Accumulation of Microcystin from Oscillatoria limnetica Lemmermann and Microcystis aeruginosa (Kützing) in Two Leafy Green Vegetable Crop Plants Lactuca sativa L. and Eruca sativa
Abstract
:1. Introduction
2. Results
2.1. Cell Count of Two Dominant Species (Microcystis aeruginosa (Kützing) and Oscillatoria limnetica Lemmermann) and Microcystin Concentration in Irrigation Water
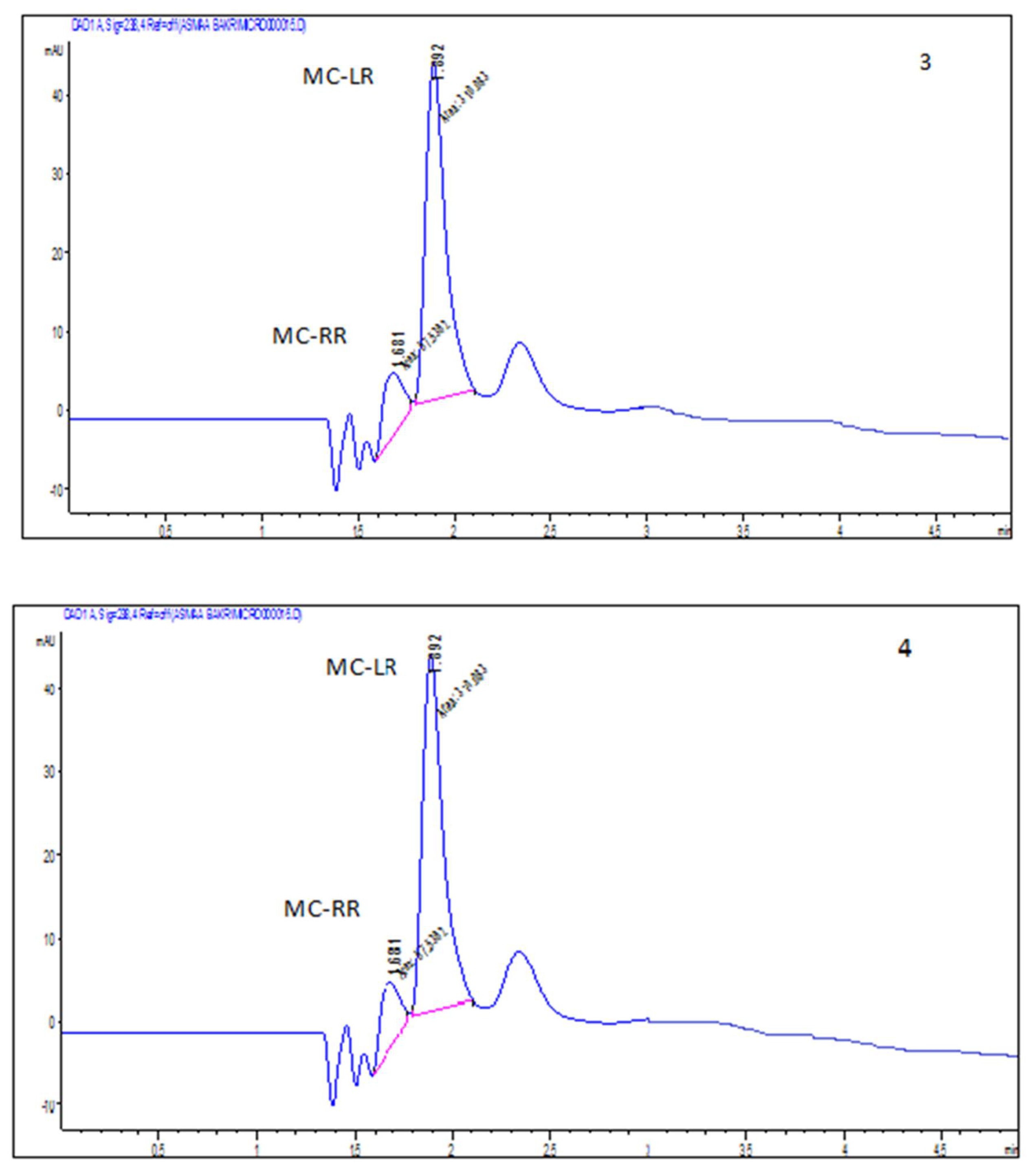
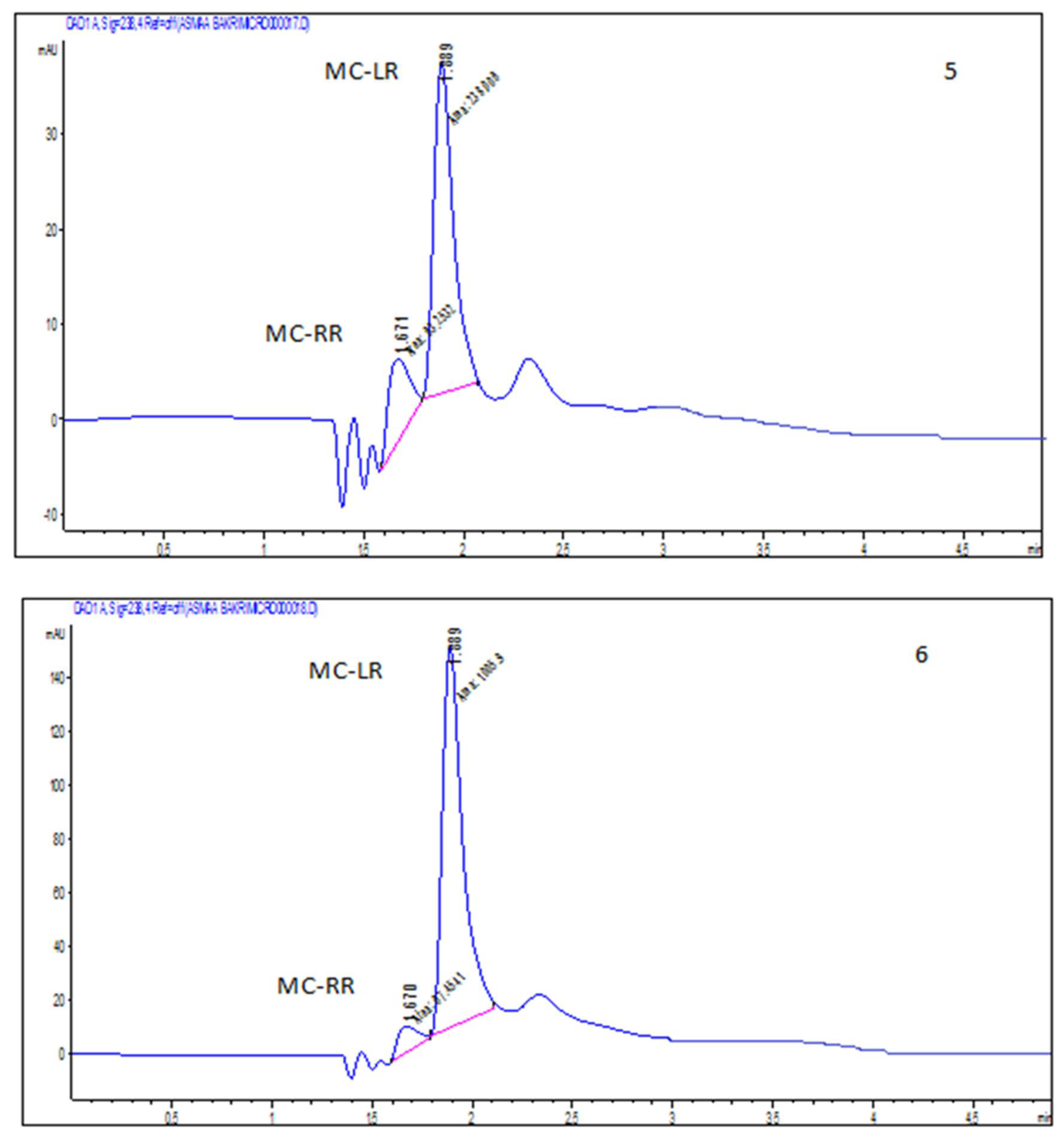
2.2. Accumulation of Microcystin-LR and RR in Edible Parts of the Two Vegetable Plants (Eruca sativa and Lactuca sativa L.)
