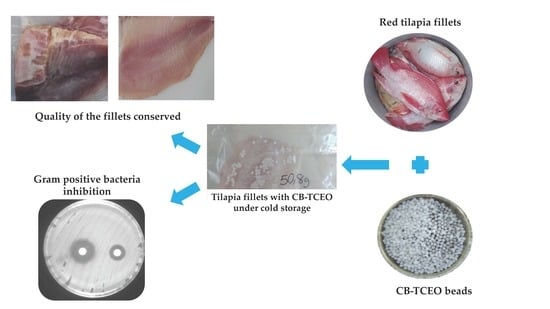
Chitosan Beads Incorporated with Essential Oil of Thymus capitatus: Stability Studies on Red Tilapia Fillets
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
Preparation of the CB-TCEO
2.3. Characterization
2.3.1. Characterization of the TCEO
2.3.2. Characterization of the CB
2.3.3. Antioxidant Activity of the CB-TCEO
2.3.4. Total Phenol Content of the CB-TCEO
2.3.5. Bacterial Inhibition In Vitro Capacity of the CB-TCEO
2.3.6. Antibacterial Analysis In Situ in Tilapia
2.3.7. Total Volatile Nitrogen (TVB-N) Analysis Performed in the Tilapia Sample
2.3.8. pH Analysis in Tilapia
2.3.9. Colorimetric Analysis in Tilapia
2.3.10. Texture Analysis in Tilapia
2.4. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Characterization of the TCEO
Chromatographic Analysis of the TCEO
3.2. Characterization of the CB
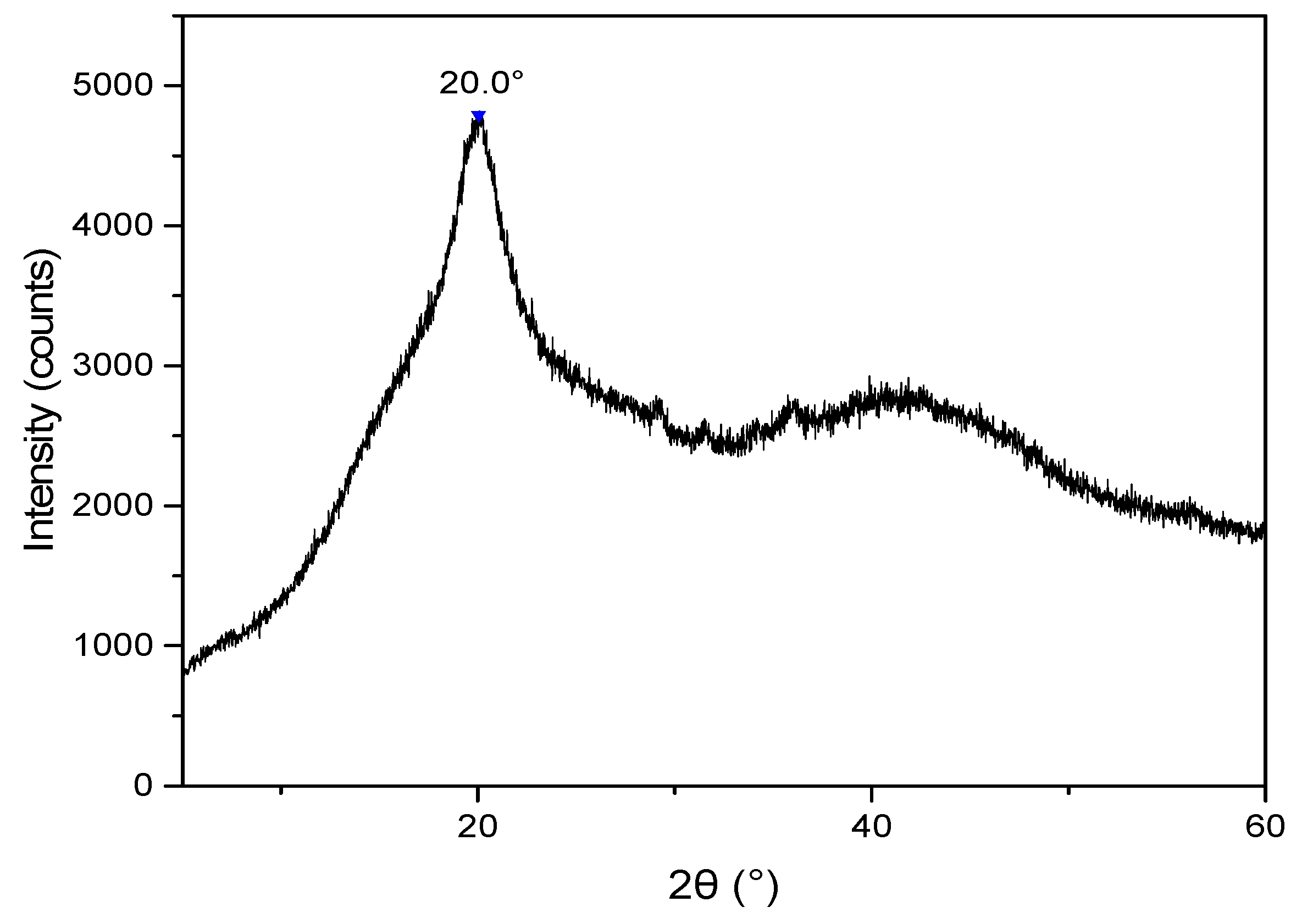
3.2.1. X-ray Diffraction Analysis of the CB
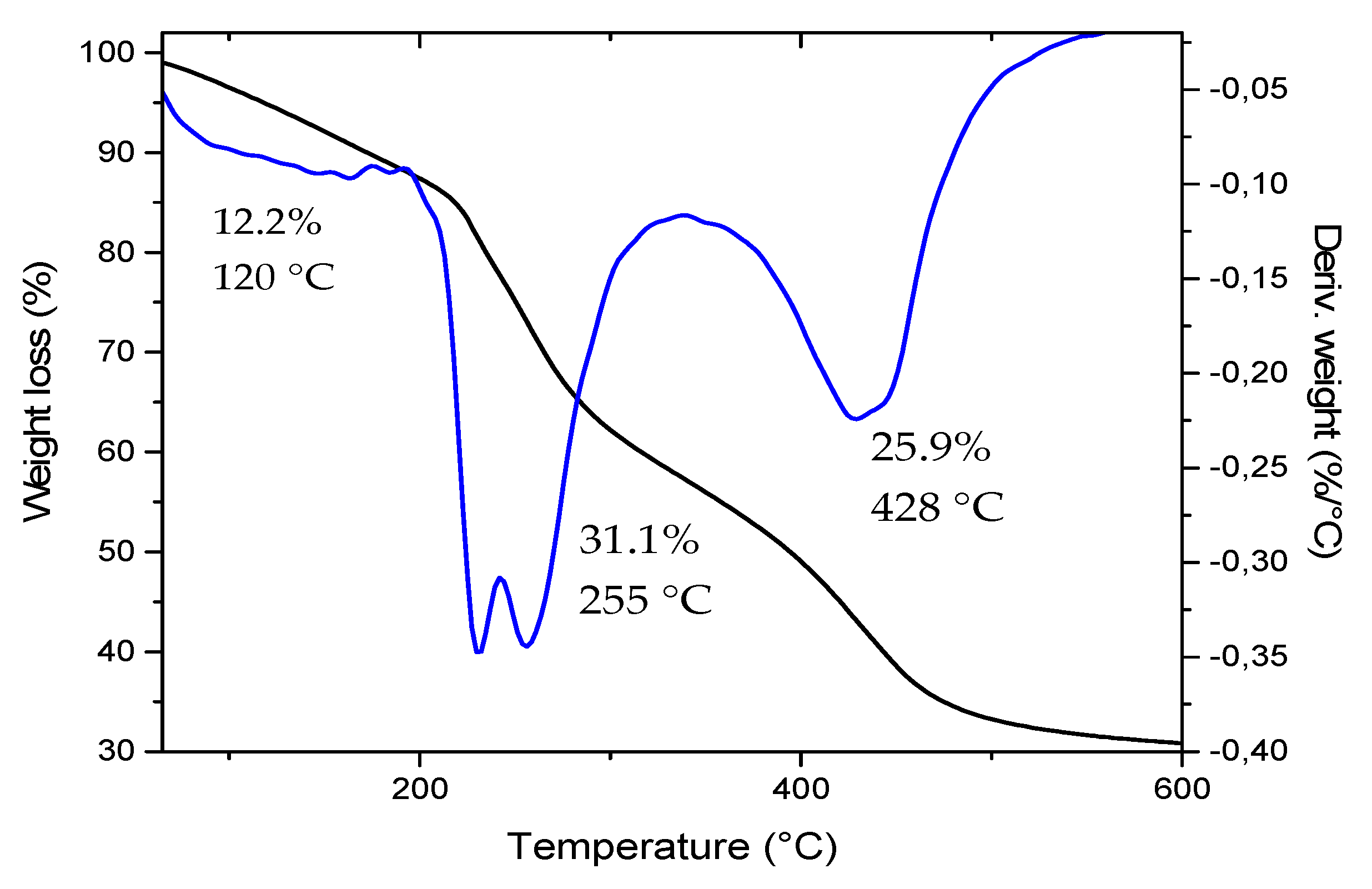
3.2.2. Thermogravimetric Analysis of the CB
3.3. Characterization of the Chitosan Beads Incorporating Thymus capitatus Essential Oil (CB-TCEO)
3.3.1. FTIR Analysis
3.3.2. Antioxidant Activity of CB-TCEO
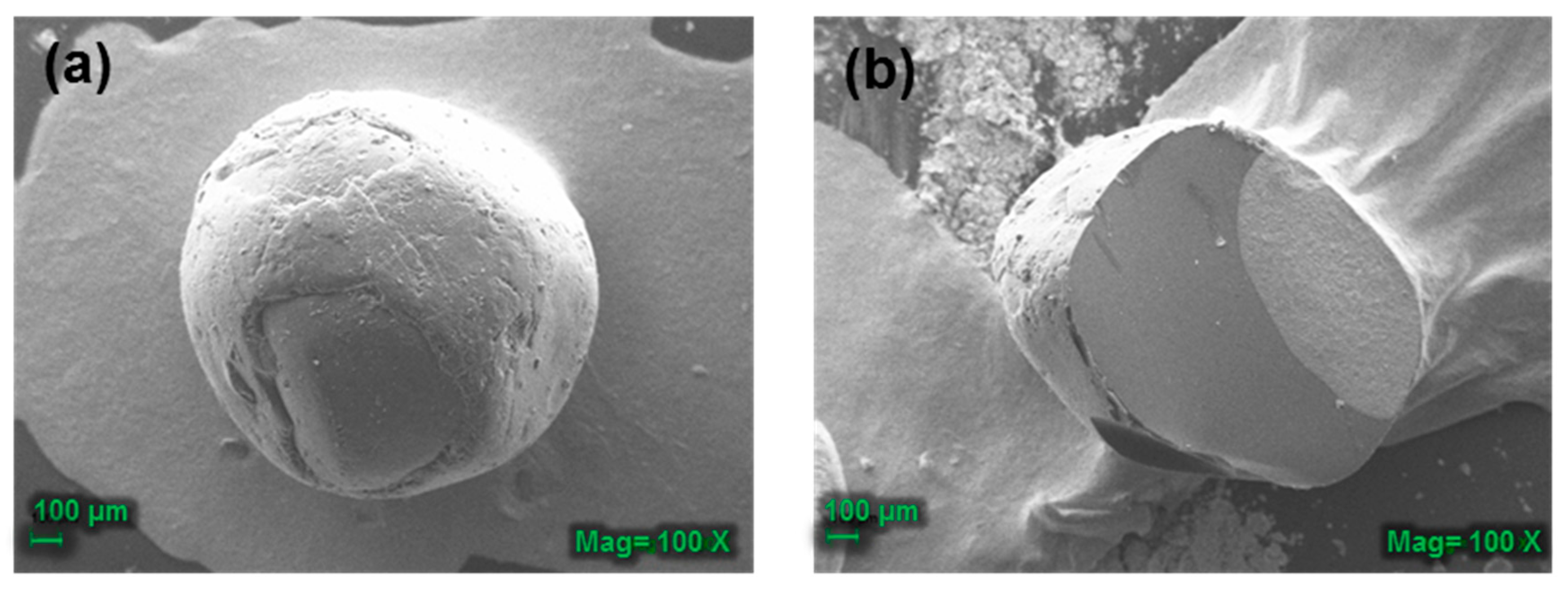
3.3.3. Scanning Electron Microscopy (SEM) Analysis of the CB-TCEO
3.3.4. Bacteria Inhibition In Vitro Capacity of the CB-TCEO
3.4. Antibacterial Analysis In Situ in Tilapia
3.4.1. Microbiological Analysis of the Muscle of the Fish Subjected to the Treatments
3.4.2. Determination of Total Volatile Nitrogen (TVB-N)
3.4.3. pH Analysis of the Tilapia Muscle
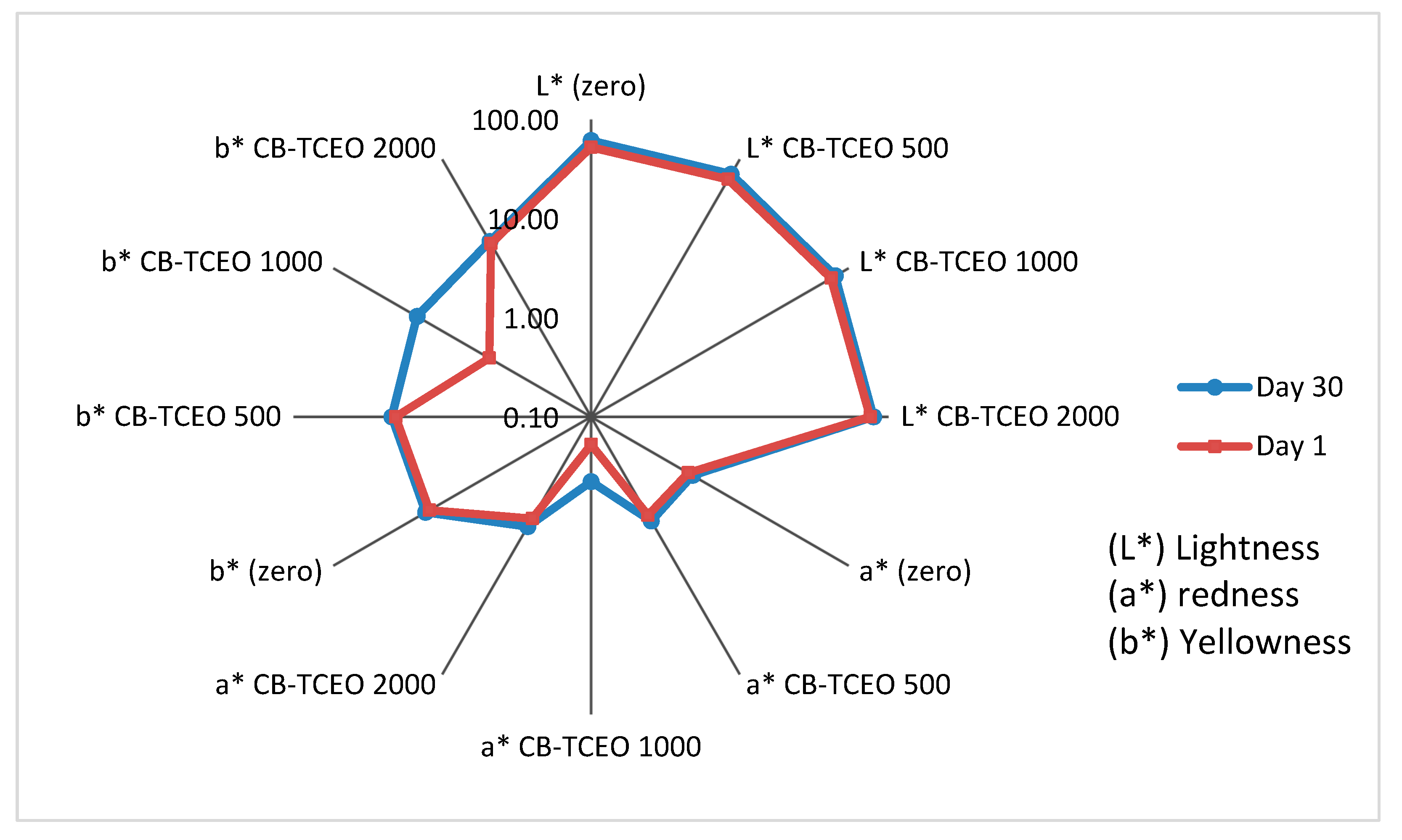
3.4.4. Colorimetric Analysis of the Tilapia Sample
