Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague–Dawley Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
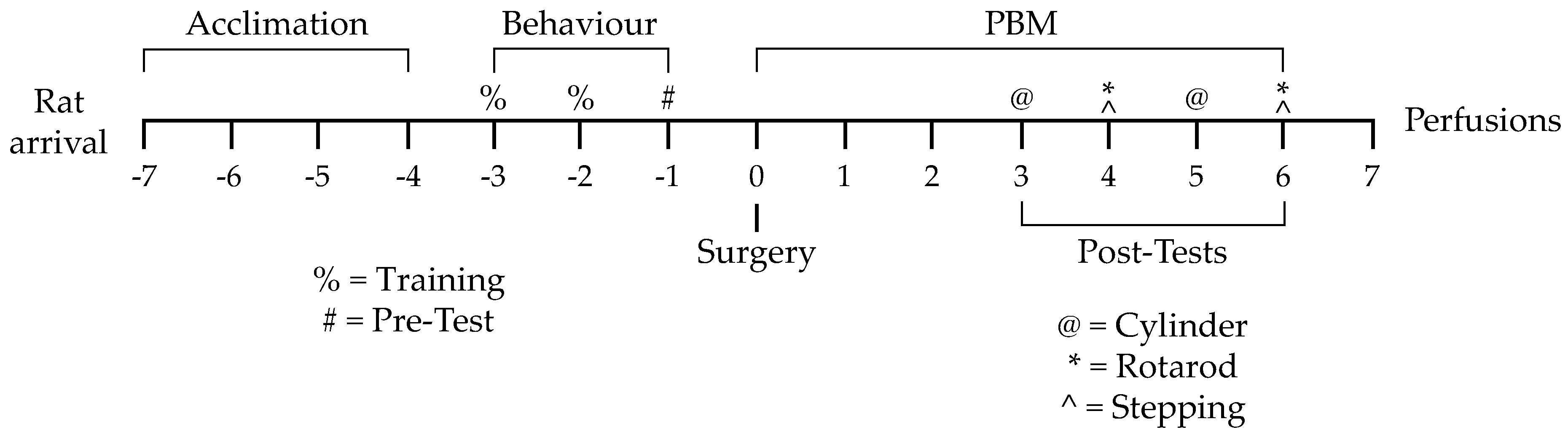
2.2. Experimental Design
2.3. Behavioural Training and Pretesting
2.4. Intracranial Microinjection Surgery
2.5. PBM and Post-Surgery Behavioural Testing
2.6. Tissue Extraction and Sectioning
2.7. Immunohistochemistry
2.8. Stereological Analysis
2.9. Behaviour Analysis
2.10. Statistical Analysis
3. Results
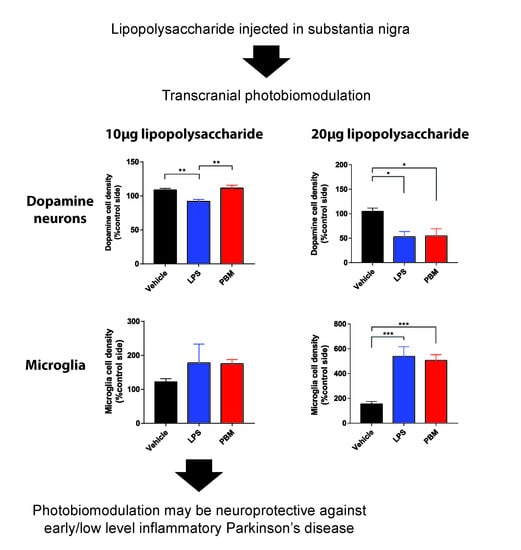
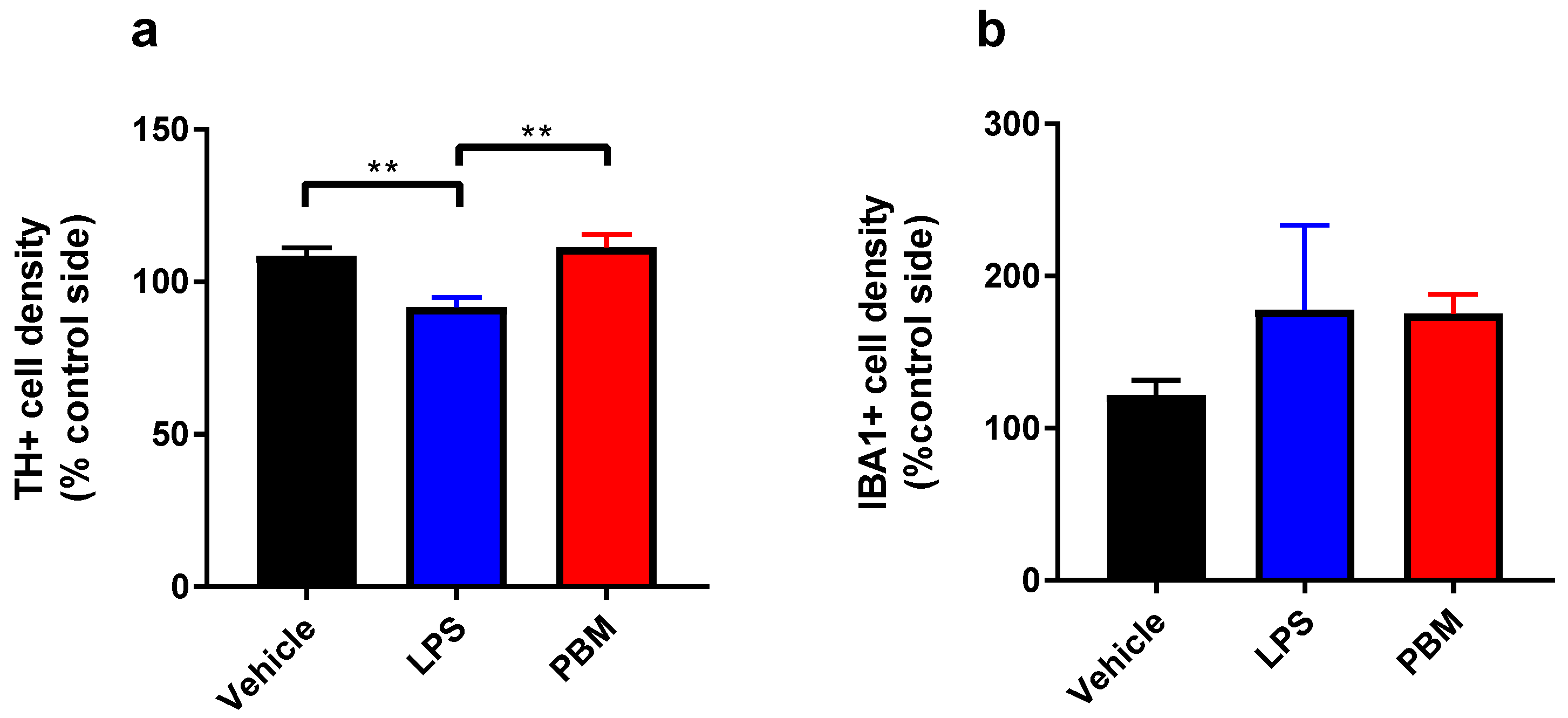
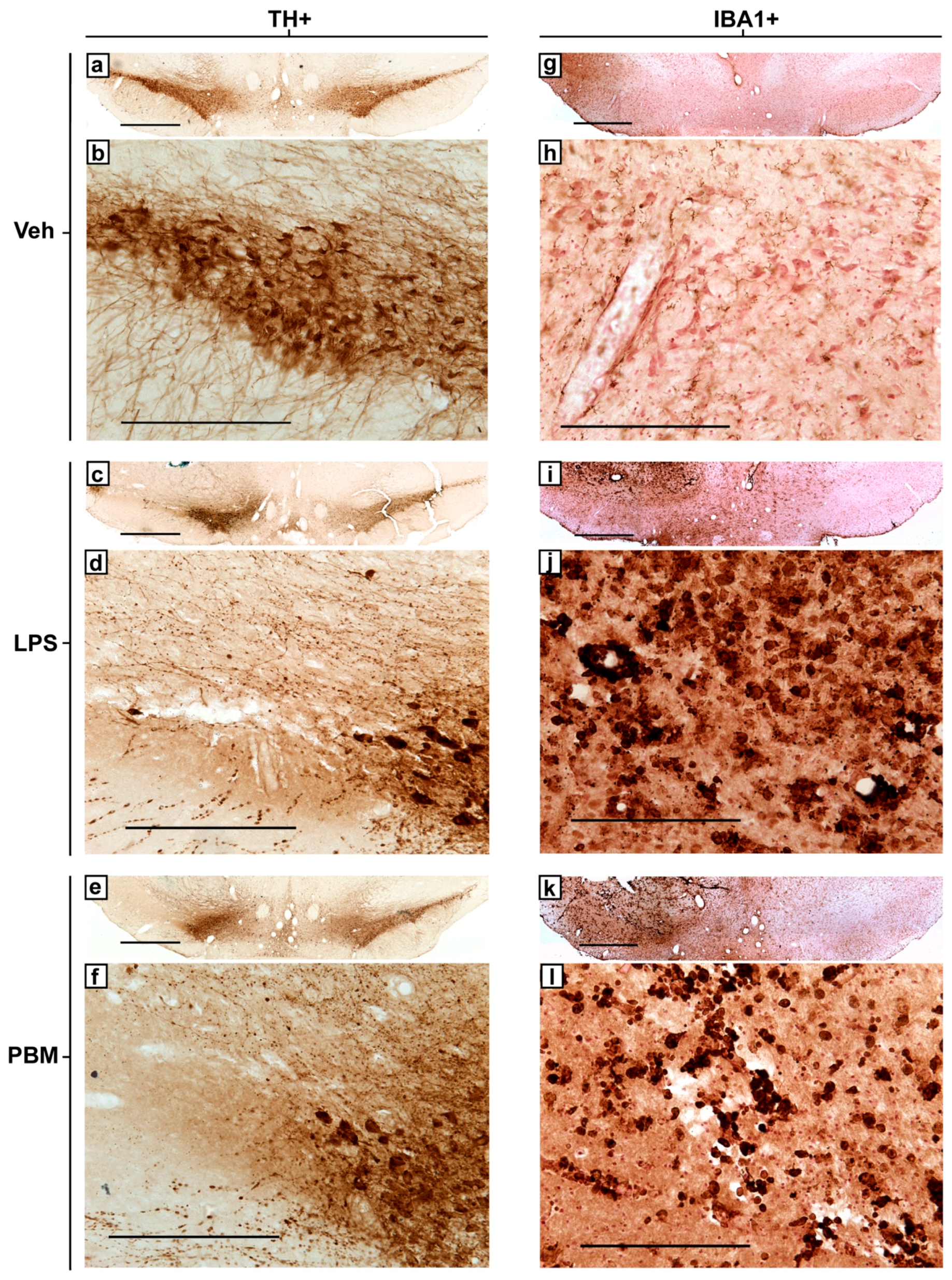
3.1. Ten Microgram LPS Paradigm
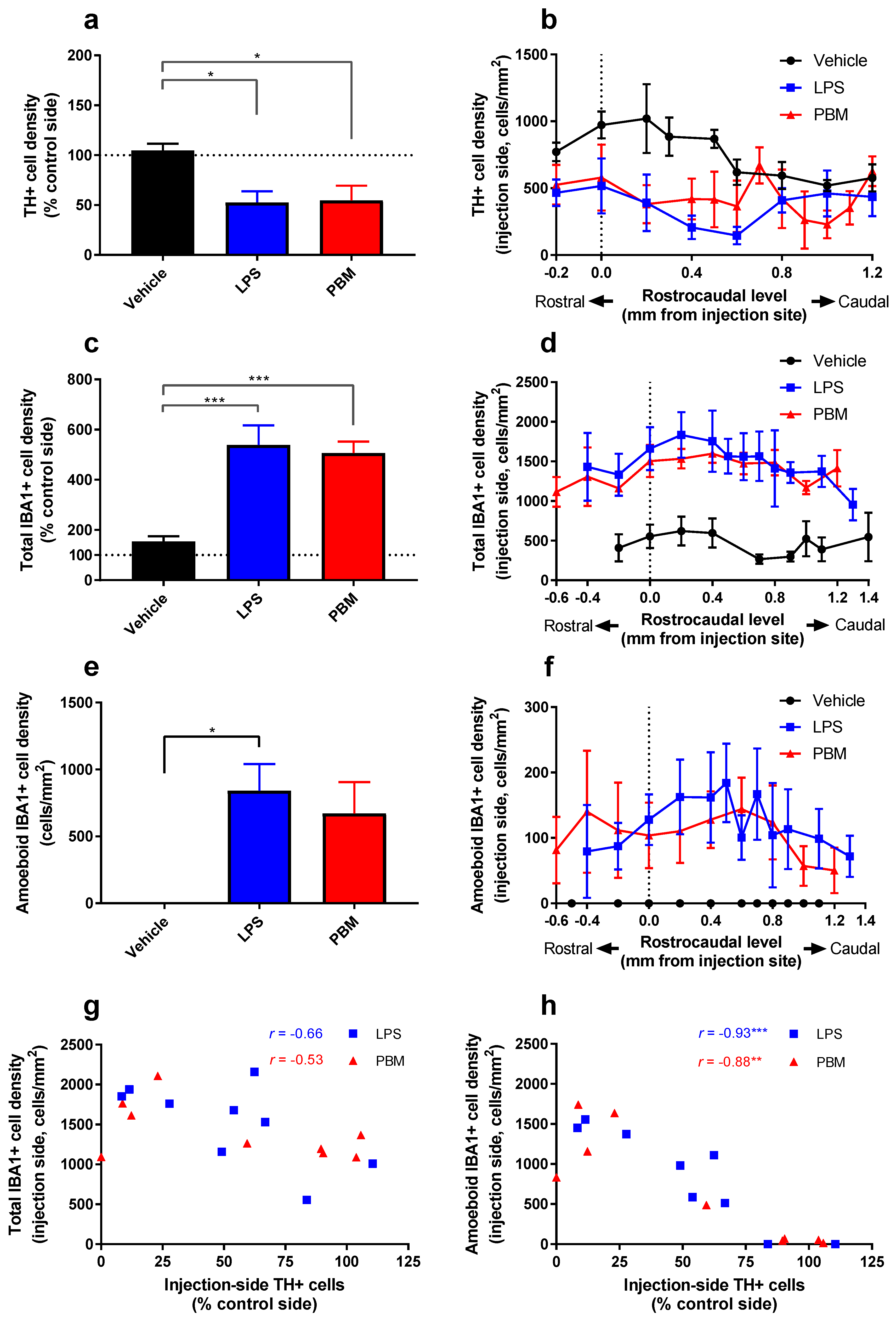
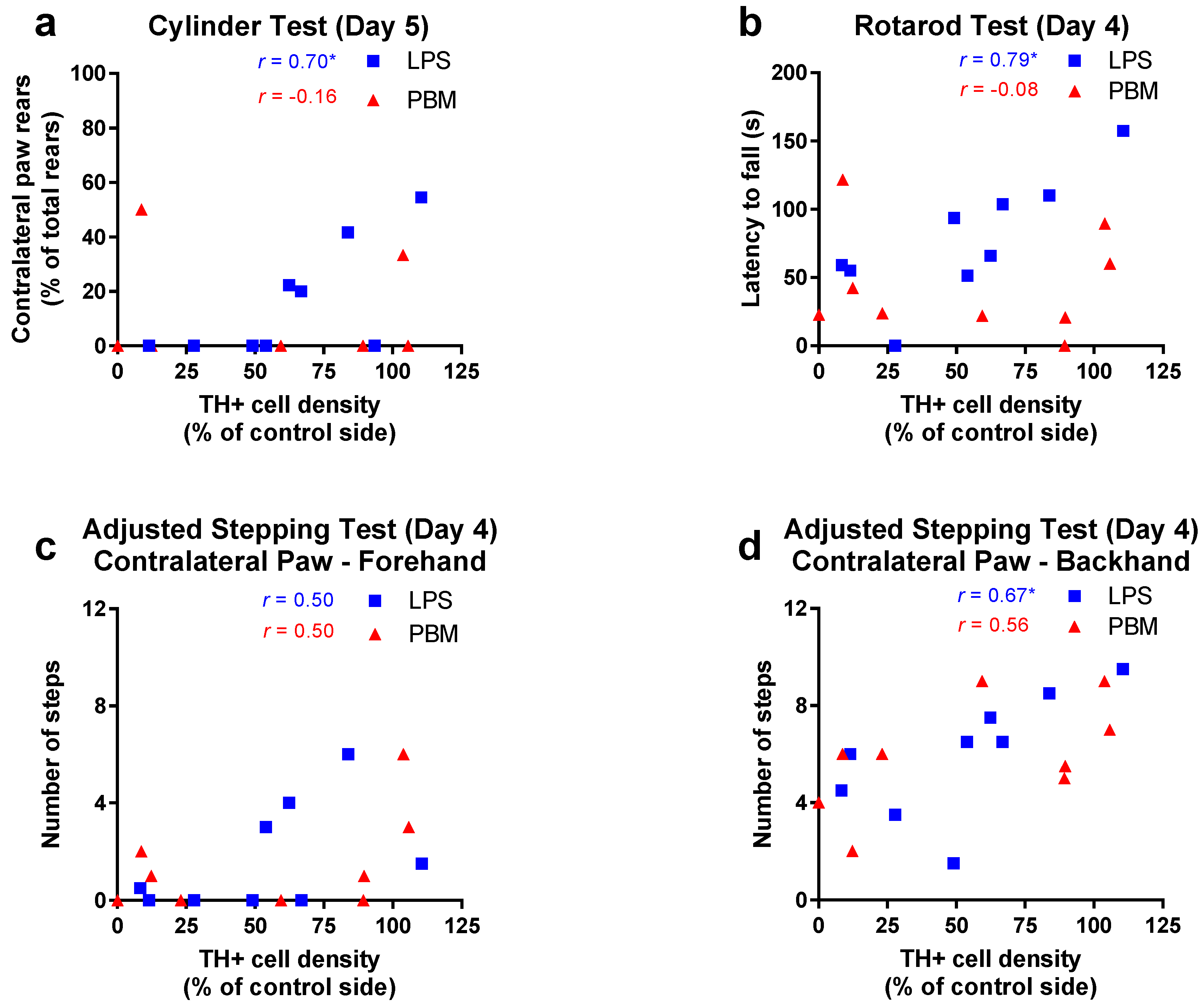
3.2. Twenty Micrograms LPS Paradigm
4. Discussion
4.1. PBM Neuroprotection
4.2. Microglial Reactivity
4.3. PBM and Motor Behaviour
4.4. Considerations of the LPS Model and Study Limitations
4.5. Clinical Prospects of PBM
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halliday, G.; Lees, A.; Stern, M. Milestones in Parkinson’s disease—Clinical and pathologic features. Mov. Disord. 2011, 26, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia Nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Croisier, E.; Moran, L.B.; Dexter, D.T.; Pearce, R.K.B.; Graeber, M.B. Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J. Neuroinflamm. 2005, 2. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Darlot, F.; Moro, C.; El Massri, N.; Chabrol, C.; Johnstone, D.M.; Reinhart, F.; Agay, D.; Torres, N.; Bekha, D.; Auboiroux, V.; et al. Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann. Neurol. 2016, 79, 59–75. [Google Scholar] [CrossRef]
