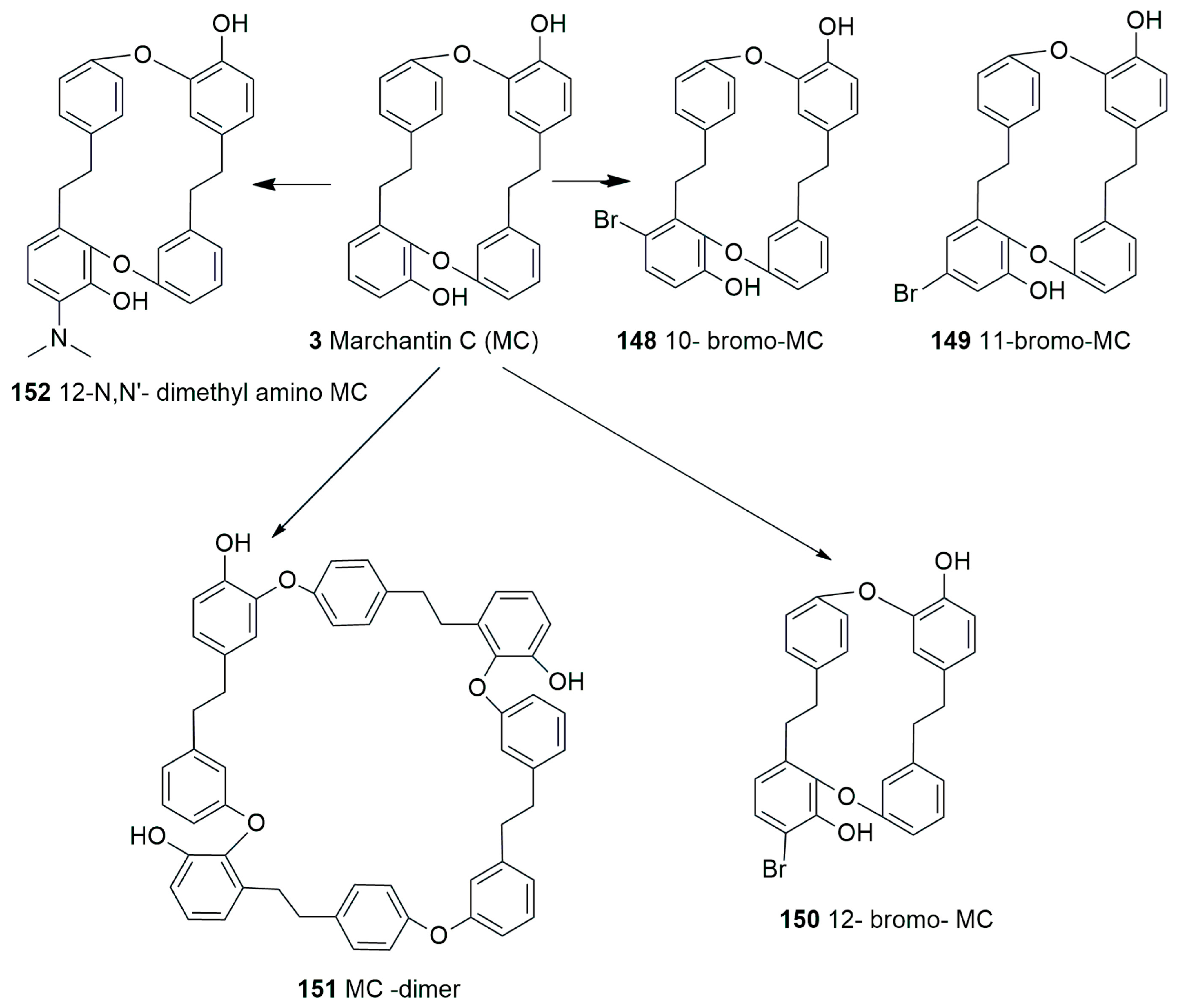
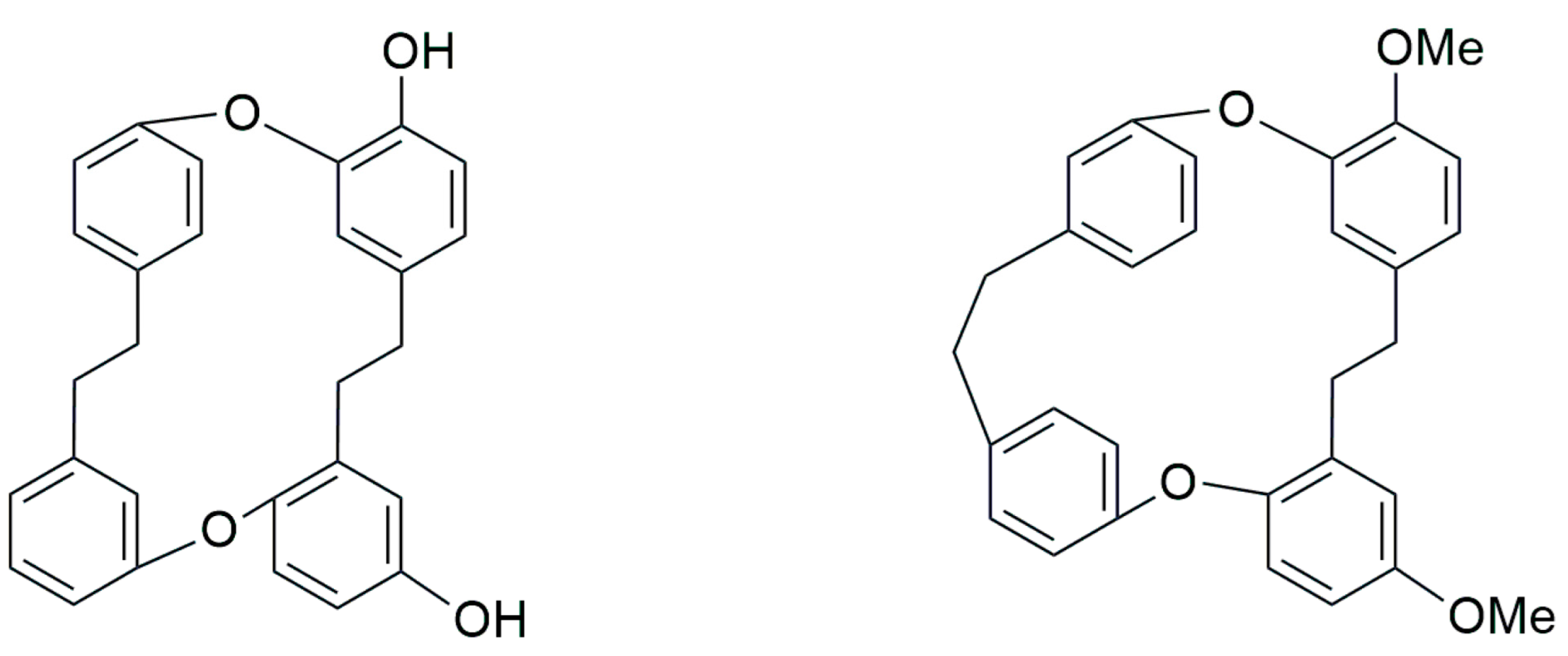
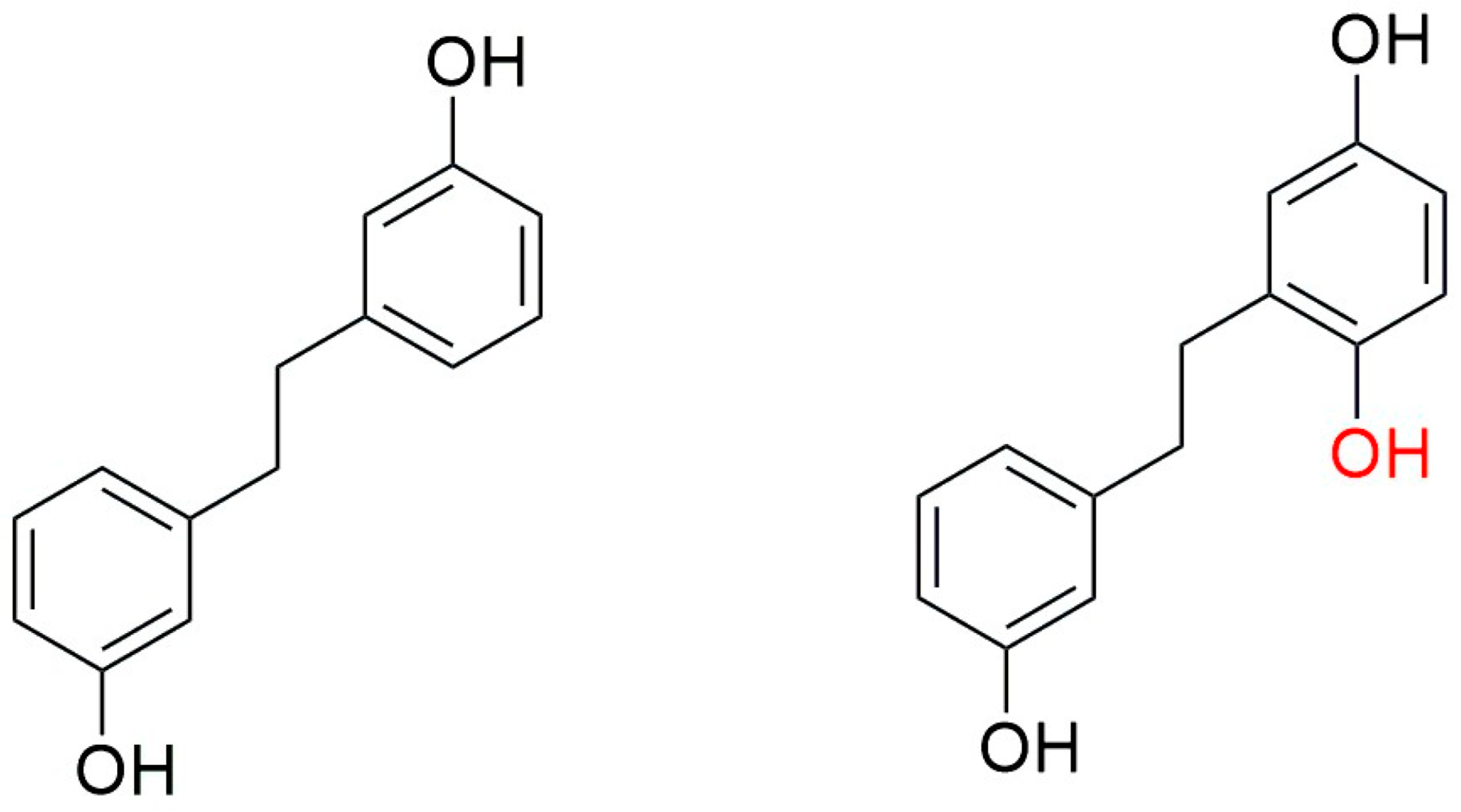
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A549 | human non-small-cell lung carcinoma cells |
| CHOP | CHOP is the acronym for achemotherapy regimen used in the treatment of non-Hodgkin lymphoma. |
| DPPH-2 | 2-diphenylpicrylhydrazyl assay |
| GRP78 | Glucose-regulated protein |
| HAT | Human African trypanosomiasis |
| JA | Jasmonic acid |
| LA | Lunularic acid |
| MATE | marchantin A trimethyl ether. |
| MDR | multi drug resistance |
| MIC | minimal inhibitory concentration |
| MRC5 | human lung fibroblast cells |
| Mp | Marchantia polymorpha |
| NDGA | Nordihydroguaiaretic acid |
References
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into Land Plant Evolution Garnered from the Marchantia Polymorpha Genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef] [PubMed]
- Renault, H.; Alber, A.; Horst, N.A.; Basilio Lopes, A.; Fich, E.A.; Kriegshauser, L.; Wiedemann, G.; Ullmann, P.; Herrgott, L.; Erhardt, M.; et al. A Phenol-Enriched Cuticle Is Ancestral to Lignin Evolution in Land Plants. Nat. Commun. 2017, 8, 14713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, X.-X.; Li, R.-Q.; Zhao, X.; Liu, Y.; Li, M.-H.; Zwaenepoel, A.; Ma, H.; Goffinet, B.; Guan, Y.-L. The Hornwort Genome and Early Land Plant Evolution. Nat. Plants 2020, 6, 107–118. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The Number of Known Plants Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-Lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Wellman, C.H.; Osterloff, P.L.; Mohiuddin, U. Fragments of the Earliest Land Plants. Nature 2003, 425, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Renzaglia, K.; Aguilar, J.C.V.; Garbary, D. Morphology Supports the Setaphyte Hypothesis: Mosses plus Liverworts Form a Natural Group. Bryophyt. Divers. Evol. 2018, 40, 11–17. [Google Scholar] [CrossRef]
- Perera-Castro, A.V.; Waterman, M.J.; Robinson, S.A.; Flexas, J. Limitations to Photosynthesis in Bryophytes: Certainties and Uncertainties Regarding Methodology. J. Exp. Bot. 2022, 73, 4592–4604. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Jibran, R.; van Klink, J.W.; Zhou, Y.; Brummell, D.A.; Albert, N.W.; Schwinn, K.E.; Chagné, D.; Landi, M.; Bowman, J.L.; et al. Stress, Senescence, and Specialized Metabolites in Bryophytes. J. Exp. Bot. 2022, 73, 4396–4411. [Google Scholar] [CrossRef]
- Xie, C.-F.; Lou, H.-X. Secondary Metabolites in Bryophytes: An Ecological Aspect. Chem. Biodivers. 2009, 6, 303–312. [Google Scholar] [CrossRef]
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The Evolution of Flavonoid Biosynthesis: A Bryophyte Perspective. Front. Plant Sci. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Sun, L.; Wu, X.; Lou, H. The Inhibitory Effect of a Macrocyclic Bisbibenzyl Riccardin D on the Biofilms of Candida albicans. Biol. Pharm. Bull. 2009, 32, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, D. Mechanisms Underlying Freezing and Desiccation Tolerance in Bryophytes. In Survival Strategies in Extreme Cold and Desiccation: Adaptation Mechanisms and Their Applications; Springer: Singapore, 2018; pp. 167–187. [Google Scholar] [CrossRef]
- Peters, K.; Treutler, H.; Döll, S.; Kindt, A.S.D.; Hankemeier, T.; Neumann, S. Chemical Diversity and Classification of Secondary Metabolites in Nine Bryophyte Species. Metabolites 2019, 9, 222. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Novakovic, M.; Bukvicki, D.; Anchang, K.Y. Bis-Bibenzyls, Bibenzyls, and Terpenoids in 33 Genera of the Marchantiophyta (Liverworts): Structures, Synthesis, and Bioactivity. J. Nat. Prod. 2022, 85, 729–762. [Google Scholar] [CrossRef] [PubMed]
- Dziwak, M.; Wróblewska, K.; Szumny, A.; Galek, R. Modern Use of Bryophytes as a Source of Secondary Metabolites. Agronomy 2022, 12, 1456. [Google Scholar] [CrossRef]
- Horn, A.; Pascal, A.; Lončarević, I.; Volpatto Marques, R.; Lu, Y.; Miguel, S.; Bourgaud, F.; Thorsteinsdóttir, M.; Cronberg, N.; Becker, J.D.; et al. Natural Products from Bryophytes: From Basic Biology to Biotechnological Applications. Crit. Rev. Plant Sci. 2021, 40, 191–217. [Google Scholar] [CrossRef]