3.4.5. Texture Profile Analysis (TPA) of the Tilapia Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Estado Mundial de la pesca y la Acuicultura: Cumplir los Objetivos de Desarrollo Sostenible; Organización de las Naciones Unidas Para la Alimentación y la Agricultura: Rome, Italy, 2016; Available online: http://www.fao.org/3/I9540ES/i9540es.pdf 2018 (accessed on 12 December 2018).
- Huss, H.H. El Pescado Fresco: Su Calidad y Cambios de su Calidad; FAO Documento Técnico de Pesca, FAO: Rome, Italy, 1999; Volume 348, ISBN 92-5-303507-2. [Google Scholar]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Sallam, K.I. Chemical, sensory, and shelf life evaluation of sliced salmon treated with salts of organic acids. Food Chem. 2007, 101, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Jamab, M.S.; Niazmand, R. Effect of essential oil of Mentha piperita and Ziziphora clinopodioides on Lactobacillus acidophilus activity as a bioyogurt starter culture. Am. Eurasian J. Agric. Environ. Sci. 2009, 6, 129–131. [Google Scholar]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Diverene, D.; Salerno, M. Effect of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crops Prod. 2016, 50, 304–311. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Márquez-Ríos, E.; Castillo-Yañez, F.J.; Graciano-Verdugo, A.Z.; Jiménez-Ruíz, E.I.; Lugo-Sánchez, M.E.; Maeda-Martínez, A.N. Impacto de las prácticas artesanales de captura y manejo postcaptura en la calidad del músculo de cazón. Interciencia 2011, 36, 672–676. [Google Scholar]
- Jeon, Y.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an edible film for quality preservation of Herring and Atlantic cod. J. Agric. Food Chem. 2002, 50, 5167–5178. [Google Scholar] [CrossRef]
