Anacardium occidentale Bark as an Antidiabetic Agent
Abstract
:1. Introduction
2. Results
2.1. HPLC-UV/DAD Analysis
2.2. Extract Standardization
2.3. In Vivo Evaluation of the Hypoglycemic Activity
2.3.1. Clinical Signs
2.3.2. Body Weight
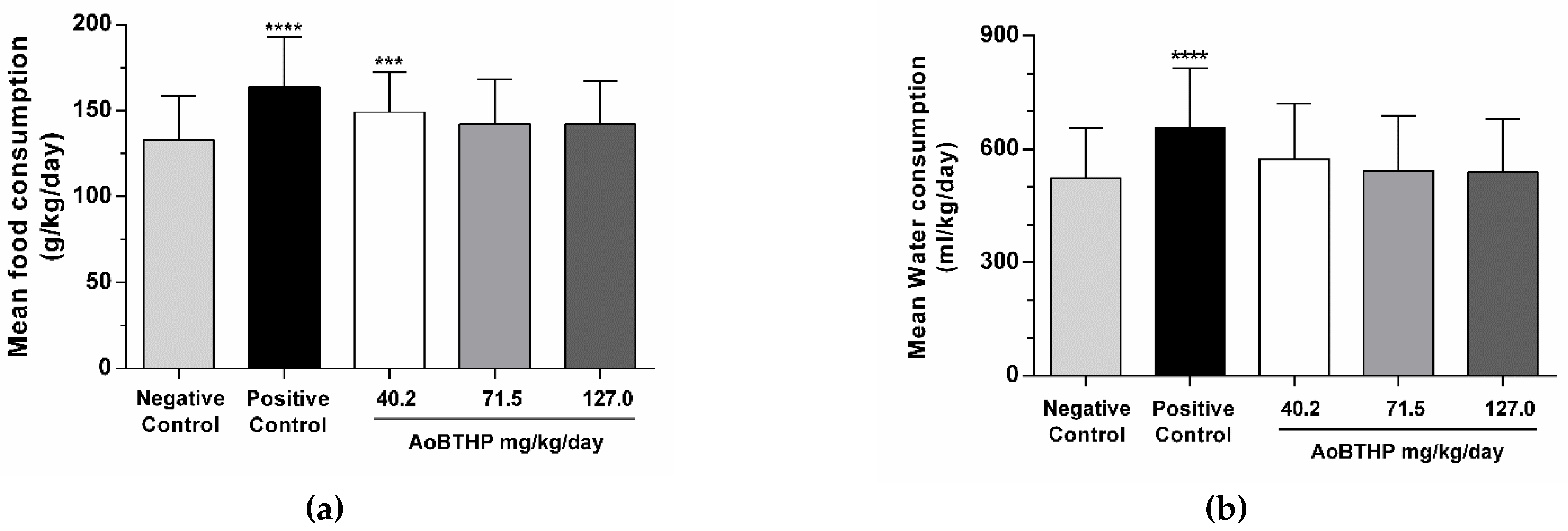
2.3.3. Food and Water Consumption
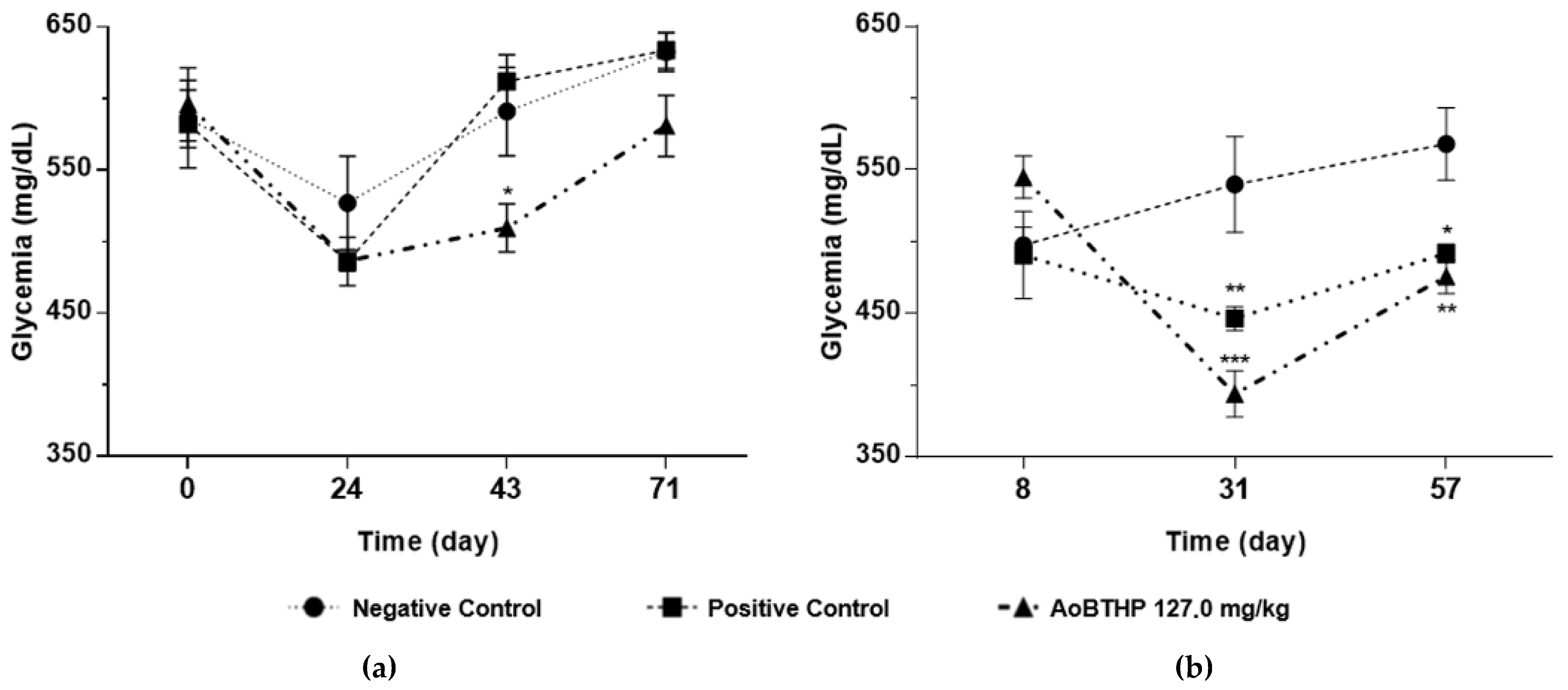
2.3.4. Biochemical Parameters
2.3.5. Organ Weights
2.3.6. Histological Analyses
2.4. In Vitro Evaluation of the Hypoglycemic Activity
2.4.1. α-Glucosidase Inhibitory Assay
2.4.2. Detection of α- and β-Glucosidase Inhibitors by Bioautography
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Plant Material
4.4. Extract Preparation
4.5. HPLC-UV Analysis
4.6. Extract Standardization
4.7. In Vivo Evaluation of the Hypoglycemic Activity
4.7.1. Experimental Protocol
4.7.2. Rationale for Dose Selection and Route of Administration
4.7.3. Necropsy
4.7.4. Biochemical Parameters
4.7.5. Histological Analyses
4.8. In Vitro Evaluation of the Hypoglycemic Activity
4.8.1. α-Glucosidase Inhibitory Assay
4.8.2. Detection of α- and β-Glucosidase Inhibitors by Bioautography
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, R.; Mitchell, B.; Gray, C.; Orbell, J.; Coulepis, T.; Muralitharan, M. Medicinal Plants of Brazil. Asia Pac. Biotech News 2007, 11, 689–706. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestry Database: A Tree Reference and Selection Guide Version 4.0. Available online: http://www.worldagroforestry.org/treedb2/AFTPDFS/Anacardium_occidentale.PDF (accessed on 25 September 2022).