2.3. Risk Assessment and Potential Health Hazards of Microcystin on Plants (Eruca sativa L. and Lactuca sativa)
3. Discussion
4. Materials and Methods
4.1. Collection of Water Samples and Isolation of Oscillatoria limnetica and Microcystis aeruginosa
4.2. Determination of Microcystin Accumulated in Plants (Lactuca sativa L. and Eruca sativa)
4.3. Calculation of Estimated Daily Intake
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmichael Wayne, W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 78–86. [Google Scholar] [CrossRef]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and Cyanotoxins: From Impacts on Aquatic Ecosystems and Human Health to Anticarcinogenic Effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Ufelmann, H.; Krüger, T.; Luckas, B.; Schrenk, D. Human and rat hepatocyte toxicity and protein phosphatase 1 and 2A inhibitory activity of naturally occurring desmethyl-microcystins and nodularins. Toxicology 2012, 293, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef] [Green Version]
- Radkova, M.; Stefanova, K.; Uzunov, B.; Gärtner, G.; Stoyneva-Gärtner, M. Morphological and Molecular Identification of Microcystin-Producing Cyanobacteria in Nine Shallow Bulgarian Water Bodies. Toxins 2020, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Hou, L.; Lin, X.; Xie, Z. Application of Novel Nanomaterials for Chemo- and Biosensing of Algal Toxins in Shellfish and Water. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 353–414. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef]
- Chorus, L.; Bartram, J. Toxic Cyanobacteria in Water; Published on behalf of WHO by E&FN Spon: London, UK; New York, NY, USA, 1999. [Google Scholar]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The Fate of Microcystins in the Environment and Challenges for Monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef] [Green Version]
- Khairy, H.; El-Sheekh, M. Toxicological Studies on Microcystin Produced by Microcystis aeruginosa: Assessment and Management. Egypt. J. Bot. 2019, 59, 551–566. [Google Scholar] [CrossRef] [Green Version]
- Runnegar, M.; Berndt, N.; Kaplowitz, N. Microcystin Uptake and Inhibition of Protein Phosphatases: Effects of Chemoprotectants and Self-Inhibition in Relation to Known Hepatic Transporters. Toxicol. Appl. Pharmacol. 1995, 134, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Williamson, T.; Sultanpuram, N.; Sendi, H. The role of liver microenvironment in hepatic metastasis. Clin. Transl. Med. 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Jaakkola, S.; Važić, T.; Häggqvist, K.; Kirkkala, T.; Ventelä, A.-M.; Kirkkala, T.; Svirčev, Z.; Meriluoto, J. Elimination of cyanobacteria and microcystins in irrigation water—effects of hydrogen peroxide treatment. Environ. Sci. Pollut. Res. 2020, 27, 8638–8652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, M.; Azevedo, J.; Pinto, E.; Neves, J.; Campos, A.; Vasconcelos, V. Effects of microcystin-LR, cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture on growth, oxidative stress and mineral content in lettuce plants (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2015, 116, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, C.; Zhang, T.; Liu, W.; Wang, L.; Chen, Y.; Wu, L.; Hegazy, A.M.; El-Sayed, A.; Zhang, X. μEvaluation of microcystin-LR absorption using an in vivo intestine model and its effect on zebrafish intestine. Aquat. Toxicol. 2019, 206, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Dissananyak, D.M.; Jayasekera, J.M.K.; Ratnayake, P.; Wickramasinghe, W.; Radella, Y.A. The Short Term Effect of Cyanobacterial Toxin Extracts on Mice Kidney. 2011. Peradeniya University Research Session PURSE -2011, Proceeding and Abstracts. 2011,24,95. Available online: https://www.sactrc.org/?p=1824 (accessed on 31 May 2022).