- Iturriaga, L.; Olabarrieta, I.; Marañón, I.M. Antimicrobial assays of natural extracts and their inhibitory effect against Listeria innocua and fish spoilage bacteria, after incorporation into biopolymer edible films. Int. J. Food Microbiol. 2012, 158, 58–64. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruíz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Chemical composition of the essential oils obtained from some spices widely used in mediterranean region. Acta Chim. Slov. 2007, 54, 921–926. [Google Scholar]
- Colin, J.C.; Nolan, C.; Holub, J.B. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J. Funct. Foods 2009, 1, 38–43. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Pongsapakworawat, T.; Bangmo, P.; Sutthasupa, S. Effect of chitosan beads incorporated with lavender or red thyme essential oils in inhibiting Botrytis cinerea and their application in the strawberry packaging system. LWT Food Sci. Technol. 2016, 74, 14–20. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Serio, A.; Delgado-Ospina, J.; Paparella, A.; Rossi, C. Chitosan films incorporated with Thymus capitatus essential oil: Mechanical properties and antimicrobial activity against degradative bacterial species isolated from tuna (Thunnus sp.) and swordfish (Xiphias gladius). J. Food Sci. Technol. 2018, 55, 4256–4265. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Hleap, J.I.; Velasco, V.A. Physicochemical parameters during storage of sausages made from red Tilapia (oreochromis sp.). Biotechnol. Sect. Agropecu. Agroind. 2012, 10, 42–50. [Google Scholar]