- Shaw, V.E.; Spana, S.; Ashkan, K.; Benabid, A.-L.; Stone, J.; Baker, G.E.; Mitrofanis, J. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 2010, 518, 25–40. [Google Scholar] [CrossRef]
- El Massri, N.; Moro, C.; Torres, N.; Darlot, F.; Agay, D.; Chabrol, C.; Johnstone, D.M.; Stone, J.; Benabid, A.-L.; Mitrofanis, J. Near-infrared light treatment reduces astrogliosis in MPTP-treated monkeys. Exp. Brain Res. 2016, 234, 3225–3232. [Google Scholar] [CrossRef]
- Reinhart, F.; Massri, N.E.; Torres, N.; Chabrol, C.; Molet, J.; Johnstone, D.M.; Stone, J.; Benabid, A.-L.; Mitrofanis, J.; Moro, C. The behavioural and neuroprotective outcomes when 670 nm and 810 nm near infrared light are applied together in MPTP-treated mice. Neurosci. Res. 2016, 117, 42–47. [Google Scholar] [CrossRef]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol B Biol. 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Liebert, A.D.; Bicknell, B.T.; Adams, R.D. Protein conformational modulation by photons: A mechanism for laser treatment effects. Med. Hypotheses 2014, 82, 275–281. [Google Scholar] [CrossRef] [PubMed]
- El Massri, N.; Johnstone, D.M.; Peoples, C.L.; Moro, C.; Reinhart, F.; Torres, N.; Stone, J.; Benabid, A.-L.; Mitrofanis, J. The effect of different doses of near infrared light on dopaminergic cell survival and gliosis in MPTP-treated mice. Int. J. Neurosci. 2016, 126, 76–87. [Google Scholar] [CrossRef] [PubMed]
- El Massri, N.; Lemgruber, A.P.; Rowe, I.J.; Moro, C.; Torres, N.; Reinhart, F.; Chabrol, C.; Benabid, A.-L.; Mitrofanis, J. Photobiomodulation-induced changes in a monkey model of Parkinson’s disease: Changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp. Brain Res. 2017, 235, 1861–1874. [Google Scholar] [CrossRef] [PubMed]
