Heterodimerization of Mu Opioid Receptor Protomer with Dopamine D2 Receptor Modulates Agonist-Induced Internalization of Mu Opioid Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Construction of Plasmids
2.3. Cell Culture and Transfection
2.4. Coimmunoprecipitation
2.5. Bioluminescence Resonance Energy Transfer1 (BRET1)
2.6. Functional Complementation Assay Using Split Luciferase
2.7. Förster Resonance Energy Transfer (FRET)
2.8. Fluorescence Recovery After Photobleaching (FRAP)
2.9. Fluorescence Live Cell Imaging
2.9.1. Measurement of Calcium Release Using Fluo-4 AM by Live Cell Imaging
2.9.2. Internalization Assay by Live Cell Imaging
2.10. Statistical Analysis
3. Results
3.1. Coimmunoprecipitation Indicates that MOR and D2R Heterodimerize in Transfected HEK 293T Cells
3.2. Fluorescently Tagged D2LR and MOR are Functionally Expressed on the Plasma Membrane of Hela Cells
3.3. Analysis of MOR-D2LR Dimerization by BRET1
3.4. Receptor Interaction Analysis by Functional Complementation of a Split Luciferase
3.5. Assessment of MOR-D2LR Interaction Using FRET
3.6. Mobility of MOR is Altered on the Plasma Membrane in the Presence of D2LR
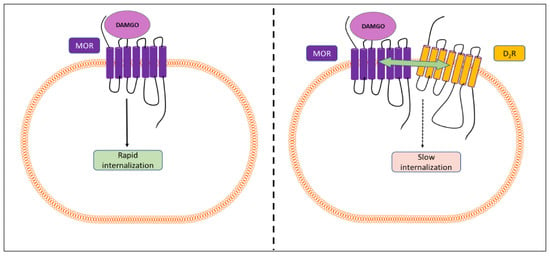
3.7. Heteromerization of MOR and D2LR Inhibits the Internalization of MOR after Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pasternak, G.; Pan, Y.-X. Mu Opioid Receptors In Pain Management. Acta Anaesthesiol. Taiwanica 2011, 49, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Mannelli, L.D.C.; Bianchi, E. The pharmacological basis of opioids. Clin. Cases Miner. Bone Metab. 2015, 12, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Cherny, N.I. Opioid analgesics: Comparative features and prescribing guidelines. Drugs 1996, 51, 713–737. [Google Scholar] [CrossRef]
- Bailey, C.P.; Connor, M. Opioids: Cellular mechanisms of tolerance and physical dependence. Curr. Opin. Pharmacol. 2005, 5, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. Opioid Receptors. Annu Rev Med. 2016, 67, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Historical Review: Opiate Addiction and Opioid Receptors. Cell Transplant. 2018, 28, 233–238. [Google Scholar] [CrossRef]
- Zawilska, J.B. An Expanding World of Novel Psychoactive Substances: Opioids. Front. Psychol. 2017, 8, 110. [Google Scholar] [CrossRef]
- Zuo, Z.Y. The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance. Anesth. Analg. 2005, 101, 728–734. [Google Scholar] [CrossRef]
- Bohn, L.M.; Gainetdinov, R.R.; Lin, F.T.; Lefkowitz, R.J.; Caron, M.G. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 2000, 408, 720–723. [Google Scholar] [CrossRef]
- Nestler, E.J. Under siege: The brain on opiates. Neuron 1996, 16, 897–900. [Google Scholar] [CrossRef]
- Bohn, L.M.; Lefkowitz, R.J.; Gainetdinov, R.R.; Peppel, K.; Caron, M.G.; Lin, F.T. Enhanced Morphine Analgesia in Mice Lacking β-Arrestin 2. Science 1999, 286, 2495–2498. [Google Scholar] [CrossRef] [PubMed]
- Raehal, K.M.; Schmid, C.L.; Groer, C.E.; Bohn, L.M. Functional Selectivity at the µ -Opioid Receptor: Implications for Understanding Opioid Analgesia and Tolerance. Pharmacol. Rev. 2011, 63, 1001–1019. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.K.; Grady, E.F.; Bunnett, N.W. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem. J. 1997, 322, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Premont, R.T.; Bohn, L.M.; Lefkowitz, R.J.; Caron, M.G. Desensitization Of G Protein–Coupled Receptors And Neuronal Functions. Annu. Rev. Neurosci. 2004, 27, 107–144. [Google Scholar] [CrossRef] [PubMed]