- Catarino, L.; Havik, P.J.; Romeiras, M.M. Medicinal Plants of Guinea-Bissau: Therapeutic Applications, Ethnic Diversity and Knowledge Transfer. J. Ethnopharmacol. 2016, 183, 71–94. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Nilo Bitu, V.; de Carvalho Nilo Bitu, V.; Matias, E.F.F.; de Lima, W.P.; da Costa Portelo, A.; Coutinho, H.D.M.; de Menezes, I.R.A. Ethnopharmacological Study of Plants Sold for Therapeutic Purposes in Public Markets in Northeast Brazil. J. Ethnopharmacol. 2015, 172, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Iwu, M.M. Handbook of African Medicinal Plants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Cano, J.H.; Volpato, G. Herbal Mixtures in the Traditional Medicine of Eastern Cuba. J. Ethnopharmacol. 2004, 90, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Chabi Sika, K.; Ahoton, L.E.; Adebo, I.; Adigoun, F.A.; Saidou, A.; Kotchoni, S.O.; Ahanchede, A. Indigenous Knowledge and Traditional Management of Cashew (Anacardium occidentale L.) Genetic Resources in Benin. J. Exp. Biol. Agric. Sci. 2013, 1, 375–382. [Google Scholar]
- Ezuruike, U.F.; Prieto, J.M. The Use of Plants in the Traditional Management of Diabetes in Nigeria: Pharmacological and Toxicological Considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannini, P.; Howes, M.-J.R.; Edwards, S.E. Medicinal Plants Used in the Traditional Management of Diabetes and Its Sequelae in Central America: A Review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Brandão, M.G.L.; Zanetti, N.N.S.; Oliveira, P.; Grael, C.F.F.; Santos, A.C.P.; Monte-Mór, R.L.M. Brazilian Medicinal Plants Described by 19th Century European Naturalists and in the Official Pharmacopoeia. J. Ethnopharmacol. 2008, 120, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.M.D. Caderno de Campo “Plantas Medicinais Usadas Em Portugal Para o Tratamento Da Diabetes”; Faculty of Pharmacy: Lisbon, Portugal, 2000. [Google Scholar]
- World Health Organization Diabetes—Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 25 September 2022).
- Alexander-Lindo, R.L.; Morrison, E.Y.S.A.; Nair, M.G. Hypoglycaemic Effect of Stigmast-4-En-3-One and Its Corresponding Alcohol from the Bark of Anacardium occidentale (Cashew). Phytother. Res. 2004, 18, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Lindo, R.L.; Nair, E.Y.S.A.; Nair, M.G.; McGrowder, D.A. Effect of the Fractions of the Hexane Bark Extract and Stigmast-4-En-3-One Isolated from Anacardium occidentale on Blood Glucose Tolerance Test in an Animal Model. Int. J. Pharmacol. 2007, 3, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Bassey, T.; Eliakim-Ikechukwu, C.; Ihentuge, C. Effect of Ethanolic Stem-Bark Extract of Anacardium occidentale (Cashew) on the Histology of the Pancreas of Diabetic Wistar Rats. J. Biol. Agric. Healthc. 2012, 2, 153–156. [Google Scholar]
- Eliakim-Ikechukwu, C.; Obri, A.; Akpa, O. Phytochemical and Micronutrient Composition of Anacardium occidentale Linn (Cashew) Stem-Bark Hydroethanolic Extract and Its Effect on the Fasting Blood Glucose Levels and Body Weight of Diabetic Wistar Rats. Internet J. Nutr. Wellness 2009, 10, 1–6. [Google Scholar]
- Ojewole, J.A.O. Laboratory Evaluation of the Hypoglycemic Effect of Anacardium occidentale Linn. (Anacardiaceae) Stem-Bark Extracts in Rats. Methods Find. Exp. Clin. Pharmacol. 2003, 25, 199–204. [Google Scholar] [CrossRef]