- El Massri, N.; Weinrich, T.W.; Kam, J.H.; Jeffery, G.; Mitrofanis, J. Photobiomodulation reduces gliosis in the basal ganglia of aged mice. Neurobiol. Aging 2018, 66, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Agrawal, T.; Huang, L.; Gupta, G.K.; Hamblin, M.R. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics 2015, 8, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad.Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, C.S.; Arbour, N.; Manusow, J.; Montgrain, V.; Blain, M.; McCrea, E.; Shapiro, A.; Antel, J.P. TLR Signaling Tailors Innate Immune Responses in Human Microglia and Astrocytes. J. Immunol. 2005, 175, 4320–4330. [Google Scholar] [CrossRef] [Green Version]
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. Lancet 1989, 333, 1269. [Google Scholar] [CrossRef]
- Castaño, A.; Herrera, A.J.; Cano, J.; Machado, A. Lipopolysaccharide Intranigral Injection Induces Inflammatory Reaction and Damage in Nigrostriatal Dopaminergic System. J. Neurochem. 1998, 70, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; Kashefi, K.; Mander, P.; Rose, S.; Jenner, P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience 2002, 110, 49–58. [Google Scholar] [CrossRef]
- Arimoto, T.; Bing, G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol. Dis. 2003, 12, 35–45. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem. Int. 2015, 87, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Naughton, C.; Moriarty, N.; Feehan, J.; O’Toole, D.; Dowd, E. Differential pattern of motor impairments in neurotoxic, environmental and inflammation-driven rat models of Parkinson’s disease. Behav. Brain Res. 2016, 296, 451–458. [Google Scholar] [CrossRef]