- Asakawa, Y. Chemical Constituents of the Bryophytes. In Progress in the Chemistry of Organic Natural Products; Asakawa, Y., Ed.; Fortschritte der Chemie Organischer Naturstoffe; Springer: Vienna, Austria, 1995; pp. 1–562. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Chemical Constituents of Bryophyta. In Chemical Constituents of Bryophytes: Bio- and Chemical Diversity, Biological Activity, and Chemosystematics; Asakawa, Y., Ludwiczuk, A., Nagashima, F., Eds.; Springer: Vienna, Austria, 2013; pp. 563–605. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y. The Isolation, Structure Elucidation, and Bio- and Total Synthesis of Bis-Bibenzyls, from Liverworts and Their Biological Activity. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Dey, A.; De, J.N. Antifungal Bryophytes: A Possible Role against Human Pathogens and in Plant Protection. Res. J. Bot. 2011, 6, 129–140. [Google Scholar] [CrossRef]
- Dey, A.; Mukherjee, A. Therapeutic Potential of Bryophytes and Derived Compounds against Cancer. J. Acute Dis. 2015, 4, 236–248. [Google Scholar] [CrossRef]
- Nandy, S.; Dey, A. Bibenzyls and Bisbybenzyls of Bryophytic Origin as Promising Source of Novel Therapeutics: Pharmacology, Synthesis and Structure-Activity. DARU J. Pharm. Sci. 2020, 28, 701–734. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Dey, A. The Ethno-Medicinal and Pharmaceutical Attributes of Bryophytes: A Review. Phytomed. Plus 2022, 2, 100255. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, D.; Barh, A.; Pankaj; Pandey, R.K.; Sharma, I.P. Bryophytes: Hoard of Remedies, an Ethno-Medicinal Review. J. Tradit. Complement. Med. 2017, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Palma, A.D.; de Falco, B. Bryophytes Used in Folk Medicine: An Ethnobotanical Overview. Horticulturae 2023, 9, 137. [Google Scholar] [CrossRef]
- Harrowven, D.C.; Kostiuk, S.L. Macrocylic Bisbibenzyl Natural Products and Their Chemical Synthesis. Nat. Prod. Rep. 2012, 29, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Song, C. Macrocyclic Bisbibenzyls: Properties and Synthesis. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 55, pp. 73–110. [Google Scholar] [CrossRef]
- Asakawa, Y.; Toyota, M.; Matsuda, R.; Takikawa, K.; Takemoto, T. Distribution of Novel Cyclic Bisbibenzyls in Marchantia and Riccardia Species. Phytochemistry 1983, 22, 1413–1415. [Google Scholar] [CrossRef]
- Tori, M.; Toyota, M.; Harrison, L.J.; Takikawa, K.; Asakawa, Y. Total Assignment of 1H and 13C NMR Spectra of Marchantins Isolated from Liverworts and Its Application to Structure Determination of Two New Macrocyclic Bis(Bibenzyls) from Plagiochasma intermedium and Riccardia multifida. Tetrahedron Lett. 1985, 26, 4735–4738. [Google Scholar] [CrossRef]
- Huang, W.-J.; Wu, C.-L.; Lin, C.-W.; Chi, L.-L.; Chen, P.-Y.; Chiu, C.-J.; Huang, C.-Y.; Chen, C.-N. Marchantin A, a Cyclic Bis(Bibenzyl Ether), Isolated from the Liverwort Marchantia emarginata Subsp. tosana Induces Apoptosis in Human MCF-7 Breast Cancer Cells. Cancer Lett. 2010, 291, 108–119. [Google Scholar] [CrossRef]
- Niu, C.; Qu, J.-B.; Lou, H.-X. Antifungal Bis [Bibenzyls] from the Chinese Liverwort Marchantia polymorpha L. Chem. Biodivers. 2006, 3, 34–40. [Google Scholar] [CrossRef]
- Lu, Z.-Q.; Fan, P.-H.; Ji, M.; Lou, H.-X. Terpenoids and Bisbibenzyls from Chinese Liverworts Conocephalum conicum and Dumortiera hirsuta. J. Asian Nat. Prod. Res. 2006, 8, 187–192. [Google Scholar] [CrossRef]
- Liu, N.; Guo, D.-X.; Wang, Y.-Y.; Wang, L.-N.; Ji, M.; Lou, H.-X. Aromatic Compounds from the Liverwort Conocephalum japonicum. Nat. Prod. Commun. 2011, 6, 49–52. [Google Scholar] [CrossRef]
- Xing, J.; Xie, C.; Qu, J.; Guo, H.; Lv, B.; Lou, H. Rapid Screening for Bisbibenzyls in Bryophyte Crude Extracts Using Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2467–2476. [Google Scholar] [CrossRef]
- Lorenzo Tejedor, M.; Mizuno, H.; Tsuyama, N.; Harada, T.; Masujima, T. In Situ Molecular Analysis of Plant Tissues by Live Single-Cell Mass Spectrometry. Anal. Chem. 2012, 84, 5221–5228. [Google Scholar] [CrossRef]
- Fujii, T.; Matsuda, S.; Tejedor, M.L.; Esaki, T.; Sakane, I.; Mizuno, H.; Tsuyama, N.; Masujima, T. Direct Metabolomics for Plant Cells by Live Single-Cell Mass Spectrometry. Nat. Protoc. 2015, 10, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Esaki, T.; Kenmoku, H.; Koeduka, T.; Kiyoyama, Y.; Masujima, T.; Asakawa, Y.; Matsui, K. Direct Evidence of Specific Localization of Sesquiterpenes and Marchantin A in Oil Body Cells of Marchantia polymorpha L. Phytochemistry 2016, 130, 77–84. [Google Scholar] [CrossRef]