- Lankoff, A.; Carmichael, W.W.; Grasman, K.A.; Yuan, M. The uptake kinetics and immunotoxic effects of microcystin-LR in human and chicken peripheral blood lymphocytes in vitro. Toxicology 2004, 204, 23–40. [Google Scholar] [CrossRef]
- Lone, Y.; Koiri, R.K.; Bhide, M. An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicol. Rep. 2015, 2, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef]
- Järvenpää, S.; Lundberg-Niinistö, C.; Spoof, L.; Sjövall, O.; Tyystjärvi, E.; Meriluoto, J. Effects of microcystins on broccoli and mustard, and analysis of accumulated toxin by liquid chromatography–mass spectrometry. Toxicon 2007, 49, 865–874. [Google Scholar] [CrossRef]
- Villatoro-Pulido, M.; Font, R.; Saha, S.; Obregón-Cano, S.; Anter, J.; Muñoz-Serrano, A.; De Haro-Bailón, A.; Alonso-Moraga, A.; Celestino, M.D.R. In vivo biological activity of rocket extracts (Eruca vesicaria subsp. sativa (Miller) Thell) and sulforaphane. Food Chem. Toxicol. 2012, 50, 1384–1392. [Google Scholar] [CrossRef]
- El-Masry, R.R.; Messiha, N.K.; El-Rokiek, K.G.; Ahmed, S.A.; Mohamed, S.A. The allelopathic effect of Eruca sativa Mill. Seed powder on growth and yield of Phaseolus vulgaris and associated weeds. Curr. Sci. Int. 2015, 4, 485–490. [Google Scholar]
- Codd, G.A.; Bell, S.G.; Kaya, K.; Ward, C.J.; Beattie, K.A.; Metcalf, J.S. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Crush, J.R.; Briggs, L.R.; Sprosen, J.M.; Nichols, S.N. Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ. Toxicol. Int. J. 2008, 23, 246–252. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Diffusion of Ehealth: Making Universal Health Coverage Achievable: Report of the Third Global Survey on Ehealth; World Health Organization: Houston, TX, USA, 2017. [Google Scholar]
- Mohamed, Z.A.; Deyab, M.; Abou-Dobara, M.; El-Sayed, A.; El-Raghi, W.M. Occurrence of cyanobacteria and microcystin toxins in raw and treated waters of the Nile River, Egypt: Implication for water treatment and human health. Environ. Sci. Pollut. Res. 2015, 22, 11716–11727. [Google Scholar] [CrossRef] [PubMed]