- Perdones, A.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.; Pinotti, A. Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Ahmad, Z.; Akil, H.M. X-ray Diffraction Studies of Cross-Linked Chitosan with Different Crosslinking Agents For Waste Water Treatment Application. AIP Conf. Proc. 2010, 1202, 106–111. [Google Scholar] [CrossRef]
- Zhang, H.; Jung, J.; Zhao, Y. Preparation and characterization of cellulose nanocrystals films incorporated with essential oil loaded β-chitosan beads. Food Hidrocoll. 2017, 69, 164–172. [Google Scholar] [CrossRef]
- Barata, J.F.B.; Pinto, R.J.B.; Serra, V.I.R.C.V.; Silvestre, A.J.D.; Trindade, T.; Grac, M.; Neves, P.M.S.; Cavaleiro, J.A.S.; Daina, S.; Sadocco, P.; et al. Fluorescent Bioactive Corrole Grafted-Chitosan Films. Biomacromolecules 2016, 17, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Valencia, C.H.; Zuluaga, F.; Valencia, M.E.; Mina, J.H.; Grande-Tovar, C.D. Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [PubMed]
- Jabri-Karoui, I.; Bettaieb, I.; Msaada, K.; Hammami, M.; Marzouk, B. Research on the phenolic compounds and antioxidant activities of Tunisian Thymus capitatus. J. Funct. Foods 2012, 4, 661–669. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.; Molina-Corral, F.; Ayala-Zavala, F.; Olivas, G.; Gónzalez-Aguilar, G. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W. Fabrication of chitosan microspheres for efficient adsorption of methyl orange. Chin. J. Chem. Eng. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Tassou, C.C.; Drosinos, E.H.; Nychas, G.J.E. Effects of Essential Oil from Mint (Mentha Piperita) on Salmonella Enteritidis and Listeria Monocytogenes in Model Food Systems at 4° and 10 °C. J. Appl. Bacteriol. 