- Asakawa, Y.; Takikawa, K.; Toyota, M.; Takemoto, T. Novel Bibenzyl Derivatives and Ent-Cuparene-Type Sesquiterpenoids from Radula Species. Phytochemistry 1982, 21, 2481–2490. [Google Scholar] [CrossRef]
- Asakawa, Y.; Kondo, K.; Takikawa, N.K.; Tori, M.; Hashimoto, T.; Ogawa, S. Prenyl Bibenzyls from the Liverwort Radula kojana. Phytochemistry 1991, 30, 219–234. [Google Scholar] [CrossRef]
- Asakawa, Y.; Toyota, M.; Taira, Z.; Takemoto, T.; Kido, M. Riccardin A and Riccardin B, Two Novel Cyclic Bis (Bibenzyls) Possessing Cytotoxicity from the Liverwort Riccardia multifida (L.) S. Gray. J. Org. Chem. 1983, 48, 2164–2167. [Google Scholar] [CrossRef]
- Nagashima, F.; Momosaki, S.; Watanabe, Y.; Toyota, M.; Huneck, S.; Asakawa, Y. Terpenoids and Aromatic Compounds from Six Liverworts. Phytochemistry 1996, 41, 207–211. [Google Scholar] [CrossRef]
- Cullmann, F.; Becker, H.; Pandolfi, E.; Roeckner, E.; Eicher, T. Bibenzyl Derivatives from Pellia epiphylla. Phytochemistry 1997, 45, 1235–1247. [Google Scholar] [CrossRef]
- Cullmann, F.; Becker, H. Prenylated Bibenzyls from the Liverwort Radula laxiramea. Z. Für Naturforschung C 1999, 54, 147–150. [Google Scholar] [CrossRef]
- Toyota, M.; Shimamura, T.; Ishii, H.; Renner, M.; Braggins, J.; Asakawa, Y. New Bibenzyl Cannabinoid from the New Zealand Liverwort Radula marginata. Chem. Pharm. Bull. 2002, 50, 1390–1392. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Murakami, K.; Gomi, Y.; Hashimoto, T.; Asakawa, Y.; Okuno, Y.; Ishikawa, T.; Hatakeyama, D.; Echigo, N.; Kuzuhara, T. Anti-Influenza Activity of Marchantins, Macrocyclic Bisbibenzyls Contained in Liverworts. PLoS ONE 2011, 6, e19825. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Tamehiro, N.; Sato, Y.; Kobayashi, T.; Ishii-Watabe, A.; Shinozaki, Y.; Nishimaki-Mogami, T.; Hashimoto, T.; Asakawa, Y.; Inoue, K.; et al. The Novel Compounds That Activate Farnesoid X Receptor: The Diversity of Their Effects on Gene Expression. J. Pharmacol. Sci. 2008, 107, 285–294. [Google Scholar] [CrossRef]
- Scher, J.M.; Burgess, E.J.; Lorimer, S.D.; Perry, N.B. A Cytotoxic Sesquiterpene and Unprecedented Sesquiterpene-Bisbibenzyl Compounds from the Liverwort Schistochila glaucescens. Tetrahedron 2002, 58, 7875–7882. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, X.; Ni, R.; Cheng, A.; Hongxiang, L. Tissue Culture of Plagiochasma appendiculatum and the Effect of Callus Differentiation on Types and Content of Bisbibenzyls. Nat. Prod. Commun. 2022, 17, 1934578X2211062. [Google Scholar] [CrossRef]
- Xie, C.-F.; Qu, J.-B.; Sun, B.; Guo, H.-F.; Lou, H.-X. Dumhirone A, an Unusual Phenylethyl Cyclohexadienone from the Chinese Liverwort Dumortiera hirsuta. Biochem. Syst. Ecol. 2007, 35, 162–165. [Google Scholar] [CrossRef]
- Kunz, S.; Becker, H. Bibenzyl Derivatives from the Liverwort Ricciocarpos natans. Phytochemistry 1994, 36, 675–677. [Google Scholar] [CrossRef]
- Yoshida, T.; Hashimoto, T.; Takaoka, S.; Kan, Y.; Tori, M.; Asakawa, Y.; Pezzuto, J.M.; Pengsuparp, T.; Cordell, G.A. Phenolic Constituents of the Liverwort: Four Novel Cyclic Bisbibenzyl Dimers from Blasia pusilla L. Tetrahedron 1996, 52, 14487–14500. [Google Scholar] [CrossRef]
- Asakawa, Y.; Tanikawa, K.; Aratani, T. New Substituted Bibenzyls of Frullania brittoniae Subsp. truncatifolia. Phytochemistry 1976, 15, 1057–1059. [Google Scholar] [CrossRef]
- Guo, D.-X.; Xiang, F.; Wang, X.-N.; Yuan, H.-Q.; Xi, G.-M.; Wang, Y.-Y.; Yu, W.-T.; Lou, H.-X. Labdane Diterpenoids and Highly Methoxylated Bibenzyls from the Liverwort Frullania inouei. Phytochemistry 2010, 71, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Hashimoto, T.; Takikawa, K.; Tori, M.; Ogawa, S. Prenyl Bibenzyls from the Liverworts Radula perrottetii and Radula complanata. Phytochemistry 1991, 30, 235–251. [Google Scholar] [CrossRef]
- Kraut, L.; Mues, R.; Zinsmeister, H.D. Prenylated Bibenzyl Derivatives from Lethocolea glossophylla and Radula voluta. Phytochemistry 1997, 45, 1249–1255, Erratum in Phytochemistry 2006, 12, 1297. [Google Scholar] [CrossRef]