- Iravani, M.M.; Leung, C.C.M.; Sadeghian, M.; Haddon, C.O.; Rose, S.; Jenner, P. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur. J. Neurosci. 2005, 22, 317–330. [Google Scholar] [CrossRef]
- Song, S.; Zhou, F.; Chen, W.R. Low-level laser therapy regulates microglial function through Src-mediated signaling pathways: Implications for neurodegenerative diseases. J. Neuroinflamm. 2012, 9, 219. [Google Scholar] [CrossRef]
- Johnstone, D.M.; El Massri, N.; Moro, C.; Spana, S.; Wang, X.S.; Torres, N.; Chabrol, C.; De Jaeger, X.; Reinhart, F.; Purushothuman, S.; et al. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism—An abscopal neuroprotective effect. Neuroscience 2014, 274, 93–101. [Google Scholar] [CrossRef]
- Naeser, M.A.; Hamblin, M.R. Traumatic Brain Injury: A Major Medical Problem That Could Be Treated Using Transcranial, Red/Near-Infrared LED Photobiomodulation. Photomed. Laser Surg. 2015, 33, 443–446. [Google Scholar] [CrossRef]
- Austin, P.J.; Betts, M.J.; Broadstock, M.; O’Neill, M.J.; Mitchell, S.N.; Duty, S. Symptomatic and neuroprotective effects following activation of nigral group III metabotropic glutamate receptors in rodent models of Parkinson’s disease. Br. J. Pharm. 2010, 160, 1741–1753. [Google Scholar] [CrossRef]
- Olsson, M.; Nikkhah, G.; Bentlage, C.; Bjorklund, A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995, 15, 3863–3875. [Google Scholar] [CrossRef]
