Assessment of DNA Topoisomerase I Unwinding Activity, Radical Scavenging Capacity, and Inhibition of Breast Cancer Cell Viability of N-alkyl-acridones and N,N′-dialkyl-9,9′-biacridylidenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Chemical Compounds and Cell Cultures Conditions
2.2. Synthetic Procedures for the Preparation of N-Alkylacridones 1–6 and N,N′-Dialkyl-9,9′-Biacridylidenes 7–12
2.3. Topoisomerase-I Mediated DNA Relaxation Assay
2.4. DNA Binding Studies
2.5. DPPH and ABTS Radical Scavenging Activity Assays
2.6. MTT Assay
2.7. Wound Healing Assay
2.8. Confocal Microscopy Imaging and Localization Study
2.9. Statistical Analysis
3. Results and Discussion
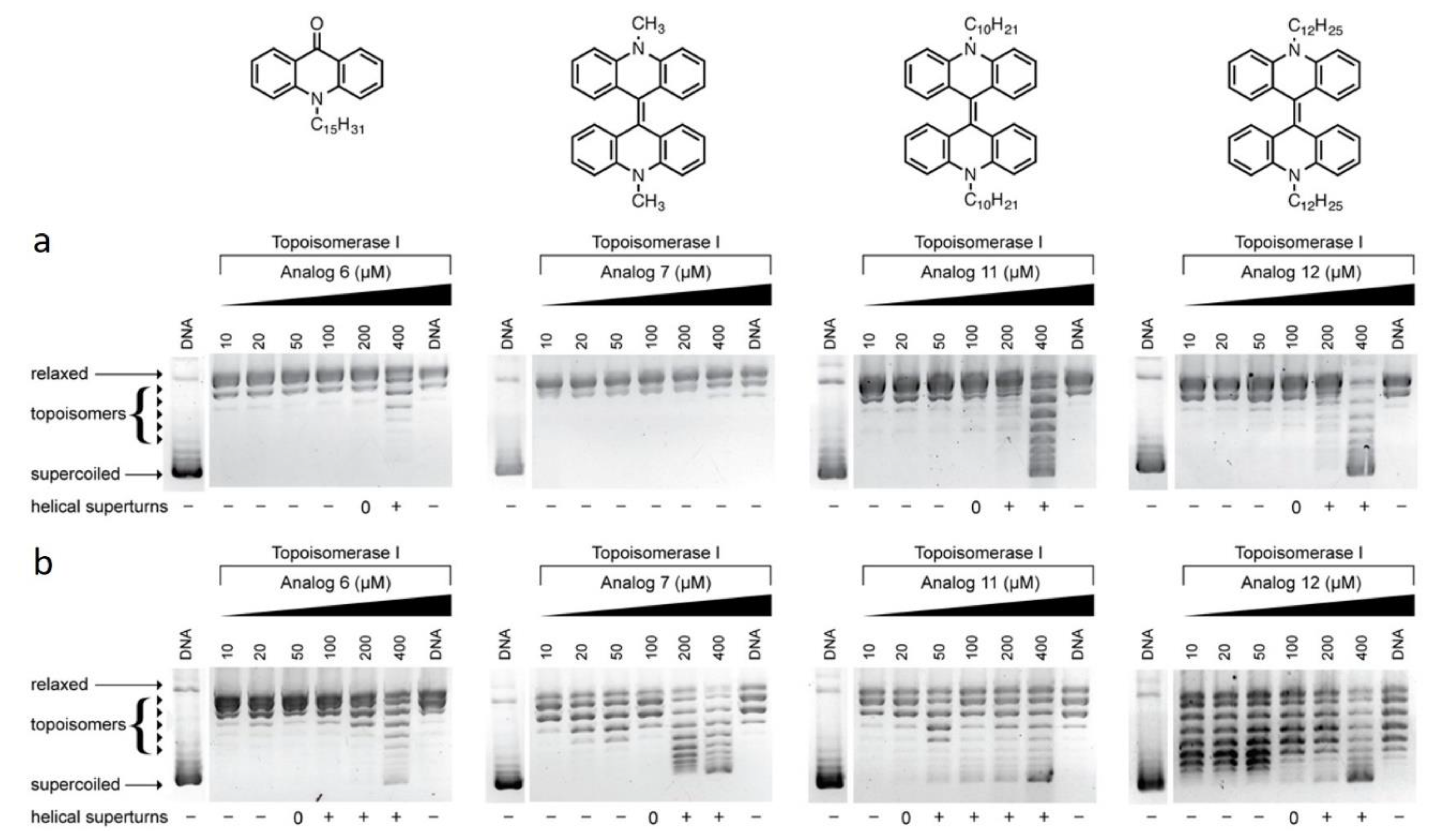
3.1. Topoisomerase-I Mediated DNA Relaxation Assay