- Aba, R.; Mugani, R.; Hejjaj, A.; de Fraissinette, N.B.; Oudra, B.; Ouazzani, N.; Campos, A.; Vasconcelos, V.; Carvalho, P.; Mandi, L. First Report on Cyanotoxin (MC-LR) Removal from Surface Water by Multi-Soil-Layering (MSL) Eco-Technology: Preliminary Results. Water 2021, 13, 1403. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Li, Y.-W.; Liu, B.-L.; Zhao, H.-M.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H.; Li, Q.X. High ecological and human health risks from microcystins in vegetable fields in southern China. Environ. Int. 2019, 133, 105142. [Google Scholar] [CrossRef]
- Mohamed, Z.; Bakr, A.; Campos, A.; Vasconcelos, V.; Mohsen, S. The growth inhibition and microcystin accumulation in bush beans (Phaselous vulgaris L.) plant irrigated with water containg toxic Chroococcus minutus. Agric. Water Manag. 2022, 261, 107381. [Google Scholar] [CrossRef]
- Cao, Q.; Steinman, A.D.; Wan, X.; Xie, L. Bioaccumulation of microcystin congeners in soil-plant system and human health risk assessment: A field study from Lake Taihu region of China. Environ. Pollut. 2018, 240, 44–50. [Google Scholar] [CrossRef]
- Lee, S.; Jiang, X.; Manubolu, M.; Riedl, K.; Ludsin, S.A.; Martin, J.F.; Lee, J. Fresh produce and their soils accumulate cyanotoxins from irrigation water: Implications for public health and food security. Food Res. Int. 2017, 102, 234–245. [Google Scholar] [CrossRef]
- Mohamed, Z.; Ahmed, Z.; Bakr, A.; Hashem, M.; Alamri, S. Detection of free and bound microcystins in tilapia fish from Egyptian fishpond farms and its related public health risk assessment. J. Consum. Prot. Food Saf. 2020, 15, 37–47. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.; Bittencourt-Oliveira, M.D.C. Potential human health risk assessment of cylindrospermopsin accumulation and depuration in lettuce and arugula. Harmful Algae 2020, 68, 217–223. [Google Scholar] [CrossRef] [PubMed]
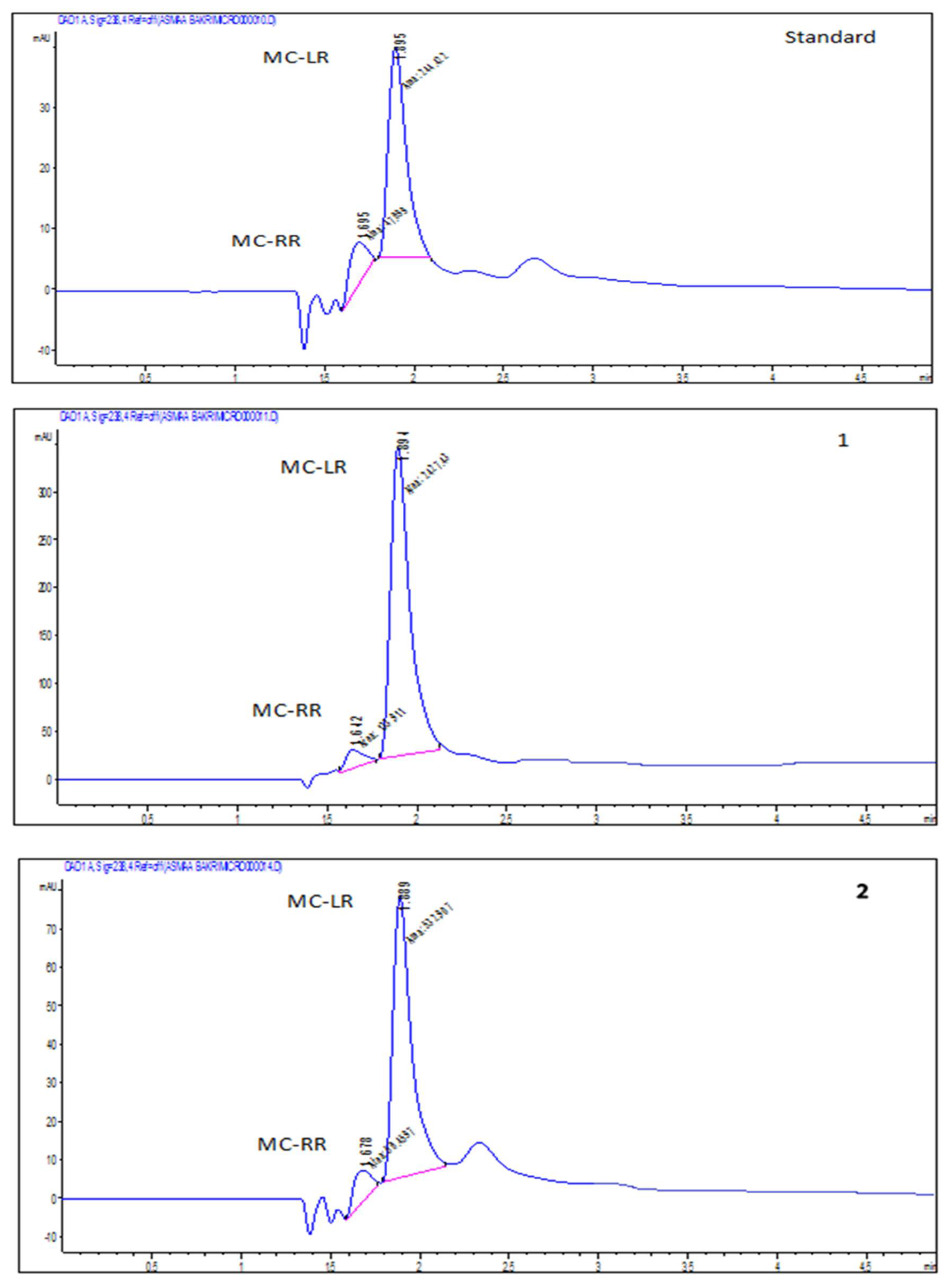
| Algal Species | Cell Number (Cells/mL) |
|---|---|
| Cyanobacteria | |
| Oscillatoria limnetica Lemmermann | 2,300,000 |
| Merismopedia minima G.Beck | 20 |
| Merismopedia tenuissima Lemmermann | 16 |
| Microcystis aeruginosa (Kützing) | 450,000 |
| Synechocystis aquatilis Sauvageau | 22 |
| Chlorophyta | |