- Rozas, G.; López-Martín, E.; Guerra, M.J.; Labandeira-García, J.L. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J. Neurosci. Methods 1998, 83, 165–175. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates—The New Coronal Set, 5th ed.; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Jenkins, P.A.; Carroll, J.D. How to Report Low-Level Laser Therapy (LLLT)/Photomedicine Dose and Beam Parameters in Clinical and Laboratory Studies. Photomed. Laser Surg. 2011, 29, 785–787. [Google Scholar] [CrossRef]
- West, M.J.; Slomianka, L.; Gundersen, H.J.G. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef]
- Reinhart, F.; El Massri, N.; Johnstone, D.M.; Stone, J.; Mitrofanis, J.; Benabid, A.-L.; Moro, C. Near-infrared light (670 nm) reduces MPTP-induced parkinsonism within a broad therapeutic time window. Exp. Brain Res. 2016, 234, 1787–1794. [Google Scholar] [CrossRef]
- Ashkan, K.; Wallace, B.A.; Mitrofanis, J.; Pollo, C.; Brard, P.-Y.; Fagret, D.; Benabid, A.-L. SPECT imaging, immunohistochemical and behavioural correlations in the primate models of Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, 266–275. [Google Scholar] [CrossRef]
- Sharma, S.; Moon, C.S.; Khogali, A.; Haidous, A.; Chabenne, A.; Ojo, C.; Jelebinkov, M.; Kurdi, Y.; Ebadi, M. Biomarkers in Parkinson’s disease (recent update). Neurochem. Int. 2013, 63, 201–229. [Google Scholar] [CrossRef]
- Surace, M.J.; Block, M.L. Targeting Microglia-Mediated Neurotoxicity: The Potential of NOX2 Inhibitors. Cell. Mol. Life Sci. 2012, 69, 2409–2427. [Google Scholar] [CrossRef]
- Vichaya, E.G.; Hunt, S.C.; Dantzer, R. Lipopolysaccharide Reduces Incentive Motivation While Boosting Preference for High Reward in Mice. Neuropsychopharmacology 2014, 39, 2884–2890. [Google Scholar] [CrossRef] [Green Version]
