Endogenous Neuropeptide Nocistatin Is a Direct Agonist of Acid-Sensing Ion Channels (ASIC1, ASIC2 and ASIC3)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis of Nocistatin
2.2. Electrophysiological Studies on Xenopus Laevis Oocytes
2.3. Data and Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Nocistatin Directly Activates ASICs the Same As Protons
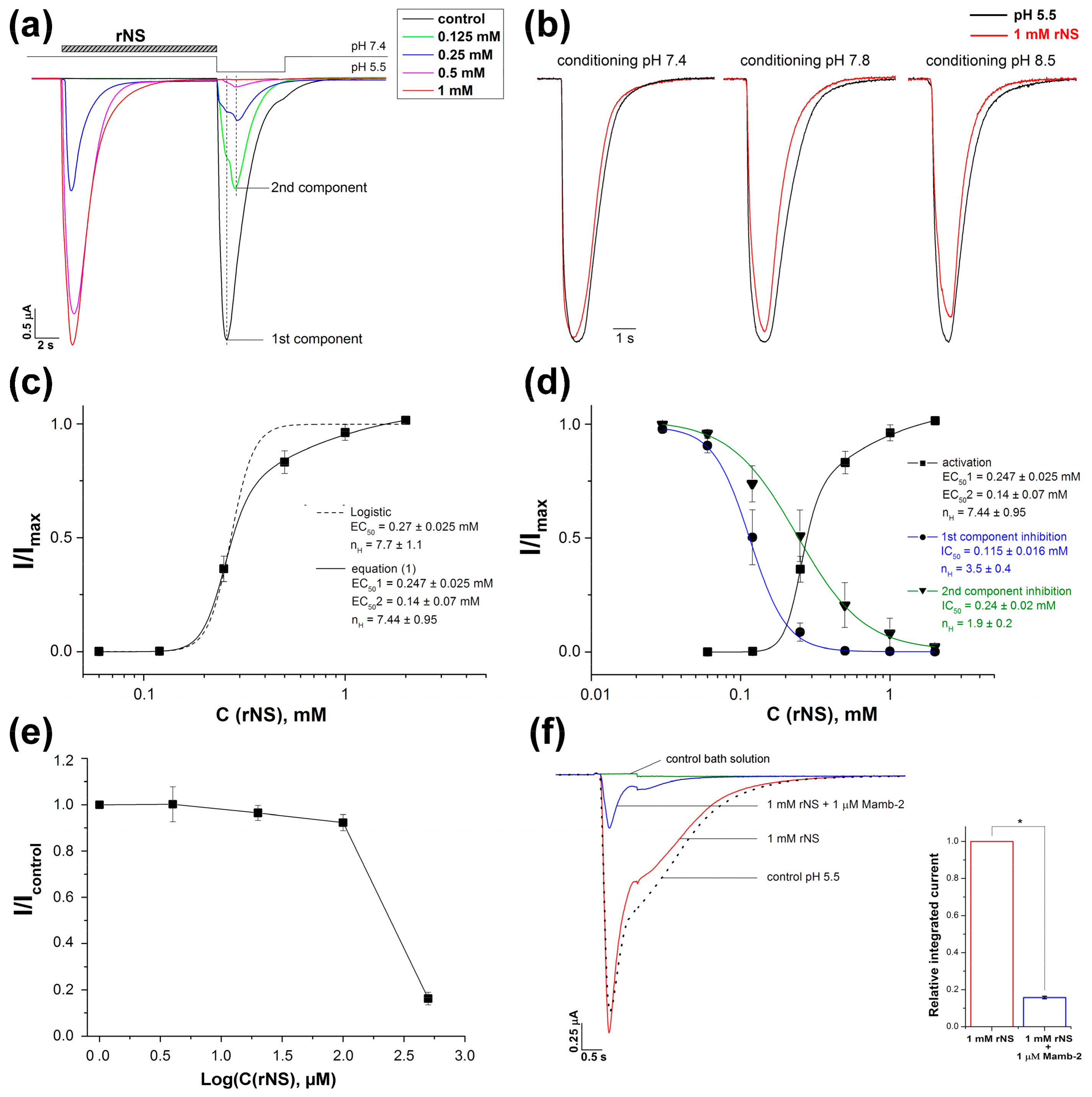
3.2. Effect of Nocistatin on Rat ASIC1a Channels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wemmie, J.A.; Price, M.P.; Welsh, M.J. Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci. 2006, 29, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, T.W.; Lee, K.G.; Gormley, M.G.; Askwith, C.C. Heteromeric ASIC channels composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J. Neurosci. 2011, 31, 9723–9734. [Google Scholar] [CrossRef] [PubMed]
- Yagi, J.; Wenk, H.N.; Naves, L.A.; McCleskey, E.W. Sustained Currents Through ASIC3 Ion Channels at the Modest pH Changes That Occur During Myocardial Ischemia. Circ. Res. 2006, 99, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deval, E.; Noël, J.; Lay, N.; Alloui, A.; Diochot, S.; Friend, V.; Jodar, M.; Lazdunski, M.; Lingueglia, E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008, 27, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Deval, E.; Noël, J.; Gasull, X.; Delaunay, A.; Alloui, A.; Friend, V.; Eschalier, A.; Lazdunski, M.; Lingueglia, E. Acid-Sensing Ion Channels in Postoperative Pain. J. Neurosci. 2011, 31, 6059–6066. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yan, W.W.; Zhang, X.L.; Liu, H.; Zhang, L.C. ASIC3 in the cerebrospinal fluid-contacting nucleus of brain parenchyma contributes to inflammatory pain in rats. Neurol. Res. 2014, 36, 270–275. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Andreev, Y.A.; Kozlov, S.A. Acid-sensing ion channels and their modulators. Biochemistry (Moscow) 2014, 79, 1528–1545. [Google Scholar] [CrossRef]
- Mazzuca, M.; Heurteaux, C.; Alloui, A.; Diochot, S.; Baron, A.; Voilley, N.; Blondeau, N.; Escoubas, P.; Gélot, A.; Cupo, A.; et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat. Neurosci. 2007, 10, 943–945. [Google Scholar] [CrossRef]
- Yan, J.; Wei, X.; Bischoff, C.; Edelmayer, R.M.; Dussor, G. pH-evoked dural afferent signaling is mediated by ASIC3 and is sensitized by mast cell mediators. Headache: J. Head Face Pain 2013, 53, 1250–1261. [Google Scholar] [CrossRef]