1995, 78, 593–600. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Lin, C.M.; Preston, J.F.; Wei, C.I. Antibacterial mechanism of allyl isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef]
- Teixeira, B.; Marquesa, A.; Ramosa, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. J. Med. Chem. 2003, 10, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, G.; Lauzon, H.L.; Martinsdottir, E.; Kristbergsson, K. Influence of storage temperature on microbial spoilage characteristics of haddock fillets (Melanogrammus aeglefinus) evaluated by multivariate quality prediction. Int. J. Food Microbiol. 2006, 111, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Sun, J.; Chen, Y.; Qiu, J.; Zhang, Y.; Chi, Y. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009, 115, 66–70. [Google Scholar] [CrossRef]
- Byrne, L.; Lau, K.; Diamond, D. Monitoring of headspace total volatile basic nitrogen from selected fish species using reflectance spectroscopic measurements of pH-sensitive films. Analyst 2002, 127, 1338–1341. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Li, D.; Luo, Y. Effect of Chitosan and Garlic Essential Oil on Microbiological and Biochemical Changes that Affect Quality in Grass Carp (Ctenopharyngodon idellus) Fillets During Storage at 4 °C. J. Aquat. Food Prod. Technol. 2018, 27, 80–90. [Google Scholar] [CrossRef]
- Sánchez, L.; Cháfer, M.; Chiralt, A.; González, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- Lindahl, G.; Lundström, K.; Tornberg, E. Contribution of pigment content, myoglobin forms and internal reflectance to the color of pork loin and ham from pure breed pigs. Meat Sci. 2001, 59, 141–151. [Google Scholar] [CrossRef]