- Balkaya, M.; Kröber, J.M.; Rex, A.; Endres, M. Assessing Post-Stroke Behavior in Mouse Models of Focal Ischemia. J. Cereb. Blood Flow Metab. 2012, 33, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, F.; El Massri, N.; Chabrol, C.; Cretallaz, C.; Johnstone, D.M.; Torres, N.; Darlot, F.; Costecalde, T.; Stone, J.; Mitrofanis, J.; et al. Intracranial application of near-infrared light in a hemi-parkinsonian rat model: the impact on behavior and cell survival. J. Neurosur. 2016, 124, 1829–1841. [Google Scholar] [CrossRef] [Green Version]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef]
- Quirk, B.J.; Desmet, K.D.; Henry, M.; Buchmann, E.; Wong-Riley, M.; Eells, J.T.; Whelan, H.T. Therapeutic effect of near infrared (NIR) light on Parkinson’s disease models. Front. Biosci. (Elite Ed.) 2012, 4, 818–823. [Google Scholar]
- Hsieh, P.F.; Chia, L.-G.; Ni, D.-R.; Cheng, L.-J.; Ho, Y.-P.; Tzeng, S.-F.; Chang, M.-H.; Hong, J.-S. Behavior, neurochemistry and histology after intranigral lipopolysaccharide injection. Neuroreport 2002, 13, 277–280. [Google Scholar] [CrossRef]
- Oueslati, A.; Lovisa, B.; Perrin, J.; Wagnières, G.; van den Bergh, H.; Tardy, Y.; Lashuel, H.A. Photobiomodulation Suppresses Alpha-Synuclein-Induced Toxicity in an AAV-Based Rat Genetic Model of Parkinson’s Disease. PLoS ONE 2015, 10, e0140880. [Google Scholar] [CrossRef]
- Claypoole, L.D.; Zimmerberg, B.; Williamson, L.L. Neonatal lipopolysaccharide treatment alters hippocampal neuroinflammation, microglia morphology and anxiety-like behavior in rats selectively bred for an infantile trait. Brain Behav. Immun. 2017, 59, 135–146. [Google Scholar] [CrossRef]