- Zheng, G.-Q.; Ho, D.K.; Elder, P.J.; Stephens, R.E.; Cottrell, C.E.; Cassady, J.M. Ohioensins and Pallidisetins: Novel Cytotoxic Agents from the Moss Polytrichum pallidisetum. J. Nat. Prod. 1994, 57, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Flegel, M.; Becker, H. Characterization of the Contents of Oil Bodies from the Liverwort Radula complanata1. Plant Biol. 2000, 2, 208–210. [Google Scholar] [CrossRef]
- Xie, C.-F.; Qu, J.-B.; Wu, X.-Z.; Liu, N.; Ji, M.; Lou, H.-X. Antifungal Macrocyclic Bis(Bibenzyls) from the Chinese Liverwort Plagiochasma intermedium L. Nat. Prod. Res. 2010, 24, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Tori, M.; Takikawa, K.; Krishnamurty, H.; Kar, S.K. Cyclic Bis (Bibenzyls) and Related Compounds from the Liverworts Marchantia polymorpha and Marchantia palmata. Phytochemistry 1987, 26, 1811–1816. [Google Scholar] [CrossRef]
- Toyota, M.; Nagashima, F.; Asakawa, Y. Fatty Acids and Cyclic Bis(Bibenzyls) from the New Zealand Liverwort Monoclea forsteri. Phytochemistry 1988, 27, 2603–2608. [Google Scholar] [CrossRef]
- So, M.-L.; Chan, W.-H.; Xia, P.-F.; Cui, Y. Two New Cyclic Bis (Bibenzyl) s, Isoriccardinquinone A and B from the Liverwort Marchantia paleacea. Nat. Prod. Lett. 2002, 16, 167–171. [Google Scholar] [CrossRef]
- Fang, L.; Guo, H.-F.; Lou, H.-X. Three New Bibenzyl Derivatives from the Chinese Liverwort Marchantia Polymorpha L. Helv. Chim. Acta 2007, 90, 748–752. [Google Scholar] [CrossRef]
- Qu, J.-B.; Sun, L.-M.; Lou, H.-X. Antifungal Variant Bis(Bibenzyl)s from the Liverwort Asterella angusta. Chin. Chem. Lett. 2013, 24, 801–803. [Google Scholar] [CrossRef]
- Wei, H.-C.; Ma, S.-J.; Wu, C.-L. Sesquiterpenoids and Cyclic Bisbibenzyls from the Liverwort Reboulia hemisphaerica. Phytochemistry 1995, 39, 91–97. [Google Scholar] [CrossRef]
- Anton, H.; Kraut, L.; Mues, R.; Morales Maria, I.Z. Phenanthrenes and Bibenzyls from a Plagiochila Species. Phytochemistry 1997, 46, 1069–1075. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kanayama, S.; Kan, Y.; Tori, M.; Asakawa, Y. Isoplagiochins C and D, New Type of Macrocyclic Bis (Bibenzyls), Having Two Biphenyl Linkages from the Liverwort Plagiochila fruticosa. Chem. Lett. 1996, 25, 741–742. [Google Scholar] [CrossRef]
- Nabeta, K.; Ohkubo, S.; Hozumi, R.; Katoh, K. Macrocyclic Bisbibenzyls in Cultured Cells of the Liverwort, Heteroscyphus planus. Phytochemistry 1998, 49, 1941–1943. [Google Scholar] [CrossRef]
- Hashimoto, T.; Irita, H.; Takaoka, S.; Tanaka, M.; Asakawa, Y. New Chlorinated Cyclic Bis(Bibenzyls) from the Liverworts Herbertus sakuraii and Mastigophora diclados. Tetrahedron 2000, 56, 3153–3159. [Google Scholar] [CrossRef]
- Toyota, M.; Kinugawa, T.; Asakawa, Y. Bibenzyl Cannabinoid and Bisbibenzyl Derivative from the Liverwort Radula perrottetii. Phytochemistry 1994, 37, 859–862. [Google Scholar] [CrossRef]
- Qu, J.; Xie, C.; Guo, H.; Yu, W.; Lou, H. Antifungal Dibenzofuran Bis(Bibenzyl)s from the Liverwort Asterella angusta. Phytochemistry 2007, 68, 1767–1774. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ikeda, H.; Takaoka, S.; Tanaka, M.; Asakawa, Y. Ptychantols A–C, Macrocyclic Bis(Bibenzyls), Possessing a Trans-Stilbene Structure from the Liverwort Ptychanthus striatus. Phytochemistry 1999, 52, 501–509. [Google Scholar] [CrossRef]
- Martini, U.; Zapp, J.; Becker, H. Chlorinated Macrocyclic Bisbibenzyls from the Liverwort Bazzania trilobata. Phytochemistry 1998, 47, 89–96. [Google Scholar] [CrossRef]
- Scher, J.; Speakman, J.; Zapp, J.; Becker, H. Bioactivity Guided Isolation of Antifungal Compounds from the Liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry 2004, 65, 2583–2588. [Google Scholar] [CrossRef]
- Kosenkova, Y.S.; Polovinka, M.P.; Komarova, N.I.; Korchagina, D.V.; Kurochkina, N.Y.; Cheremushkina, V.A.; Salakhutdinov, N.F. Riccardin C, a Bisbibenzyl Compound from Primula macrocalyx. Chem. Nat. Compd. 2007, 43, 712–713. [Google Scholar] [CrossRef]
- Oiso, Y.; Toyota, M.; Asakawa, Y. Occurrence of a Bis-Bibenzyl Derivative in the Japanese Fern Hymenophyllum barbatum: First Isolation and Identification of Perrottetin H from the Pteridophytes. ChemInform 2010, 30. [Google Scholar] [CrossRef]