- Suárez-Mahecha, H.; De Francisco, A.; Beirão, L.H.; Pardo-Carrasco, S.; Córtes-Rodríguez, M. Post mortem loss of texture of fish meat during cold storage. Acta Biol. Colomb. 2007, 12, 3–18. [Google Scholar]
- Castillo-Yañez, F.J.; Pacheco-Aguilar, R.; Marquez-Ríos, E.; Lugo-Sánchez, M.E.; Lozano-Taylor, J. Freshness loss in sierra fish (Scomberomorus sierra) muscle stored in ice as affected by postcapture handling practices. J. Food Biochem. 2007, 31, 56–67. [Google Scholar] [CrossRef]
- Rahman, M.; Al-Farsi, S. Instrumental texture profile analysis (TPA) of date flesh as a function of moisture content. J. Food Eng. 2005, 66, 505–511. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Sample | DPPH µmol Trolox (eq/g TCEO) | ABTS µmol Trolox (eq/g TCEO) | TPC (mg GAE/g TCEO) |
|---|---|---|---|
| TCEO | 1833 ± 32 a | 12.50 ± 0.008 e | 0.32 ± 0.03 a |
| CB | 2085 ± 27 b | 6.89 ± 0.006 a | 1.92 ± 0.05 b |
| CB-TCEO 500 | 6200 ± 23 c | 7.53 ± 0.011 b | 3.36 ± 0.06 c |
| CB-TCEO 1000 | 7624 ± 21 d | 8.11 ± 0.06 c | 3.51 ± 0.06 d |
| CB-TCEO 2000 | 9239 ± 20 e | 8.95 ± 0.05 d | 4.25 ± 0.07 e |
| Sample | The Diameter of the Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|
| B. cereus | S. aureus | S. enterica | E. coli | V. parahaemolyticus | |
| CB | - | - | - | - | - |
| CB-TCEO 500 | 9.4 ± 1.5 a | 5.7 ± 1.0 a | 4.2 ± 0.8 a | 2.0 ± 0.5 a | - |
| CB-TCEO 1000 | 22.9 ± 1.9 b | 19.8 ± 2.1 b | 17.5 ± 2.5 b | 15.3 ± 1.9 b | 13.1 ± 1.6 a |
| CB-TCEO 2000 | 37.2 ± 2.5 c | 34.1 ± 2.3 c | 30.8 ± 3.9 c | 25.6 ± 2.5 c | 23.5 ± 2.7 b |