- Waldmann, R.; Bassilana, F.; de Weille, J.; Champigny, G.; Heurteaux, C.; Lazdunski, M. Molecular Cloning of a Non-inactivating Proton-gated Na + Channel Specific for Sensory Neurons. J. Biol. Chem. 1997, 272, 20975–20978. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.P.; Benson, C.J.; Adelman, J.P.; McCleskey, E.W. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Babini, E.; Paukert, M.; Gründer, S.; Geisler, H.-S. Alternative Splicing and Interaction with Di- and Polyvalent Cations Control the Dynamic Range of Acid-sensing Ion Channel 1 (ASIC1). J. Boil. Chem. 2002, 277, 41597–41603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alijevic, O.; Kellenberger, S. Subtype-specific Modulation of Acid-sensing Ion Channel (ASIC) Function by 2-Guanidine-4-methylquinazoline. J. Boil. Chem. 2012, 287, 36059–36070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.-P.; Xiong, Z.-G. Physiological and pathological functions of acid-sensing ion channels in the central nervous system. Curr. Drug Targets 2012, 13, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bartoi, T.; Augustinowski, K.; Polleichtner, G.; Gründer, S.; Ulbrich, M.H. Acid-sensing ion channel (ASIC) 1a/2a heteromers have a flexible 2:1/1:2 stoichiometry. Proc. Natl. Acad. Sci. USA 2014, 111, 8281–8286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlen, C.J.; Chesler, A.T.; Sharif-Naeini, R.; Medzihradszky, K.F.; Zhou, S.; King, D.; Sánchez, E.E.; Burlingame, A.L.; Basbaum, A.I.; Julius, D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 2011, 479, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Z.; Li, W.-G.; Cao, H.; Feng, E.-G.; Yu, F.; Liu, H.; Jiang, H.; Xu, T.-L. A Nonproton Ligand Sensor in the Acid-Sensing Ion Channel. Neuron 2010, 68, 61–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, E.C.; Wang, Y.; Yang, L.Q.; So, K.H.; Lo, Y.-C.; Wu, S.-N. Multiple regulatory actions of 2-guanidine-4-methylquinazoline (GMQ), an agonist of acid-sensing ion channel type 3, on ionic currents in pituitary GH 3 cells and in olfactory sensory (Rolf B1. T) neurons. Biochem. Pharmacol. 2018, 151, 79–88. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Koshelev, S.G.; Andreev, Y.A.; Dubinnyi, M.A.; Kublitski, V.S.; Efremov, R.G.; Sobolevsky, A.I.; Kozlov, S.A. Proton-independent activation of acid-sensing ion channel 3 by an alkaloid, lindoldhamine, from Laurus nobilis. Br. J. Pharmacol. 