- De Vries, T.J.; Ril, G.H.T.T.; Van Der Laan, J.W.; Mulder, A.H.; Schoffelmeer, A.N. Chronic exposure to morphine and naltrexone induces changes in catecholaminergic neurotransmission in rat brain without altering mu-opioid receptor sensitivity. Life Sci. 1993, 52, 1685–1693. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine Receptors: From Structure to Function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D. Opioid–Dopamine Interactions: Implications for Substance Use Disorders and Their Treatment. Boil. Psychiatry 2010, 68, 685–686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koob, G.F. Neural Mechanisms of Drug Reinforcement. Ann. New York Acad. Sci. 1992, 654, 171–191. [Google Scholar] [CrossRef]
- Rivera, A.; Gago, B.; Suarez-Boomgaard, D.; Yoshitake, T.; Roales-Bujan, R.; Valderrama-Carvajal, A.; Bilbao, A.; Medina-Luque, J.; Diaz-Cabiale, Z.; Van Craenenbroeck, K.; et al. Dopamine D-4 receptor stimulation prevents nigrostriatal dopamine pathway activation by morphine: Relevance for drug addiction. Addict. Biol. 2017, 22, 1232–1245. [Google Scholar] [CrossRef]
- Dai, W.-L.; Liu, X.-T.; Bao, Y.-N.; Yan, B.; Jiang, N.; Yu, B.-Y.; Liu, J.-H. Selective blockade of spinal D2DR by levo-corydalmine attenuates morphine tolerance via suppressing PI3K/Akt-MAPK signaling in a MOR-dependent manner. Exp. Mol. Med. 2018, 50, 148. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Reyes, J.; Almanza, A.; Segura-Chama, P.; Pellicer, F.; Mercado, F. D2-like receptor agonist synergizes the mu-opioid agonist spinal antinociception in nociceptive, inflammatory and neuropathic models of pain in the rat. Eur. J. Pharmacol. 2019, 853, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.; Marín, A.R.; Dalton, J.A.R.; Giraldo, J.; Stove, C. Distinct Dopamine D2 Receptor Antagonists Differentially Impact D2 Receptor Oligomerization. Int. J. Mol. Sci. 2019, 20, 1686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; O’Dowd, B.F.; Rajaram, R.D.; Nguyen, T.; George, S.R. D2 Dopamine Receptor Homodimerization Is Mediated by Multiple Sites of Interaction, Including an Intermolecular Interaction Involving Transmembrane Domain 4†. Biochemistry 2003, 42, 11023–11031. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Pearsall, E.A.; Hurst, D.P.; Zhang, Y.H.; Chu, J.; Zhou, Y.L.; Reggio, P.H.; Loh, H.H.; Law, P.Y. Palmitoylation and membrane cholesterol stabilize mu-opioid receptor homodimerization and G protein coupling. Bmc Cell Biol. 2012, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Rodriguez, D.; Romero-Fernandez, W.; Kapla, J.; Jaiteh, M.; Ranganathan, A.; Lazarova, T.; Fuxe, K.; Carlsson, J. Mapping the Interface of a GPCR Dimer: A Structural Model of the A2A Adenosine and D2 Dopamine Receptor Heteromer. Front. Pharmacol. 2018, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Canals, M.; Marcellino, D.; Fanelli, F.; Ciruela, F.; de Benedetti, P.; Goldberg, S.R.; Neve, K.; Fuxe, K.; Agnati, L.F.; Woods, A.S.; et al. Adenosine A(2A)-dopamine D2 receptor-receptor heteromerization - Qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003, 278, 46741–46749. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Polit, A.; Kedracka-Krok, S.; Wedzony, K.; Maćkowiak, M.; Dziedzicka-Wasylewska, M. Hetero-dimerization of serotonin 5-HT2A and dopamine D2 receptors. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2010, 1803, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Bonaventura, J.; Farré, D.; Sanchez, M.; Simola, N.; Mallol, J.; Lluís, C.; Costa, G.; Baqi, Y.; Müller, C.E.; et al. l-DOPA disrupts adenosine A2A–cannabinoid CB1–dopamine D2 receptor heteromer cross-talk in the striatum of hemiparkinsonian rats: Biochemical and behavioral studies. Exp. Neurol. 2014, 253, 180–191. [Google Scholar] [CrossRef]