- Olatunji, L.A.; Okwusidi, J.I.; Soladoye, A.O. Antidiabetic Effect of Anacardium occidentale. Stem-Bark in Fructose-Diabetic Rats. Pharm. Biol. 2005, 43, 589–593. [Google Scholar] [CrossRef]
- Chaves, M.H.; Citó, A.M.; das, G.L.; Lopes, J.A.D.; da Costa, D.A.; de Oliveira, C.A.A.; e Francisco, A.F.C.; Brito Júnior, E.M. Fenóis Totais, Atividade Antioxidante e Constituintes Químicos de Extratos de Anacardium occidentale L. Anacardiaceae. Rev. Bras. Farm. 2010, 20, 106–112. [Google Scholar] [CrossRef]
- Coutinho, H.; Barbosa-Filho, V.; Waczuk, E.; Leite, N.; Costa, J.G.; Lacerda, S.; Adedara, I.; Kamdem, J.P.; Posser, T.; Menezes, I. Phytocompounds and Modulatory Effects of Anacardium microcarpum (Cajui) on Antibiotic Drugs Used in Clinical Infections. Drug Des. Dev. Ther. 2015, 9, 5965–5972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Encarnação, S.; de Mello-Sampayo, C.; Graça, N.A.G.; Catarino, L.; da Silva, I.B.M.; Lima, B.S.; Silva, O.M.D. Total Phenolic Content, Antioxidant Activity and Pre-Clinical Safety Evaluation of an Anacardium occidentale Stem Bark Portuguese Hypoglycemic Traditional Herbal Preparation. Ind. Crops Prod. 2016, 82, 171–178. [Google Scholar] [CrossRef]
- Obaineh, M. Phytochemical Constituents and Medicinal Properties of Different Extracts of Anacardium occidentale and Psidium Guajava. Asian J. Biomed. Pharm. 2013, 3, 1–4. [Google Scholar]
- Vilar, M.; de Souza, G.; Vilar, D.; Leite, J.; Raffin, F.; Barbosa-Filho, J.; Nogueira, F.; Rodrigues-Mascarenhas, S.; Moura, T. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules 2016, 21, 1087. [Google Scholar] [CrossRef] [Green Version]
- Shehu, A.; Ponnapalli, M.G.; Mahboob, M.; Prabhakar, P.V.; Olatunji, G.A. New N-Nonadecanoyl-β-Sitosterol and Other Constituents from the Stem-Bark of Anacardium occidentale. Nat. Prod. Res. 2021, 35, 1357–1363. [Google Scholar] [CrossRef]
- Encarnação, S.; Malmir, M.; Sousa, D.; da Silva, I.; Mello-Sampayo, C.; Serrano, R.; Lima, B.; Silva, O. Phenol Content, Antioxidant and α- and β-Glucosidase Inhibitory Activities of an Anacardium occidentale Stem Bark Traditional Herbal Preparation. Planta Med. 2014, 80, 5–9. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Brunton, L. Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 13th ed.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Crandall, J.; Shamoon, H. Diabetes Mellitus. In Goldman-Cecil Medicine, 2-Volume Set; Elsevier Inc.: Philadelphia, PA, USA, 2016; pp. 1527–1548.e3. [Google Scholar]
- Nadkarni, P.; Weinstock, R.S. Carbohydrates. In Henry’s Clinical Diagnosis and Management by Laboratory Methods; McPherson, R.A., Pincus, M.R., Eds.; Elsevier Inc.: Maryland Heights, MO, USA, 2016; Volume 1, pp. 205–220.e2. [Google Scholar]
- Hupfeld, C.J.; Olefsky, J.M. Type 2 Diabetes Mellitus. In Endocrinology: Adult and Pediatric; Jameson, J.L., de Groot, L.J., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 691–714.e6. [Google Scholar]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, K.S.; Burant, C.F. Type 2 Diabetes Mellitus. In Williams Textbook of Endocrinology; Melmed, S., Polonsky, K.S., Larsen, P.R., Kronenberg, H.M., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2016; pp. 1385–1450. [Google Scholar]
- Ferri, F. Diabetes Mellitus. In Ferri’s Clinical Advisor 2017; Elsevier: Philadelphia, PA, USA, 2016; pp. 364–370.e4. [Google Scholar]