2018, 175, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Marra, S.; Ferru-Clément, R.; Breuil, V.; Delaunay, A.; Christin, M.; Friend, V.; Sebille, S.; Cognard, C.; Ferreira, T.; Roux, C.; et al. Non-acidic activation of pain-related Acid-Sensing Ion Channel 3 by lipids. EMBO J. 2016, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Koshelev, S.G.; Andreev, Y.A.; Kozlov, S.A. Endogenous Isoquinoline Alkaloids Agonists of Acid-Sensing Ion Channel Type 3. Front. Mol. Neurosci. 2017, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Vyvers, A.; Schmidt, A.; Wiemuth, D.; Gründer, S. Screening of 109 neuropeptides on ASICs reveals no direct agonists and dynorphin A, YFMRFamide and endomorphin-1 as modulators. Sci. Rep. 2018, 8, 18000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boom, A.; Mollereau, C.; Meunier, J.-C.; Vassart, G.; Parmentier, M.; Vanderhaeghen, J.-J.; Schiffmann, S.; Schiffmann, S. Distribution of the nociceptin and nocistatin precursor transcript in the mouse central nervous system. Neuroscience 1999, 91, 991–1007. [Google Scholar] [CrossRef]
- Andoh, T.; Itoh, M.; Kuraishi, Y. Nociceptin gene expression in rat dorsal root ganglia induced by peripheral inflammation. Neuroreport 1997, 8, 2793–2796. [Google Scholar] [CrossRef] [PubMed]
- Okuda-Ashitaka, E.; Ito, S. Nocistatin: Milestone of one decade of research. Curr. Pharm. Des. 2015, 21, 868–884. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Inoue, K. Nociceptin/orphanin FQ and nocistatin on learning and memory impairment induced by scopolamine in mice. Br. J. Pharmacol. 1999, 127, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Gavioli, E.C.; Rae, A.G.; Calo’, G.; Guerrini, R.; De Lima, T.C.M. Central injections of nocistatin or its C-terminal hexapeptide exert anxiogenic-like effect on behaviour of mice in the plus-maze test. Br. J. Pharmacol. 2002, 136, 764–772. [Google Scholar] [CrossRef]
- Okuda-Ashitaka, E.; Minami, T.; Tachibana, S.; Yoshihara, Y.; Nishiuchi, Y.; Kimura, T.; Ito, S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature 1998, 392, 286–289. [Google Scholar] [CrossRef]
- Inoue, M.; Kawashima, T.; Allen, R.G.; Ueda, H. Nocistatin and Prepro-Nociceptin/Orphanin FQ 160–187 Cause Nociception through Activation of Gi/o in Capsaicin-Sensitive and of Gs in Capsaicin-Insensitive Nociceptors, Respectively. J. Pharmacol. Exp. Ther. 2003, 306, 141–146. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Yeh, T.; Chou, A.; Weng, Y.; Wang, H. Nocistatin excites rostral agranular insular cortex-periaqueductal gray projection neurons by enhancing transient receptor potential cation conductance via Gαq/11-PLC-protein kinase C pathway. Neuroscience 2010, 168, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Okuda-Ashitaka, E.; Minami, T.; Tsubouchi, S.; Kiyonari, H.; Iwamatsu, A.; Noda, T.; Handa, H.; Ito, S. Identification of NIPSNAP1 as a Nocistatin-interacting Protein Involving Pain Transmission. J. Boil. Chem. 2012, 287, 10403–10413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santillan, M. On the Use of the Hill Functions in Mathematical Models of Gene Regulatory Networks. Math. Model. Nat. Phenom. 2008, 3, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Osmakov, D.I.; Koshelev, S.G.; Lyukmanova, E.N.; Shulepko, M.A.; Andreev, Y.A.; Illes, P.; Kozlov, S.A. Multiple Modulation of Acid-Sensing Ion Channel 1a by the Alkaloid Daurisoline. Biomolecules 2019, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.; Lee, C.-H.; Kalbacher, H.; Tian, Y.; Hung, C.-H.; Schmidt, A.; Prokop, L.; Kauferstein, S.; Mebs, D.; Chen, C.-C.; et al. Identification of a cono-RFamide from the venom of Conus textile that targets ASIC3 and enhances muscle pain. Proc. Natl. Acad. Sci. USA 2017, 114, E3507–E3515. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Chen, J.; Askwith, C.C.; Hruska-Hageman, A.M.; Price, M.P.; Nolan, B.C.; Yoder, P.G.; Lamani, E.; Hoshi, T.; Freeman, J.H.; et al. The Acid-Activated Ion Channel ASIC Contributes to Synaptic Plasticity, Learning, and Memory. Neuron 2002, 34, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yu, Y.; Li, W.-G.; Yu, F.; Cao, H.; Xu, T.-L.; Jiang, H. Inherent Dynamics of the Acid-Sensing Ion Channel 1 Correlates with the Gating Mechanism. PLoS Boil. 2009, 7, e1000151. [Google Scholar] [CrossRef]
- Schuhmacher, L.-N.; Smith, E.S.J. Expression of acid-sensing ion channels and selection of reference genes in mouse and naked mole rat. Mol. Brain 2016, 9, 99. [Google Scholar] [CrossRef]
- Sherwood, T.W.; Askwith, C.C. Endogenous arginine-phenylalanine-amide-related peptides alter steady-state desensitization of ASIC1a. J. Biol. Chem. 2008, 283, 1818–1830. [Google Scholar] [CrossRef]
- Diochot, S.; Baron, A.; Rash, L.D.; Deval, E.; Escoubas, P.; Scarzello, S.; Salinas, M.; Lazdunski, M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004, 23, 1516–1525. [Google Scholar] [CrossRef]