- Doyle, H.H.; Eidson, L.N.; Sinkiewicz, D.M.; Murphy, A.Z. Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. J. Neurosci. 2017, 37, 3202–3214. [Google Scholar] [CrossRef]
- Girard-Joyal, O.; Ismail, N. Effect of LPS treatment on tyrosine hydroxylase expression and Parkinson-like behaviors. Horm. Behav. 2017, 89, 1–12. [Google Scholar] [CrossRef]
- Pitzschke, A.; Lovisa, B.; Seydoux, O.; Zellweger, M.; Pfleiderer, M.; Tardy, Y.; Wagnières, G. Red and NIR light dosimetry in the human deep brain. Phys. Med. Biol. 2015, 60, 2921–2937. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, C.; Hamilton, D.; Nicklason, F.; El Massri, N.; Mitrofanis, J. Exploring the use of transcranial photobiomodulation in Parkinson’s disease patients. Neural. Regen. Res. 2018, 13, 1738–1740. [Google Scholar] [CrossRef]

| Device Information | |
|---|---|
| Manufacturer | Quantum WARP Light Devices |
| Model | Quantum Light WARP 10 |
| Number of emitters | 48 |
| Emitter type | LED |
| Spatial distribution of emitters | 48 emitters spaced in a square grid pattern 5 mm apart within a circular aperture |
| Beam delivery | Handheld |
| Irradiation Parameters | |
| Centre wavelength [nm] | 675 |
| Spectral bandwidth [nm] | 23 |
| Operating mode | Continuous wave |
| Average Radiant Power [mW] | 500 ^ |
| Irradiance at aperture [mW/cm2] | 50 ^ |
| Beam shape | Circular |
| Beam profile | Gaussian |
| Treatment Parameters | |
| Irradiance at target [mW/cm2] | 40.84 |
| Exposure duration [s] | 88 |
| Radiant exposure [J/cm2] | 3.594 |
| Radiant energy [J] | 35.94 |
| Number of points irradiated | 1 |
| Area irradiated [cm2] | 10 |
| Application technique | Held 1 cm above skin |