3.2. DNA Binding Study
3.3. DPPH and ABTS Radical Scavenging Activity
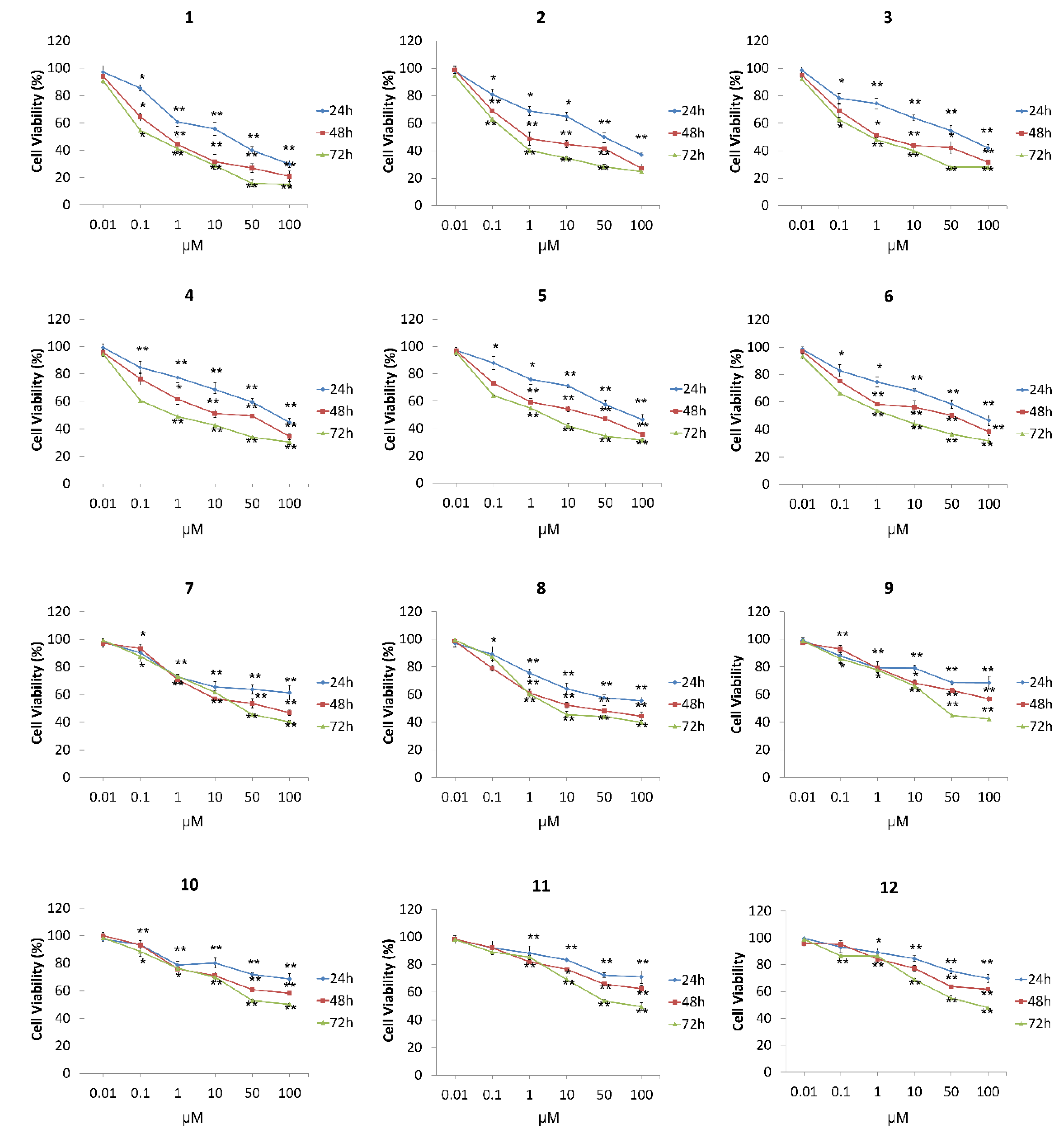
3.4. MTT Assay
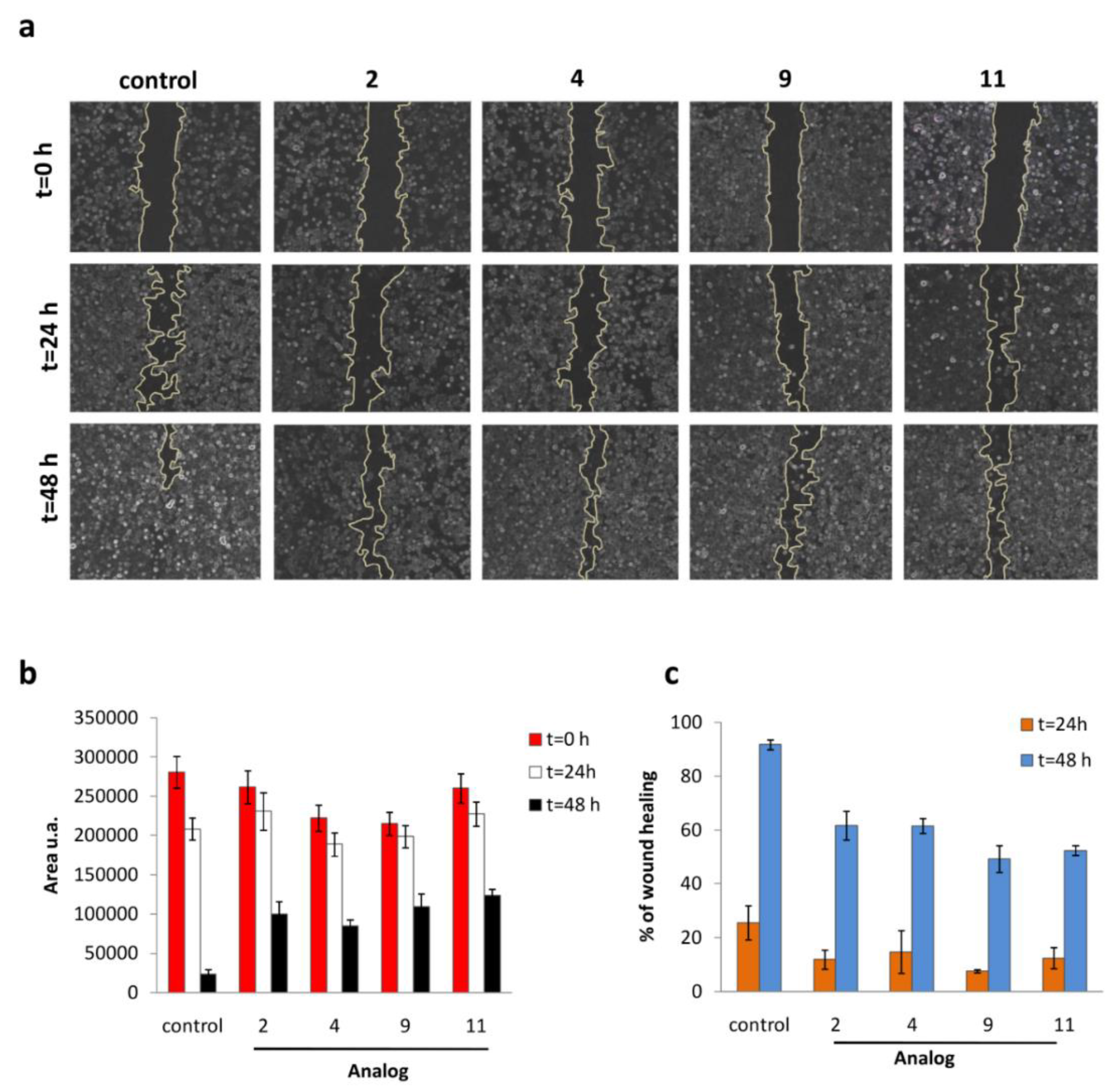
3.5. Wound-Healing Assay
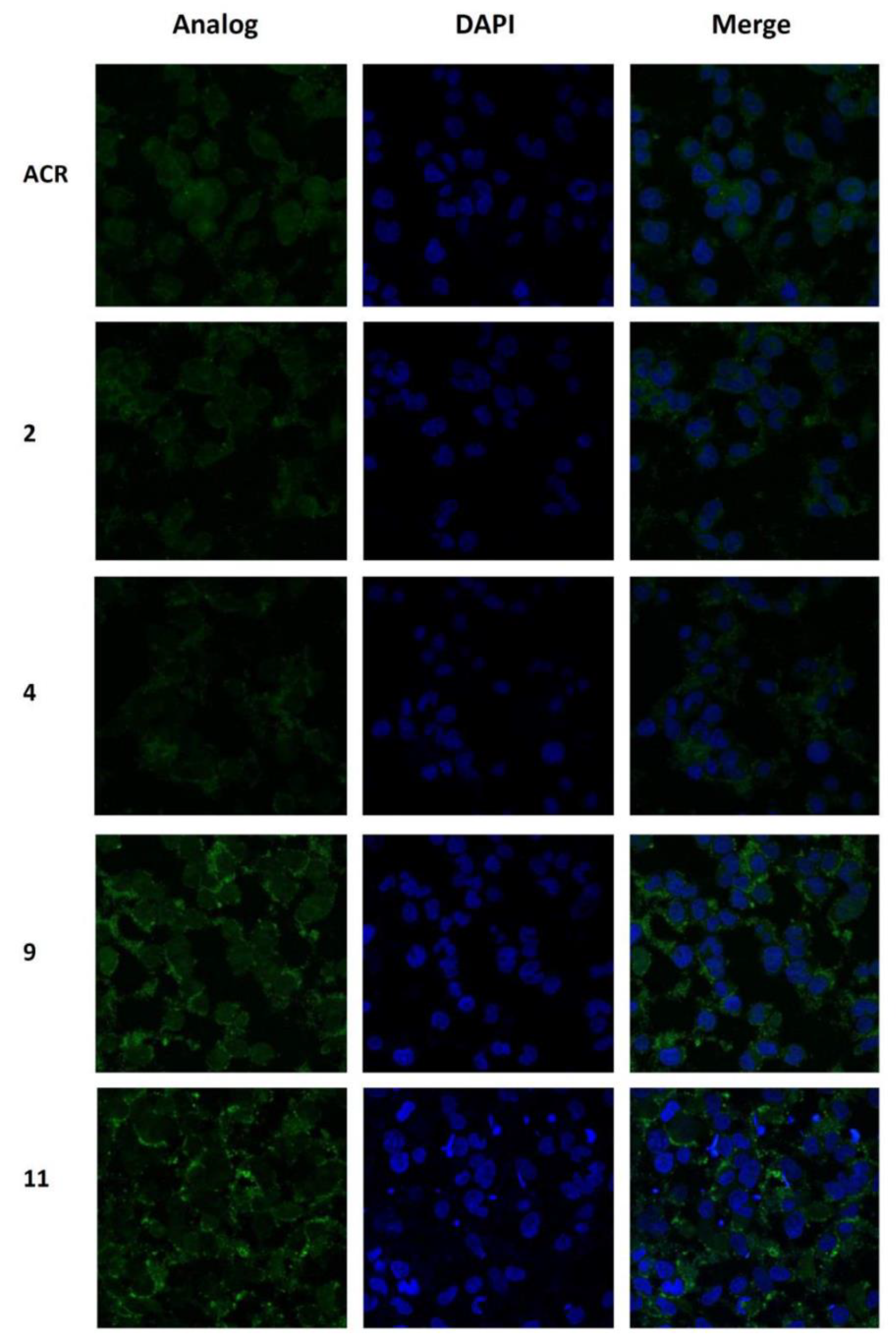
3.6. Confocal Microscopy Imaging and Localization Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, Y.F.; Chu, H.J.; Kuang, G.F.; Jiang, G.J.; Che, Y.C. Inhibition effects of acridone on the growth of breast cancer cells in vivo. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2356–2363. [Google Scholar] [PubMed]
- Bin, Z.; Kang, C.; Ning, W.; Chunmei, G.; Qinsheng, S.; Lulu, L.; Yuzong, C.; Chunyan, T.; Hongxia, L.; Yuyang, J. Molecular design, synthesis and biological research of novel