- Kearn, C.S.; Blake-Palmer, K.; Scotter, E.; Mackie, K.; Glass, M. Concurrent Stimulation of Cannabinoid CB1 and Dopamine D2 Receptors Enhances Heterodimer Formation: A Mechanism for Receptor Cross-Talk? Mol. Pharmacol. 2005, 67, 1697–1704. [Google Scholar] [CrossRef]
- George, S.R.; Fan, T.; Xie, Z.D.; Tse, R.; Tam, V.; Varghese, G.; O’Dowd, B.F. Oligomerization of mu- and delta-opioid receptors - Generation of novel functional properties. J. Biol. Chem. 2000, 275, 26128–26135. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Q.; He, Q.H.; Han, J.S.; Su, L.; Wan, Y. Heteromerization of mu-opioid receptor and cholecystokinin B receptor through the third transmembrane domain of the mu-opioid receptor contributes to the anti-opioid effects of cholecystokinin octapeptide. Exp. Mol. Med. 2018, 50, 64. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Valderrama-Carvajal, A.; Suárez-Boomgaard, D.; Shumilov, K.; RealÁngeles, M.; Fuxe, K.; Gago, B. On the Study of D4R-MOR Receptor–Receptor Interaction in the Rat Caudate Putamen: Relevance on Morphine Addiction. In Animal Models of Neurotrauma; Humana Press: New York, NY, USA, 2018; Volume 140, pp. 25–39. [Google Scholar]
- Borroto-Escuela, D.O.; Van Craenenbroeck, K.; Romero-Fernandez, W.; Guidolin, D.; Woods, A.S.; Rivera, A.; Haegeman, G.; Agnati, L.F.; Tarakanov, A.O.; Fuxe, K.; et al. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor–receptor interactions. Biochem. Biophys. Res. Commun. 2011, 404, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.-L.; Xiong, F.; Yan, B.; Cao, Z.-Y.; Liu, W.-T.; Liu, J.-H.; Yu, B.-Y. Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord. Sci. Rep. 2016, 6, 38746. [Google Scholar] [CrossRef] [PubMed]
- Skieterska, K.; Duchou, J.; Lintermans, B.; Van Craenenbroeck, K. Detection of G Protein-Coupled Receptor (GPCR) Dimerization by Coimmunoprecipitation. Echinoderms Part B 2013, 117, 323–340. [Google Scholar]
- Salahpour, A.; Angers, S.; Bouvier, M. Functional Significance of Oligomerization of G-protein-coupled Receptors. Trends Endocrinol. Metab. 2000, 11, 163–168. [Google Scholar] [CrossRef]
- Pei, L.; Li, S.; Wang, M.; Diwan, M.; Anisman, H.; Fletcher, P.J.; Nobrega, J.N.; Liu, F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat. Med. 2010, 16, 1393–1395. [Google Scholar] [CrossRef]
- Los, G.V.; Encell, L.P.; McDougall, M.G.; Hartzell, D.D.; Karassina, N.; Zimprich, C.; Wood, M.G.; Learish, R.; Ohane, R.F.; Urh, M.; et al. HatoTag: A novel protein labeling technology for cell imaging and protein analysis. Acs Chem Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef]
- Choy, E.; Chiu, V.K.; Silletti, J.; Feoktistov, M.; Morimoto, T.; Michaelson, D.; Ivanov, I.E.; Philips, M.R. Endomembrane trafficking of Ras: The CAAX motif targets proteins to the ER and Golgi. Cell 1999, 98, 69–80. [Google Scholar] [CrossRef]
- Giese, B.; Au-Yeung, C.-K.; Herrmann, A.; Diefenbach, S.; Haan, C.; Küster, A.; Wortmann, S.B.; Roderburg, C.; Heinrich, P.C.; Behrmann, I.; et al. Long Term Association of the Cytokine Receptor gp130 and the Janus Kinase Jak1 Revealed by FRAP Analysis. J. Boil. Chem. 2003, 278, 39205–39213. [Google Scholar] [CrossRef]
- Reits, E.A.; Neefjes, J.J. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Bio. 2001, 3, E145–E147. [Google Scholar] [CrossRef] [PubMed]
- Lippincott-Schwartz, J.; Snapp, E.; Kenworthy, A. Studying protein dynamics in living cells. Nat. Rev. Mol. Cell Boil. 2001, 2, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Mauri, M.; Parenti, M.; Chini, B. Analysis of GPCR Dimerization Using Acceptor Photobleaching Resonance Energy Transfer Techniques. Methods Enzymol. 2013, 521, 311–327. [Google Scholar] [PubMed]