| Chloramphenicol 250 mgL−1 | 40.0 ± 1.6 c | 39.5 ± 2.0 d | 39.7 ± 2.1 d | 37.2 ± 1.8 d | 36.1 ± 3.2 c |
| Day | CB-TCEO | Microorganism (UFC/g) | |||
|---|---|---|---|---|---|
| Salmonella spp. * | Vibrio cholerae * | Coliforms Yotal | S. aureus Coagulase-Positive | ||
| Requirement NTC 1322 | Absence | Absence | <10 | <100 | |
| 1 | 0 | 10 | 10 | <10 | <100 |
| 500 | <10 | <10 | Absence | <10 | |
| 1000 | <10 | <10 | Absence | <10 | |
| 2000 | Absence | Absence | Absence | Absence | |
| 5 | 0 | <100 | <100 | <10 | <100 |
| 500 | <10 | <10 | Absence | <10 | |
| 1000 | <10 | <10 | Absence | <10 | |
| 2000 | Absence | Absence | Absence | Absence | |
| 9 | 0 | <100 | <100 | <100 | <1000 |
| 500 | <10 | <10 | Absence | <100 | |
| 1000 | <10 | <10 | Absence | <10 | |
| 2000 | Absence | Absence | Absence | Absence | |
| 13 | 0 | <1000 | <1000 | <100 | <1000 |
| 500 | <100 | <100 | Absence | <100 | |
| 1000 | <10 | <10 | Absence | <10 | |
| 2000 | Absence | Absence | Absence | Absence | |
| 16 | 0 | <1000 | <1000 | <1000 | <10,000 |
| 500 | <100 | <100 | Absence | <100 | |
| 1000 | <10 | <10 | Absence | <10 | |
| 2000 | Absence | Absence | Absence | Absence | |
| 19 | 0 | <10,000 | <10,000 | <1000 | <10,000 |
| 500 | <100 | <100 | Absence | <100 | |
| 1000 | <100 | <100 | Absence | <10 | |
| 2000 | Absence | Absence | Absence | Absence | |
| 23 | 0 | <10,000 | <10,000 | <1000 | <10,000 |
| 500 | <100 | <100 | Absence | <1000 | |
| 1000 | <100 | <100 | Absence | <100 | |
| 2000 | Absence | Absence | Absence | Absence | |
| 27 | 0 | <10,000 | <10,000 | <1000 | <100,000 |
| 500 | <1000 | <1000 | Absence | <1000 | |
| 1000 | <100 | <100 | Absence | <100 | |
| 2000 | Absence | Absence | Absence | Absence | |