- Diochot, S.; Baron, A.; Salinas, M.; Douguet, D.; Scarzello, S.; Dabert-Gay, A.-S.; Debayle, D.; Friend, V.; Alloui, A.; Lazdunski, M.; et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012, 490, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Yoder, N.; Yoshioka, C.; Gouaux, E. Gating mechanisms of acid sensing ion channels. Nature 2018, 555, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.H.; Li, C.; Govindasamy, M.; Neo, H.J.; Lee, T.L.; Low, C.M.; Tachibana, S. Elevated prepronociceptin, nociceptin/orphanin FQ and nocistatin concentrations in rat chronic constriction nerve injury and diabetic neuropathic pain models. Neurosci. Lett. 2012, 506, 104–106. [Google Scholar] [CrossRef]
- Gründer, S.; Pusch, M. Biophysical properties of acid-sensing ion channels (ASICs). Neuropharmacol 2015, 94, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T. Total Number of Synapses in the Adult Human Neocortex. Undergrad. J. Math. Model. 2010, 3. [Google Scholar] [CrossRef]
- Hay, D.L.; Walker, C.S. CGRP and its receptors. Headache J. Head Face Pain 2017, 57, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, T.W.; Askwith, C.C. Dynorphin opioid peptides enhance acid sensing ion channel 1a activity and acidosis induced neuronal death. J. Neurosci. 2009, 29, 14371–14380. [Google Scholar] [CrossRef]
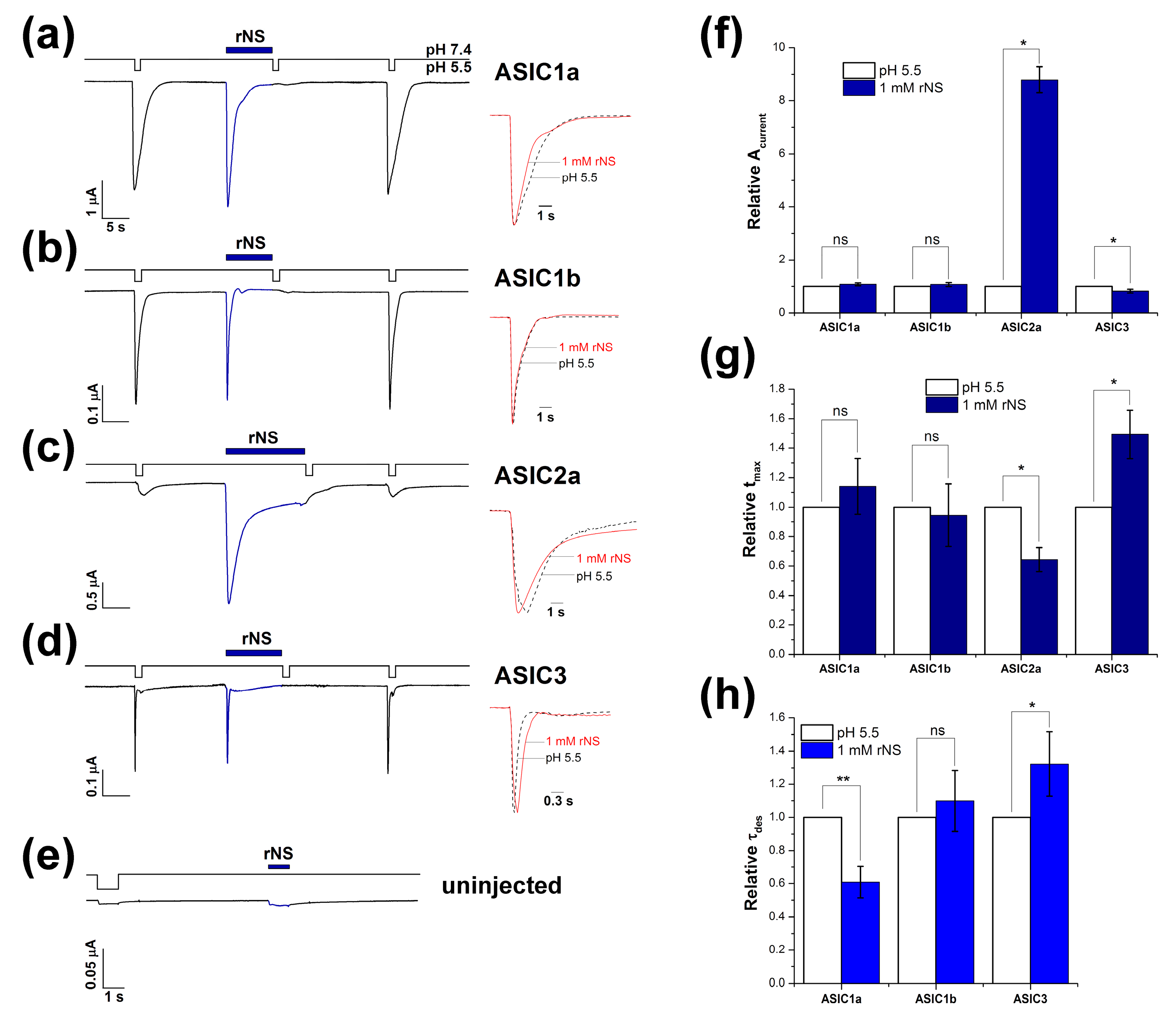
| Isoform | Amplitude, nA | tmax, s | τdes, s | |||
|---|---|---|---|---|---|---|
| pH 5.5 | rNS | pH 5.5 | rNS | pH 5.5 | rNS | |
| ASIC1a | 3300 ± 522 | 3525 ± 497 | 0.47 ± 0.08 | 0.52 ± 0.11 | 2.6 ± 0.4 | 1.4 ± 0.1 |
| ASIC1b | 762 ± 212 | 869 ± 285 | 0.32 ± 0.06 | 0.27 ± 0.03 | 0.51 ± 0.09 | 0.5 ± 0.06 |
| ASIC2a | 182 ± 20 (1039 ± 43) 1 | 1609 ± 246 | 1.45 ± 0.19 (1.27 ± 0.15) 1 | 0.94 ± 0.19 | ND (7.6 ± 0.5) 1 | 2.22 ± 0.16 |
| ASIC3 | 210 ± 62 | 165 ± 46 | 0.17 ± 0.02 | 0.25 ± 0.03 | 0.33 ± 0.09 | 0.4 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmakov, D.I.; Koshelev, S.G.; Ivanov, I.A.; Andreev, Y.A.; Kozlov, S.A. Endogenous Neuropeptide Nocistatin Is a Direct Agonist of Acid-Sensing Ion Channels (ASIC1, ASIC2 and ASIC3). Biomolecules 2019, 9, 401. https://doi.org/10.3390/biom9090401
Osmakov DI, Koshelev SG, Ivanov IA, Andreev YA, Kozlov SA. Endogenous Neuropeptide Nocistatin Is a Direct Agonist of Acid-Sensing Ion Channels (ASIC1, ASIC2 and ASIC3). Biomolecules. 2019; 9(9):401. https://doi.org/10.3390/biom9090401
Chicago/Turabian StyleOsmakov, Dmitry I., Sergey G. Koshelev, Igor A. Ivanov, Yaroslav A. Andreev, and Sergey A. Kozlov. 2019. "Endogenous Neuropeptide Nocistatin Is a Direct Agonist of Acid-Sensing Ion Channels (ASIC1, ASIC2 and ASIC3)" Biomolecules 9, no. 9: 401. https://doi.org/10.3390/biom9090401