| Ankistrodesmus gracilis (Reinsch) | 20 |
| Chlorella vulgaris Beyerinck | 80 |
| Chlorococcum lobatum (Korshikov) | 88 |
| Pediastrum duplex Meyen | 96 |
| Scenedesmus ellipsoideus Chodat | 10 |
| Ankistrodesmus gracilis (Reinsch) | 20 |
| Chlorella vulgaris Beyerinck | 80 |
| Chlorococcum lobatum (Korshikov) | 88 |
| Pediastrum duplex Meyen | 96 |
| Bacillariophyta | |
| Cyclotella sp. | 5 |
| Fragillaria sp. | 12 |
| Melosira sp. | 8 |
| Navicula sp. | 6 |
| Nitzschia sp. | 4 |
| Tribonema sp. | 4 |
| Samples | MC-RR (μg/L) | MC-LR (μg/L) | Total (μg/L) |
|---|---|---|---|
| Intracellular MCs (Oscillatoria limnetica) | 18,000 | 40,000 | 58,000 |
| Extracellular MCs (Oscillatoria limnetica) | 2300 | 5005 | 7305 |
| Intracellular MCs of (Microcystis aeruginosa) | 1000 | 87,000 | 88,000 |
| Extracellular MCs (Microcystis aeruginosa) | 20 | 285 | 305 |
| MC-RR (ng g−1 FW) | MC-LR (ng g−1 FW) | Total (ng g−1 FW) | |
|---|---|---|---|
| MCs accumulated E. sativa | 89 | 1000 | 1089 |
| MCs accumulated L. sativa | 56 | 988 | 1044 |
| EDI (Adults) ng/g FW | EDI (Children) ng/g FW | |
|---|---|---|
| MCs accumulated E. sativa | 3630 | 4356 |
| MCs accumulated L. sativa | 3480 | 4176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakr, A.; Alzain, M.N.; Alzamel, N.M.; Loutfy, N. Accumulation of Microcystin from Oscillatoria limnetica Lemmermann and Microcystis aeruginosa (Kützing) in Two Leafy Green Vegetable Crop Plants Lactuca sativa L. and Eruca sativa. Plants 2022, 11, 1733. https://doi.org/10.3390/plants11131733
Bakr A, Alzain MN, Alzamel NM, Loutfy N. Accumulation of Microcystin from Oscillatoria limnetica Lemmermann and Microcystis aeruginosa (Kützing) in Two Leafy Green Vegetable Crop Plants Lactuca sativa L. and Eruca sativa. Plants. 2022; 11(13):1733. https://doi.org/10.3390/plants11131733
Chicago/Turabian StyleBakr, Asmaa, Mashail Nasser Alzain, Nurah M. Alzamel, and Naglaa Loutfy. 2022. "Accumulation of Microcystin from Oscillatoria limnetica Lemmermann and Microcystis aeruginosa (Kützing) in Two Leafy Green Vegetable Crop Plants Lactuca sativa L. and Eruca sativa" Plants 11, no. 13: 1733. https://doi.org/10.3390/plants11131733