- Prinster, S.C.; Hague, C.; Hall, R.A. Heterodimerization of G Protein-Coupled Receptors: Specificity and Functional Significance. Pharmacol. Rev. 2005, 57, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; O’Dowd, B.F.; George, S.R. Homo- and hetero-oligomerization of G protein-coupled receptors. Life Sci. 2003, 74, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.A.; Devi, L.A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 1999, 399, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Gupta, A.; Filipovska, J.; Szeto, H.H.; Pintar, J.E.; Devi, L.A. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. USA 2004, 101, 5135–5139. [Google Scholar] [CrossRef]
- Gomes, I.; Jordan, B.A.; Gupta, A.; Trapaidze, N.; Nagy, V.; Devi, L.A. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J. Neurosci. 2000, 20, RC110. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Rivera, A.; Diaz-Cabiale, Z.; Filip, M.; Gago, B.; Roberts, D.C.S.; Langel, U.; Genedani, S.; Ferraro, L.; et al. Receptor–receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res. Rev. 2008, 58, 415–452. [Google Scholar] [CrossRef]
- Jordan, B.A.; Gomes, I.; Rios, C.; Filipovska, J.; Devi, L.A. Functional interactions between mu opioid and alpha(2A)-adrenergic receptors. Mol. Pharmacol. 2003, 64, 1317–1324. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Koch, T.; Schröder, H.; Laugsch, M.; Höllt, V.; Schulz, S. Heterodimerization of Somatostatin and Opioid Receptors Cross-modulates Phosphorylation, Internalization, and Desensitization. J. Boil. Chem. 2002, 277, 19762–19772. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Vasudevan, L.; Huysentruyt, J.; Risseeuw, M.D.P.; Stove, C.; Vanderheyden, P.M.L.; Van Craenenbroeck, K.; Van Calenbergh, S. Design, Synthesis, and Biological Evaluation of Bivalent Ligands Targeting Dopamine D2 -Like Receptors and the μ-Opioid Receptor. Chem. Med. Chem. 2018, 13, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Niewiarowska-Sendo, A.; Polit, A.; Piwowar, M.; Tworzydło, M.; Kozik, A.; Guevara-Lora, I. Bradykinin B2 and dopamine D2 receptors form a functional dimer. BBA Mol. Cell Res. 2017, 1864, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, S.; Klotz, K.-N.; Engelhardt, S.; Lohse, M.J.; Bünemann, M. Analysis of receptor oligomerization by FRAP microscopy. Nat. Methods 2009, 6, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.G.; Miller, L.J. Secretin Receptor Dimerization. Prototypic of Class B GPCR Behaviour. In G-Protein-Coupled Receptor Dimers; Herrick-Davis, K., Milligan, G., Di Giovanni, G., Eds.; Springer: Berlin, Germany, 2017; pp. 273–287. [Google Scholar]
- Kasai, R.S.; Kusumi, A. Single-molecule imaging revealed dynamic GPCR dimerization. Curr. Opin. Cell Boil. 2014, 27, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kasai, R.S.; Ito, S.V.; Awane, R.M.; Fujiwara, T.K.; Kusumi, A. The Class-A GPCR Dopamine D2 Receptor Forms Transient Dimers Stabilized by Agonists: Detection by Single-Molecule Tracking. Cell Biochem. Biophys. 2018, 76, 29–37. [Google Scholar] [CrossRef]
- Christie, M.J. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Brit. J. Pharmacol. 2008, 154, 384–396. [Google Scholar] [CrossRef]
- Connor, M.; Osborne, P.B.; Christie, M.J. mu-Opioid receptor desensitization: Is morphine different? Brit. J. Pharmacol. 2004, 143, 685–696. [Google Scholar] [CrossRef]
- Kelly, E.; Bailey, C.P.; Henderson, G. Agonist-selective mechanisms of GPCR desensitization. Brit. J. Pharmacol. 2008, 153, S379–S388. [Google Scholar] [CrossRef]