- Heinrichs, J.; Anton, H.; Gradstein, S.R.; Mues, R. Systematics of Plagiochila Sect. glaucescentes Carl (Hepaticae) from Tropical America: A Morphological and Chemotaxonomical Approach. Plant Syst. Evol. 2000, 220, 115–138. [Google Scholar] [CrossRef]
- Asakawa, Y.; Nagashima, F.; Ludwiczuk, A. Distribution of Bibenzyls, Prenyl Bibenzyls, Bis-Bibenzyls, and Terpenoids in the Liverwort Genus Radula. J. Nat. Prod. 2020, 83, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Espley, R.; Gertsch, J.; Whare, T.; Stehle, F.; Kayser, O. Demystifying the Liverwort Radula marginata, a Critical Review on Its Taxonomy, Genetics, Cannabinoid Phytochemistry and Pharmacology. Phytochem. Rev. 2019, 18, 953–965. [Google Scholar] [CrossRef]
- Asakawa, Y. Liverworts-Potential Source of Medicinal Compounds. Curr. Pharm. Des. 2008, 14, 3067–3088. [Google Scholar] [CrossRef]
- Sawyer, J.S.; Schmittling, E.A.; Palkowitz, J.A.; Smith, W.J. Synthesis of Diaryl Ethers, Diaryl Thioethers, and Diarylamines Mediated by Potassium Fluoride−Alumina and 18-Crown-6: Expansion of Scope and Utility. J. Org. Chem. 1998, 63, 6338–6343. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Kodama, M.; Shiobara, Y.; Sumitomo, H.; Matsumura, K.; Tsukamoto, M.; Harada, C. Total Syntheses of Marchantin A and Riccardin B, Cytotoxic Bis(Bibenzyls) from Liverworts. J. Org. Chem. 1988, 53, 72–77. [Google Scholar] [CrossRef]
- Kámory, E.; Keserü, G.M.; Papp, B. Isolation and Antibacterial Activity of Marchantin A, a Cyclic Bis(Bibenzyl) Constituent of Hungarian Marchantia polymorpha. Planta Med. 1995, 61, 387–388. [Google Scholar] [CrossRef]
- Asakawa, Y.; Tori, M.; Masuya, T.; Frahm, J.P. Ent Sesquiterpenoids and Cyclic Bisbibenzyls from the German Liverwort Marchantia polymorpha. Phytochemistry 1990, 29, 1577–1584. [Google Scholar] [CrossRef]
- Tori, M.; Aoki, M.; Asakawa, Y. Chenopodene, Marchantin P and Riccardin G from the Liverwort Marchantia chenopoda. Phytochemistry 1994, 36, 73–76. [Google Scholar] [CrossRef]
- Asakawa, Y.; Matsuda, R. Riccardin C, a Novel Cyclic Bibenzyl Derivative from Reboulia hemisphaerica. Phytochemistry 1982, 21, 2143–2144. [Google Scholar] [CrossRef]
- Gottsegen, Á.; Nógrádi, M.; Vermes, B.; Kajtár-Peredy, M.; Bihátsi-Karsai, É. Total Syntheses of Riccardins A, B, and C, Cytotoxic Macrocyclic Bis (Bibenzyls) from Liverworts. J. Chem. Soc. Perkin Trans. 1990, 1, 315–320. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tori, M.; Asakawa, Y.; Fukazawa, Y. Plagiochins A, B, C, and D, New Type of Macrocyclic Bis(Bibenzyls) Having a Biphenyl Linkage between the Ortho Positions to the Benzyl Methylenes, from the Liverwort Plagiochila acanthophylla Subsp. Japonica. Tetrahedron Lett. 1987, 28, 6295–6298. [Google Scholar] [CrossRef]
- Speicher, A.; Groh, M.; Zapp, J.; Schaumlöffel, A.; Knauer, M.; Bringmann, G. A Synthesis-Driven Structure Revision of ‘Plagiochin E’, a Highly Bioactive Bisbibenzyl. Synlett 2009, 2009, 1852–1858. [Google Scholar] [CrossRef]
- Keserü, G.M.; Nógrádi, M. Biosynthesis and Molecular Strain. A Computational Study on the Conformation of Cyclic Bis(Bibenzyl) Constituents of Liverwort Species. Phytochemistry 1992, 31, 1573–1576. [Google Scholar] [CrossRef]
- Musman, M.; Tanaka, J.; Higa, T. New Sesquiterpene Carbonimidic Dichlorides and Related Compounds from the Sponge Stylotella aurantium. J. Nat. Prod. 2001, 64, 111–113. [Google Scholar] [CrossRef]
- Banerjee, R.D.; Sen, S.P. Antibiotic Activity of Bryophytes. Bryophytes 1979, 82, 141–153. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Hussein, Y.; Abou Sabaa, G.; Abdel-Monaem, A.-S. An Evaluation of the Antibacterial and Antiviral Activities of Some Bryophytes. Egypt. J. Microbiol. 2017, 52, 63–86. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, S.; Sharma, R.; Vats, S.; Alam, A. Gas Chromatography-Mass Spectrometry (GC–MS) Profiling of Aqueous Methanol Fraction of Plagiochasma appendiculatum Lehm. & Lindenb. and Sphagnum fimbriatum Wilson for Probable Antiviral Potential. Vegetos 2023, 36, 87–92. [Google Scholar] [CrossRef]