| Sample | pH/Day | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 9 | 13 | 16 | 19 | 23 | 27 | |
| CB | 6.51 ± 0.01 a | 6.58 ± 0.02 c | 6.61 ± 0.03 b | 6.65 ± 0.02 c | 6.72 ± 0.05 c | 6.80 ± 0.02 c | 6.85 ± 0.03 c | 6.90 ± 0.03 c |
| CB-TCEO 500 | 6.51 ± 0.01 a | 6.53 ± 0.03 b | 6.57 ± 0.04 b | 6.60 ± 0.03 b | 6.64 ± 0.02 b | 6.71 ± 0.01 b | 6.76 ± 0.03 b | 6.81 ± 0.02 b |
| CB-TCEO 1000 | 6.51 ± 0.02 a | 6.47 ± 0.03 a | 6.51 ± 0.03 a | 6.52 ± 0.02 a | 6.58 ± 0.03 a | 6.63 ± 0.03 a | 6.68 ± 0.01 a | 6.73 ± 0.03 a |
| CB-TCEO 2000 | 6.50 ± 0.02 a | 6.44 ± 0.04 a | 6.47 ± 0.04 a | 6.50 ± 0.03 a | 6.55 ± 0.05 a | 6.60 ± 0.02 a | 6.65 ± 0.04 a | 6.70 ± 0.01 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia Junca, M.A.; Valencia, C.; Flórez López, E.; Delgado-Ospina, J.; Zapata, P.A.; Solano, M.; Grande Tovar, C.D. Chitosan Beads Incorporated with Essential Oil of Thymus capitatus: Stability Studies on Red Tilapia Fillets. Biomolecules 2019, 9, 458. https://doi.org/10.3390/biom9090458
Valencia Junca MA, Valencia C, Flórez López E, Delgado-Ospina J, Zapata PA, Solano M, Grande Tovar CD. Chitosan Beads Incorporated with Essential Oil of Thymus capitatus: Stability Studies on Red Tilapia Fillets. Biomolecules. 2019; 9(9):458. https://doi.org/10.3390/biom9090458
Chicago/Turabian StyleValencia Junca, Mayra Alejandra, Cesar Valencia, Edwin Flórez López, Johannes Delgado-Ospina, Paula A. Zapata, Moisés Solano, and Carlos David Grande Tovar. 2019. "Chitosan Beads Incorporated with Essential Oil of Thymus capitatus: Stability Studies on Red Tilapia Fillets" Biomolecules 9, no. 9: 458. https://doi.org/10.3390/biom9090458