- Apovian, C.M.; Okemah, J.; O’Neil, P.M. Body Weight Considerations in the Management of Type 2 Diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef] [Green Version]
- Maas, J. New Approaches in Research and Development of Anti-Diabetic Drugs: An Industry Perspective. Ther. Adv. Endocrinol. Metab. 2012, 3, 109–112. [Google Scholar] [CrossRef]
- Ghorbani, A. Best Herbs for Managing Diabetes: A Review of Clinical Studies. Braz. J. Pharm. Sci. 2013, 49, 413–422. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; Howes, M.-J.R. Plants Used in the Treatment of Diabetes. In Traditional Herbal Medicines for Modern Times—Antidiabetic Plants; Soumyanath, A., Ed.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 19–82. [Google Scholar]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.; Miraldi, E.; Biagi, M. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodeker, G.; Bhat, K.K.S.; Burley, J.; Vantomme, P. Medicinal Plants for Forest Conservation and Health Care; Food and Agriculture Organization of the United Nations: Rome, Italy, 1997; Volume 11. [Google Scholar]
- Kennedy, A.J.; Ellacott, K.L.J.; King, V.L.; Hasty, A.H. Mouse Models of the Metabolic Syndrome. Dis. Model. Mech. 2010, 3, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.J.; Batdorf, H.M.; Burk, D.H.; Noland, R.C.; Eder, A.E.; Boulos, M.S.; Karlstad, M.D.; Jason Collier, J. Db/Db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. J. Diabetes Res. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal Models of Metabolic Syndrome: A Review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Poretsky, L. Principles of Diabetes Mellitus, 3rd ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Katzung, B.; Trevor, A.J. Basic & Clinical Pharmacology, 13th ed.; McGraw Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Hanefeld, M. Pioglitazone and Sulfonylureas: Effectively Treating Type 2 Diabetes. Int. J. Clin. Pract. 2007, 61, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mun’im, A.; Katrin, A.; Andriani, A.; Mahmudah, K.F.; Mashita, M. Screening of α- Glucosidase Inhibitory Activity of Some Indonesian Medicinal Plants. Int. J. Med. Aromat. Plants 2013, 3, 144–150. [Google Scholar]
- Toyomizu, M.; Sugiyama, S.; Jin, R.L.; Nakatsu, T. α-Glucosidase and Aldose Reductase Inhibitors: Constituents of Cashew, Anacardium occidentale, Nut Shell Liquids. Phytother. Res. 1993, 7, 252–254. [Google Scholar] [CrossRef]
- Tedong, L.; Madiraju, P.; Martineau, L.C.; Vallerand, D.; Arnason, J.T.; Desire, D.D.P.; Lavoie, L.; Kamtchouing, P.; Haddad, P.S. Hydro-Ethanolic Extract of Cashew Tree (Anacardium occidentale) Nut and Its Principal Compound, Anacardic Acid, Stimulate Glucose Uptake in C2C12 Muscle Cells. Mol. Nutr. Food Res. 2010, 54, 1753–1762. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic Potential of Gallic Acid from Emblica officinalis: Improved Glucose Transporters and Insulin Sensitivity through PPAR-γ and Akt Signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of Gallic Acid on Alpha-Amylase and Alpha-Glucosidase Inhibitory Properties of Acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef]
- Inzucchi, S.; Lupsa, B. Clinical Presentation, Diagnosis, and Initial Evaluation of Diabetes Mellitus in Adults. UpToDate. Available online: https://www.uptodate.com/contents/clinical-presentation-diagnosis-and-initial-evaluation-of-diabetes-mellitus-in-adults?search=diabetes&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 25 September 2022).