- Commisso, M.; Guarino, F.; Marchi, L.; Muto, A.; Piro, A.; Degola, F. Bryo-Activities: A Review on How Bryophytes Are Contributing to the Arsenal of Natural Bioactive Compounds against Fungi. Plants 2021, 10, 203. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, A.; Sun, L.; Lou, H. Effect of Plagiochin E, an Antifungal Macrocyclic Bis(Bibenzyl), on Cell Wall Chitin Synthesis in Candida albicans. Acta Pharmacol. Sin. 2009, 29, 1478–1485. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, C.; Yuan, H.; Li, B.; Gao, J.; Qu, X.; Sun, B.; Cheng, Y.; Li, S.; Li, X.; et al. Marchantin C, a Novel Microtubule Inhibitor from Liverwort with Anti-Tumor Activity Both In Vivo and In Vitro. Cancer Lett. 2009, 276, 160–170. [Google Scholar] [CrossRef]
- Dey, A.; De, J. Antioxidative Potential of Bryophytes: Stress Tolerance and Commercial Perspectives: A Review. Pharmacologia 2012, 3, 151–159. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Lobo-Echeverri, T.; Rojano, B.; Vargas, L.; Fernandez, M.; Gaviria, C.A.; Núñez, V. Correlation of the Inhibitory Activity of Phospholipase A2 Snake Venom and the Antioxidant Activity of Colombian Plant Extracts. Rev. Bras. Farmacogn. 2010, 20, 910–916. [Google Scholar] [CrossRef]
- Otoguro, K.; Ishiyama, A.; Iwatsuki, M.; Namatame, M.; Nishihara-Tukashima, A.; Kiyohara, H.; Hashimoto, T.; Asakawa, Y.; Ōmura, S.; Yamada, H. In Vitro Antitrypanosomal Activity of Bis(Bibenzyls)s and Bibenzyls from Liverworts against Trypanosoma brucei. J. Nat. Med. 2012, 66, 377–382. [Google Scholar] [CrossRef]
- Taira, Z.; Takei, M.; Endo, K.; Hashimoto, T.; Sakiya, Y.; Asakawa, Y. Marchantin A Trimethyl Ether: Its Molecular Structure and Tubocurarine-like Skeletal Muscle Relaxation Activity. Chem. Pharm. Bull. 1994, 42, 52–56. [Google Scholar] [CrossRef]
- Hara, K.; Schmidt, F.I.; Crow, M.; Brownlee, G.G. Amino Acid Residues in the N-Terminal Region of the PA Subunit of Influenza A Virus RNA Polymerase Play a Critical Role in Protein Stability, Endonuclease Activity, Cap Binding, and Virion RNA Promoter Binding. J. Virol. 2006, 80, 7789–7798. [Google Scholar] [CrossRef]
- Iwai, Y.; Takahashi, H.; Hatakeyama, D.; Motoshima, K.; Ishikawa, M.; Sugita, K.; Hashimoto, Y.; Harada, Y.; Itamura, S.; Odagiri, T. Anti-Influenza Activity of Phenethylphenylphthalimide Analogs Derived from Thalidomide. Bioorg. Med. Chem. 2010, 18, 5379–5390. [Google Scholar] [CrossRef]
- DuBois, R.M.; Slavish, P.J.; Baughman, B.M.; Yun, M.-K.; Bao, J.; Webby, R.J.; Webb, T.R.; White, S.W. Structural and Biochemical Basis for Development of Influenza Virus Inhibitors Targeting the PA Endonuclease. PLoS Pathog. 2012, 8, e1002830. [Google Scholar] [CrossRef]
- Kowalinski, E.; Zubieta, C.; Wolkerstorfer, A.; Szolar, O.H.J.; Ruigrok, R.W.H.; Cusack, S. Structural Analysis of Specific Metal Chelating Inhibitor Binding to the Endonuclease Domain of Influenza pH1N1 Polymerase. PLoS Pathog. 2012, 8, e1002831. [Google Scholar] [CrossRef]
- Stevaert, A.; Dallocchio, R.; Dessì, A.; Pala, N.; Rogolino, D.; Sechi, M.; Naesens, L. Mutational Analysis of the Binding Pockets of the Diketo Acid Inhibitor L-742,001 in the Influenza Virus PA Endonuclease. J. Virol. 2013, 87, 10524–10538. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Quang, D.N.; Takeshi, N.; Hashimoto, T.; Kohchi, C.; Soma, G.-I.; Asakawa, Y. Bis (Bibenzyls) from Liverworts Inhibit Lipopolysaccharide-Induced Inducible NOS in RAW 264.7 Cells: A Study of Structure−Activity Relationships and Molecular Mechanism. J. Nat. Prod. 2005, 68, 1779–1781. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Osika, P.; Asakawa, Y.; Antosiewicz, B.; Głowniak, K.; Ludwiczuk, A. Evaluation of Anti-Melanoma and Tyrosinase Inhibitory Properties of Marchantin A, a Natural Macrocyclic Bisbibenzyl Isolated from Marchantia Species. Phytochem. Lett. 2019, 31, 192–195. [Google Scholar] [CrossRef]
- Jensen, S.; Omarsdottir, S.; Þorsteinsdóttir, J.; Ogmundsdottir, H.; Olafsdottir, E. Synergistic Cytotoxic Effect of the Microtubule Inhibitor Marchantin A from Marchantia polymorpha and the Aurora Kinase Inhibitor MLN8237 on Breast Cancer Cells In Vitro. Planta Medica 2012, 78, 448–454. [Google Scholar] [CrossRef]
- Lorimer, S.D.; Perry, N.B.; Tangney, R.S. An Antifungal Bibenzyl from the New Zealand Liverwort, Plagiochila stephensoniana. Bioactivity-Directed Isolation, Synthesis, and Analysis. J. Nat. Prod. 1993, 56, 1444–1450. [Google Scholar] [CrossRef]
- Lou, H.-X.; Li, G.-Y.; Wang, F.-Q. A Cytotoxic Diterpenoid and Antifungal Phenolic Compounds from Frullania muscicola Steph. J. Asian Nat. Prod. Res. 2002, 4, 87–94. [Google Scholar] [CrossRef]