| Number of treatments | 13 |
| Total radiant energy [J] | 467.2 |
| Cell Count Measure | 10 µg LPS | 20 µg LPS | ||
|---|---|---|---|---|
| LPS | PBM | LPS | PBM | |
| TH+ cell density (% contralateral side) | ↓ | - | ↓ | ↓ |
| IBA1+ cell density (% contralateral side) | - | - | ↑ | ↑ |
| Behaviour Measure | 10 µg LPS | 20 µg LPS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-Test 1 | Post-Test 2 | Post-Test 1 | Post-Test 2 | ||||||||||
| Veh | LPS | PBM | Veh | LPS | PBM | Veh | LPS | PBM | Veh | LPS | PBM | ||
| Cylinder | - | - | - | - | - | - | - | ↓ | ↓ | - | ↓ ^ | - | |
| Rotarod | - | - | - | - | - | - | - | ↓ ^ | ↓ | - | ↓ | - | |
| Stepping | IF 1 | - | - | - | - | - | - | - | - | - | - | - | - |
| IB 2 | - | - | - | - | - | - | - | - | - | - | - | - | |
| CF 3 | - | - | - | - | - | - | - | ↓ | ↓ | - | - | - | |
| CB 4 | - | - | - | - | - | - | - | ↓ ^ | ↓ | - | ↓ | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, J.A.; Austin, P.J. Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague–Dawley Rat. Biomolecules 2019, 9, 381. https://doi.org/10.3390/biom9080381
O’Brien JA, Austin PJ. Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague–Dawley Rat. Biomolecules. 2019; 9(8):381. https://doi.org/10.3390/biom9080381
Chicago/Turabian StyleO’Brien, Jayden A., and Paul J. Austin. 2019. "Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague–Dawley Rat" Biomolecules 9, no. 8: 381. https://doi.org/10.3390/biom9080381
APA StyleO’Brien, J. A., & Austin, P. J. (2019). Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague–Dawley Rat. Biomolecules, 9(8), 381. https://doi.org/10.3390/biom9080381