- European Directorate for the Quality of Medicines and Healthcare. Herbal Drugs: Sampling and Sample Preparation. In European Pharmacopoeia 9.0; Council of Europe: Strasbourg, France, 2016; pp. 291–292. [Google Scholar]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in Wood: Comparison of Different Estimation Methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- European Parliament. Council of the European Union Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. OJEU 2010, L276, 33–79. [Google Scholar]
- Ministério da Agricultura do Mar do Ambiente e do Ordenamento do Território. Decree-Law No. 113/2013 Implementing EU Directive No. 2010/63 on Animal Protection for Scientific Purposes; DR, 1.ª série, 151, Decreto-Lei n.º 113/2013,4709–4739; FAO: Geneva, Switzerland, 2013. [Google Scholar]
- Center for Drug Evaluation and Research Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Starting Materials Used in the Production of Viral Vaccines for the Prevention and Treatment of Infectious Diseases. Biotechnol. Law Rep. 2006, 25, 697–723. [CrossRef]
- Azmi, M.B.; Qureshi, S.A. Methanolic Root Extract of Rauwolfia serpentina Benth Improves the Glycemic, Antiatherogenic, and Cardioprotective Indices in Alloxan-Induced Diabetic Mice. Adv. Pharmacol. Sci. 2012, 2012, 376429. [Google Scholar]
- Semwal, D.K.; Rawat, U.; Badoni, R.; Semwal, R.; Singh, R. Anti-Hyperglycemic Effect of Stephania glabra Tubers in Alloxan Induced Diabetic Mice. J. Med. 2010, 11, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Rouzbehan, S.; Moein, S.; Homaei, A.; Reza Moein, M. Kinetics of α-Glucosidase Inhibition by Different Fractions of Three Species of Labiatae Extracts: A New Diabetes Treatment Model Kinetics of α-Glucosidase Inhibition by Different Fractions of Three Species of Labiatae Extracts. Pharm. Biol. 2017, 55, 1483–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões-Pires, C.A.; Hmicha, B.; Marston, A.; Hostettmann, K. A TLC Bioautographic Method for the Detection of α- and β-Glucosidase Inhibitors in Plant Extracts. Phytochem. Anal. 1990, 20, 511–515. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Plenum Press: New York, NY, USA, 1989; pp. 141–144. [Google Scholar]
- Silva, O.M.D. Estudo Etnofarmacológico de Espécies Da Flora Da Guiné-Bissau Com Actividade Antimicrobiana. Ph.D. Thesis, Faculty of Pharmacy, Universidade de Lisboa, Lisbon, Portugal, 2004; pp. 275–276. [Google Scholar]


| Type of Extraction | Extract Dose and Route of Administration | Duration of Study | Positive Control | Animal Model | Reference |
|---|---|---|---|---|---|
| Methanol, Aqueous | 100–800 mg/kg p.o. | 24 h; >24 h | Insulin 5 UI/kg s.c.; glibenclamide 0.2 mg/kg p.o. | Streptozotocin 90 mg/kg i.p. Rat | [17] |
| Hexane | 20 and 30 mg/kg i.v. | 2 h | ----- | Healthy dog | [13] |
| Methanol | 200 mg/kg p.o. | 21 days | ----- | Fructose 25% w/w in diet; rat | [18] |
| Hexane | 300 mg/kg p.o.; 30 mg/kg i.v. | 2 h | ----- | Healthy dog | [14] |
| Hydroethanol (80%) | 500 mg/kg p.o. | 28 days | Insulin 5 IU/kg | Streptozotocin 65 mg/kg i.p.; rat | [16] |
| Ethanol | 300 and 500 mg/kg p.o. twice daily | 28 days | ----- | Streptozotocin 65 mg/kg s.c.; rat | [15] |
| Week | Negative Control | Positive Control | AoBTHP mg/kg/Day | ||
|---|---|---|---|---|---|
| 40.2 | 71.5 | 127.0 | |||