- Gibbons, S. Anti-Staphylococcal Plant Natural Products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef]
- Guo, X.-L.; Leng, P.; Yang, Y.; Yu, L.-G.; Lou, H.-X. Plagiochin E, a Botanic-Derived Phenolic Compound, Reverses Fungal Resistance to Fluconazole Relating to the Efflux Pump. J. Appl. Microbiol. 2008, 104, 831–838. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Chang, W.-Q.; Cheng, A.-X.; Sun, L.-M.; Lou, H.-X. Plagiochin E, an Antifungal Active Macrocyclic Bis(Bibenzyl), Induced Apoptosis in Candida albicans through a Metacaspase-Dependent Apoptotic Pathway. Biochim. Biophys. Acta 2010, 1800, 439–447. [Google Scholar] [CrossRef]
- Novakovic, M.; Bukvicki, D.; Andjelkovic, B.; Ilic-Tomic, T.; Veljic, M.; Tesevic, V.; Asakawa, Y. Cytotoxic Activity of Riccardin and Perrottetin Derivatives from the Liverwort Lunularia cruciata. J. Nat. Prod. 2019, 82, 694–701. [Google Scholar] [CrossRef]
- Xue, X.; Qu, X.-J.; Gao, Z.-H.; Sun, C.-C.; Liu, H.; Zhao, C.-R.; Cheng, Y.-N.; Lou, H.-X. Riccardin D, a Novel Macrocyclic Bisbibenzyl, Induces Apoptosis of Human Leukemia Cells by Targeting DNA Topoisomerase II. Investig. New Drugs 2012, 30, 212–222. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Xue, X.; Cheng, Y.; Liu, H.; Zhao, C.; Lou, H.; Qu, X. Inhibition of Angiogenesis Involves in Anticancer Activity of Riccardin D, a Macrocyclic Bisbibenzyl, in Human Lung Carcinoma. Eur. J. Pharmacol. 2011, 667, 136–143. [Google Scholar] [CrossRef]
- Liu, H.-P.; Gao, Z.-H.; Cui, S.-X.; Sun, D.-F.; Wang, Y.; Zhao, C.-R.; Lou, H.-X.; Qu, X.-J. Inhibition of Intestinal Adenoma Formation in APCMin/+ Mice by Riccardin D, a Natural Product Derived from Liverwort Plant Dumortiera hirsuta. PLoS ONE 2012, 7, e33243. [Google Scholar] [CrossRef]
- Liu, H.; Li, G.; Zhang, B.; Sun, D.; Wu, J.; Chen, F.; Kong, F.; Luan, Y.; Jiang, W.; Wang, R.; et al. Suppression of the NF-κB Signaling Pathway in Colon Cancer Cells by the Natural Compound Riccardin D from Dumortiera hirsuta. Mol. Med. Rep. 2018, 17, 5837–5843. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Hao, J.; Tian, K.; Wang, W.; Lou, H.; Yuan, H. Induction of DNA Damage and P21-Dependent Senescence by Riccardin D Is a Novel Mechanism Contributing to Its Growth Suppression in Prostate Cancer Cells In Vitro and In Vivo. Cancer Chemother. Pharmacol. 2014, 73, 397–407. [Google Scholar] [CrossRef]
- Yue, B.; Zhao, C.-R.; Xu, H.-M.; Li, Y.-Y.; Cheng, Y.-N.; Ke, H.-N.; Yuan, Y.; Wang, R.-Q.; Shi, Y.-Q.; Lou, H.-X.; et al. Riccardin D-26, a Synthesized Macrocyclic Bisbibenzyl Compound, Inhibits Human Oral Squamous Carcinoma Cells KB and KB/VCR: In Vitro and in Vivo Studies. Biochim. Biophys. Acta 2013, 1830, 2194–2203. [Google Scholar] [CrossRef]
- Schwartner, C.; Bors, W.; Michel, C.; Franck, U.; Müller-Jakic, B.; Nenninger, A.; Asakawa, Y.; Wagner, H. Effect of Marchantins and Related Compounds on 5-Lipoxygenase and Cyclooxygenase and Their Antioxidant Properties: A Structure Activity Relationship Study. Phytomedicine 1995, 2, 113–117. [Google Scholar] [CrossRef]
- Hsiao, G.; Teng, C.-M.; Wu, C.-L.; Ko, F.-N. Marchantin H as a Natural Antioxidant and Free Radical Scavenger. Arch. Biochem. Biophys. 1996, 334, 18–26. [Google Scholar] [CrossRef]
- Keseru, G.M.; Nógrádi, M. The Biological Activity of Cyclic Bis(Bibenzyls): A Rational Approach. Bioorg. Med. Chem. 1995, 3, 1511–1517. [Google Scholar] [CrossRef]
- Morita, H.; Tomizawa, Y.; Tsuchiya, T.; Hirasawa, Y.; Hashimoto, T.; Asakawa, Y. Antimitotic Activity of Two Macrocyclic Bis (Bibenzyls), Isoplagiochins A and B from the Liverwort Plagiochila fruticosa. Bioorg. Med. Chem. Lett. 2009, 19, 493–496. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Liao, Y.-X.; Qu, X.-J.; Yuan, H.-Q.; Li, S.; Qu, J.-B.; Lou, H.-X. Marchantin C, a Macrocyclic Bisbibenzyl, Induces Apoptosis of Human Glioma A172 Cells. Cancer Lett. 2008, 262, 173–182. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, B.; Wang, Y.; Cui, M.; Zhang, L.; Cui, C.; Wang, Y.; Liu, X.; Lou, H. Synthesis of Macrocyclic Bisbibenzyl Derivatives and Their Anticancer Effects as Anti-Tubulin Agents. Bioorg. Med. Chem. 2012, 20, 2382–2391. [Google Scholar] [CrossRef]