- Koch, T.; Höllt, V. Role of receptor internalization in opioid tolerance and dependence. Pharmacol. Ther. 2008, 117, 199–206. [Google Scholar] [CrossRef]
- Williams, J.T.; Ingram, S.L.; Henderson, G.; Chavkin, C.; von Zastrow, M.; Schulz, S.; Koch, T.; Evans, C.J.; Christie, M.J. Regulation of mu-Opioid Receptors: Desensitization, Phosphorylation, Internalization, and Tolerance. Pharmacol. Rev. 2013, 65, 223–254. [Google Scholar] [CrossRef] [PubMed]
- Grecksch, G.; Just, S.; Pierstorff, C.; Imhof, A.K.; Gluck, L.; Doll, C.; Lupp, A.; Becker, A.; Koch, T.; Stumm, R.; et al. Analgesic Tolerance to High-Efficacy Agonists But Not to Morphine Is Diminished in Phosphorylation-Deficient S375A mu-Opioid Receptor Knock-In Mice. J. Neurosci. 2011, 31, 13890–13896. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.; Rivero, G.; Baptist, M.; Llorente, J.; Al-Sabah, S.; Krasel, C.; Dewey, W.L.; Bailey, C.P.; Rosethorne, E.M.; Charlton, S.J.; et al. mu-Opioid Receptors: Correlation of Agonist Efficacy for Signalling with Ability to Activate Internalization. Mol. Pharmacol. 2010, 78, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.M.; Gainetdinov, R.R.; Caron, M.G. G protein-coupled receptor kinase/beta-arrestin systems and drugs of abuse - Psychostimulant and opiate studies in knockout mice. Neuromol. Med. 2004, 5, 41–50. [Google Scholar] [CrossRef]
- Whistler, J.L.; von Zastrow, M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc. Natl. Acad. Sci. USA 1998, 95, 9914–9919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ferguson, S.S.G.; Barak, L.S.; Bodduluri, S.R.; Laporte, S.A.; Law, P.Y.; Caron, M.G. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc. Natl. Acad. Sci. USA 1998, 95, 7157–7162. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Liu, X.; Tian, X.; Yang, H.; Gao, F. Enhancement of morphine analgesia and prevention of morphine tolerance by downregulation of β-arrestin 2 with antigene RNAs in mice. Int. J. Neurosci. 2014, 125, 56–65. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Liu, C.; Kang, J.; Yang, J.; Pei, G.; Wu, C. Improvement of Morphine-Mediated Analgesia by Inhibition of β-Arrestin 2 Expression in Mice Periaqueductal Gray Matter. Int. J. Mol. Sci. 2009, 10, 954–963. [Google Scholar] [CrossRef]
- Raehal, K.M.; Walker, J.K.L.; Bohn, L.M. Morphine Side Effects in β-Arrestin 2 Knockout Mice. J. Pharmacol. Exp. Ther. 2005, 314, 1195–1201. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasudevan, L.; Borroto-Escuela, D.O.; Huysentruyt, J.; Fuxe, K.; Saini, D.K.; Stove, C. Heterodimerization of Mu Opioid Receptor Protomer with Dopamine D2 Receptor Modulates Agonist-Induced Internalization of Mu Opioid Receptor. Biomolecules 2019, 9, 368. https://doi.org/10.3390/biom9080368
Vasudevan L, Borroto-Escuela DO, Huysentruyt J, Fuxe K, Saini DK, Stove C. Heterodimerization of Mu Opioid Receptor Protomer with Dopamine D2 Receptor Modulates Agonist-Induced Internalization of Mu Opioid Receptor. Biomolecules. 2019; 9(8):368. https://doi.org/10.3390/biom9080368
Chicago/Turabian StyleVasudevan, Lakshmi, Dasiel O. Borroto-Escuela, Jelle Huysentruyt, Kjell Fuxe, Deepak K. Saini, and Christophe Stove. 2019. "Heterodimerization of Mu Opioid Receptor Protomer with Dopamine D2 Receptor Modulates Agonist-Induced Internalization of Mu Opioid Receptor" Biomolecules 9, no. 8: 368. https://doi.org/10.3390/biom9080368
APA StyleVasudevan, L., Borroto-Escuela, D. O., Huysentruyt, J., Fuxe, K., Saini, D. K., & Stove, C. (2019). Heterodimerization of Mu Opioid Receptor Protomer with Dopamine D2 Receptor Modulates Agonist-Induced Internalization of Mu Opioid Receptor. Biomolecules, 9(8), 368. https://doi.org/10.3390/biom9080368