| 0 | 40.0 ± 1.8 | 38.2 ± 2.6 | 37.9 ± 3.1 | 38.3 ± 2.0 | 37.4 ± 1.9 |
| 1 to 3 | 39.4 ± 1.5 | 36.4 ± 2.7 | 37.3 ± 2.9 | 37.2 ± 1.6 | 36.2 ± 1.8 |
| 4 to 6 | 41.8 ± 2.1 | 37.2 ± 2.0 | 38.9 ± 2.6 | 39.5 ± 1.3 | 37.5 ± 1.0 |
| 7 to 9 | 43.2 ± 2.6 | 38.0 ± 1.8 | 39.9 ± 2.3 | 39.6 ± 0.8 | 39.7 ± 1.1 |
| 10 to 12 | 44.8 ± 2.4 | 39.2 ± 1.9 | 41.2 ± 2.6 | 40.1 ± 1.0 | 40.8 ± 1.2 |
| 13 | 47.2 ± 2.7 | 41.6 ± 2.2 | 42.6 ± 2.7 | 42.1 ± 1.3 | 41.9 ± 1.1 |
| Day | Negative Control | Positive Control | AoBTHP mg/kg/Day | ||
|---|---|---|---|---|---|
| 40.2 | 71.5 | 127.0 | |||
| 0 | 586 ± 23 | 582 ± 34 | 552 ±30 | 576 ± 27 | 578 ± 31 |
| 24 | 527 ± 37 | 486 ± 19 # | 524 ± 23 | 486 ± 15 † | 487 ± 9 ** |
| 43 | 591 ± 34 | 612 ± 21 | 545 ± 36 | 565 ± 32 | 510 ± 17 * |
| 71 | 633 ± 15 | 634 ± 14 | 602 ± 23 | 618 ± 22 | 581 ± 21 |
| Day | Negative Control | Positive Control | AoBTHP mg/kg/Day | ||
|---|---|---|---|---|---|
| 40.2 | 71.5 | 127.0 | |||
| 8 | 498 ± 14 | 491 ± 34 | 466 ± 18 | 496 ± 21 | 545 ± 16 |
| 31 | 540 ± 37 | 447 ± 9 ** | 449 ± 39 ** | 442 ± 30 ** | 394 ± 18 **** |
| 57 | 568 ± 28 | 492 ± 7 * | 492 ± 12 * | 486 ± 16 * | 476 ± 13 ** |
| Biochemical Parameter | Negative Control | Positive Control | AoBTHP mg/kg/Day | ||
|---|---|---|---|---|---|
| 40.2 | 71.5 | 127.0 | |||
| ALT (I.U/L) | 122 ± 27 | 119 ± 18 | 150 ± 54 | 123 ± 20 | 139 ± 48 |
| AST (I.U/L) | 277 ± 96 | 320 ± 105 | 584 ± 158 | 414 ± 84 | 338 ± 85 |
| Cholesterol | 172 ± 44 | 134 ± 39 | 142 ± 29 | 175 ± 49 | 149 ± 0.0 |
| Serum creatinine (mg/dL) | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.4 ± 0.3 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Serum urea (mg/dL) | 27 ± 3.0 | 21 ± 3.0 | 50 ± 27 | 27 ± 4.0 | 21 ± 1.0 |
| Triglycerides (mg/dL) | 301 ± 45 | 282 ± 57 | 323 ± 52 | 225 ± 65 | 257 ± 41 |
| Organ | Negative Control | Positive Control | AoBTHP mg/kg/Day | ||
|---|---|---|---|---|---|
| 40.2 | 71.5 | 127.0 | |||
| Heart | 0.24 ± 0.01 | 0.28 ± 0.01 | 0.20 ± 0.06 | 0.28 ± 0.01 | 0.25 ± 0.01 |
| Kidneys | 0.90 ± 0.03 | 1.05 ± 0.06 | 0.96 ± 0.09 | 0.88 ± 0.04 | 0.87 ± 0.02 |
| Liver | 6.24 ± 0.17 | 6.10 ± 0.14 | 5.63 ± 0.51 | 5.74 ± 0.38 | 5.57 ± 0.29 |
| Pancreas | 0.42 ± 0.06 | 0.39 ± 0.12 | 0.32 ± 0.05 | 0.38 ± 0.03 | 0.35 ± 0.03 |
| Spleen | 0.18 ± 0.03 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.15 ± 0.03 | 0.10 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encarnação, S.; De Mello-Sampayo, C.; Carrapiço, B.; São Braz, B.; Jordão, A.P.; Peleteiro, C.; Catarino, L.; Silva, I.B.M.d.; Gouveia, L.F.; Lima, B.S.; et al. Anacardium occidentale Bark as an Antidiabetic Agent. Plants 2022, 11, 2637. https://doi.org/10.3390/plants11192637
Encarnação S, De Mello-Sampayo C, Carrapiço B, São Braz B, Jordão AP, Peleteiro C, Catarino L, Silva IBMd, Gouveia LF, Lima BS, et al. Anacardium occidentale Bark as an Antidiabetic Agent. Plants. 2022; 11(19):2637. https://doi.org/10.3390/plants11192637
Chicago/Turabian StyleEncarnação, Sofia, Cristina De Mello-Sampayo, Belmira Carrapiço, Berta São Braz, Ana Patrícia Jordão, Conceição Peleteiro, Luís Catarino, Isabel B. Moreira da Silva, Luís F. Gouveia, Beatriz Silva Lima, and et al. 2022. "Anacardium occidentale Bark as an Antidiabetic Agent" Plants 11, no. 19: 2637. https://doi.org/10.3390/plants11192637