- Zhang, T.-W.; Xing, L.; Tang, J.-L.; Lu, J.-X.; Liu, C.-X. Marchantin M Induces Apoptosis of Prostate Cancer Cells through Endoplasmic Reticulum Stress. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3570. [Google Scholar] [CrossRef]
- Li, X.; Sun, B.; Zhu, C.-J.; Yuan, H.-Q.; Shi, Y.-Q.; Gao, J.; Li, S.-J.; Lou, H.-X. Reversal of P-Glycoprotein-Mediated Multidrug Resistance by Macrocyclic Bisbibenzyl Derivatives in Adriamycin-Resistant Human Myelogenous Leukemia (K562/A02) Cells. Toxicol. Vitr. 2009, 23, 29–36. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, H.; Xi, G.; Ma, Y.; Lou, H. Synthesis and Multidrug Resistance Reversal Activity of Dihydroptychantol A and Its Novel Derivatives. Bioorg. Med. Chem. 2009, 17, 4981–4989. [Google Scholar] [CrossRef]
- Li, X.; Wu, W.K.; Sun, B.; Cui, M.; Liu, S.; Gao, J.; Lou, H. Dihydroptychantol A, a Macrocyclic Bisbibenzyl Derivative, Induces Autophagy and Following Apoptosis Associated with P53 Pathway in Human Osteosarcoma U2OS Cells. Toxicol. Appl. Pharmacol. 2011, 251, 146–154. [Google Scholar] [CrossRef]
- Pang, Y.; Si, M.; Sun, B.; Niu, L.; Xu, X.; Lu, T.; Yuan, H.; Lou, H. DHA2, a Synthesized Derivative of Bisbibenzyl, Exerts Antitumor Activity against Ovarian Cancer through Inhibition of XIAP and Akt/mTOR Pathway. Food Chem. Toxicol. 2014, 69, 163–174. [Google Scholar] [CrossRef]
- Xu, A.; Hu, Z.; Qu, J.; Liu, S.; Syed, A.K.A.; Yuan, H.; Lou, H. Cyclic Bisbibenzyls Induce Growth Arrest and Apoptosis of Human Prostate Cancer PC3 Cells. Acta Pharmacol. Sin. 2010, 31, 609–615. [Google Scholar] [CrossRef]
- Vollár, M.; Gyovai, A.; Szűcs, P.; Zupkó, I.; Marschall, M.; Csupor-Löffler, B.; Bérdi, P.; Vecsernyés, A.; Csorba, A.; Liktor-Busa, E.; et al. Antiproliferative and Antimicrobial Activities of Selected Bryophytes. Molecules 2018, 23, 1520. [Google Scholar] [CrossRef]
- Roldos, V.; Nakayama, H.; Rolón, M.; Montero-Torres, A.; Trucco, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G.; et al. Activity of a Hydroxybibenzyl Bryophyte Constituent against Leishmania spp. and Trypanosoma cruzi: In Silico, in Vitro and in Vivo Activity Studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Gao, Y.; Zhou, J.-C.; Xu, Z.-J.; Qiao, Y.-N.; Zhang, J.-Z.; Lou, H.-X. Diverse Prenylated Bibenzyl Enantiomers from the Chinese Liverwort Radula apiculata and Their Cytotoxic Activities. J. Nat. Prod. 2021, 84, 1459–1468. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Gao, Y.; Zhu, R.-X.; Qiao, Y.-N.; Zhou, J.-C.; Zhang, J.-Z.; Li, Y.; Li, S.-W.; Fan, S.-H.; Lou, H.-X. Prenylated Bibenzyls from the Chinese Liverwort Radula constricta and Their Mitochondria-Derived Paraptotic Cytotoxic Activities. J. Nat. Prod. 2019, 82, 1741–1751. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhu, R.; Zhang, J.; Zhou, J.; Hongxiang, L. Bibenzyl-Based Meroterpenoid Enantiomers from the Chinese Liverwort Radula sumatrana. J. Nat. Prod. 2017, 80, 3143–3150. [Google Scholar] [CrossRef]
- Ivković, I.; Novaković, M.; Veljić, M.; Mojsin, M.; Stevanović, M.; Marin, P.D.; Bukvički, D. Bis-Bibenzyls from the Liverwort Pellia endiviifolia and Their Biological Activity. Plants 2021, 10, 1063. [Google Scholar] [CrossRef]
- Asif, A.; Ishtiaq, S.; Kamran, S.H.; Youssef, F.S.; Lashkar, M.O.; Ahmed, S.A.; Ashour, M.L. UHPLC–QTOF–MS Metabolic Profiling of Marchantia polymorpha and Evaluation of Its Hepatoprotective Activity Using Paracetamol-Induced Liver Injury in Mice. ACS Omega 2023, 8, 19037–19046. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, K.; Khan, M.I.; Paul, R.; Ghoshal, U.; Asakawa, Y. Recent Advances in the Phytochemistry of Bryophytes: Distribution, Structures and Biological Activity of Bibenzyl and Bisbibenzyl Compounds. Plants 2023, 12, 4173. https://doi.org/10.3390/plants12244173
Sen K, Khan MI, Paul R, Ghoshal U, Asakawa Y. Recent Advances in the Phytochemistry of Bryophytes: Distribution, Structures and Biological Activity of Bibenzyl and Bisbibenzyl Compounds. Plants. 2023; 12(24):4173. https://doi.org/10.3390/plants12244173
Chicago/Turabian StyleSen, Kakali, Mohammad Imtiyaj Khan, Raja Paul, Utsha Ghoshal, and Yoshinori Asakawa. 2023. "Recent Advances in the Phytochemistry of Bryophytes: Distribution, Structures and Biological Activity of Bibenzyl and Bisbibenzyl Compounds" Plants 12, no. 24: 4173. https://doi.org/10.3390/plants12244173