pyridyl acridones as potent DNA-binding and apoptosis-inducing agents. Eur. J. Med. Chem. 2015, 93, 214–226. [Google Scholar]
- Gensicka-Kowalewska, M.; Cholewiński, G.; Dzierzbicka, K. Recent developments in the synthesis and biological activity of acridine/acridone analogues. RSC Adv. 2017, 7, 15776–15804. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, W.; He, S.; Li, S.; Liu, F.; Jiang, Y. Synthesis and antiproliferative activity of 2,7-diamino l0-(3,5-dimethoxy)benzyl-9(10H)-acridone derivatives as potent telomeric G-quadruplex DNA ligands. Bioorg. Med. Chem. 2011, 19, 3312–3319. [Google Scholar] [CrossRef]
- Mayur, Y.C.; Zaheeruddin; Peters, G.J.; Lemos, C.; Kathmann, I.; Prasad, V.V. Synthesis of 2-fluoro N(10)-substituted acridones and their cytotoxicity studies in sensitive and resistant cancer cell lines and their DNA intercalation studies. Arch. Pharm. 2009, 342, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Goodell, J.R.; Ougolkov, A.V.; Hiasa, H.; Kaur, H.; Remmel, R.; Billadeau, D.D.; Ferguson, D.M. Acridine-based agents with topoisomerase II activity inhibit pancreatic cancer cell proliferation and induce apoptosis. J. Med. Chem. 2008, 51, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef]
- Khadka, D.B.; Cho, W.J. Topoisomerase inhibitors as anticancer agents: A patent update. Expert Opin. Ther. Pat. 2013, 23, 1033–1056. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Nikokavouras, J. Reactions of lucigenin in protic solvents in the presence of amines. J. Prakt. Chem.-Chem. Zeitung 1994, 336, 506–509. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Nikokavouras, J. Synthesis of N,N′-dialkyl-9,9′-biacridylidenes and 9,9′-biacridinium nitrates containing long alkyl chains. J. Prakt. Chem. 1993, 335, 633–636. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Nikokavouras, J. Synthesis of novel protected hemiaminal N-methoxymeothyl-N′-methyl-9,9′-biacridylidene from lucigenin. Tetrahedron. Lett. 1993, 34, 1371–1372. [Google Scholar] [CrossRef]
- Zuin Fantoni, N.; Molphy, Z.; Slator, C.; Menounou, G.; Toniolo, G.; Mitrikas, G.; McKee, V.; Chatgilialoglu, C.; Kellett, A. Polypyridyl-based copper phenanthrene complexes: A new type of stabilized artificial chemical nuclease. Chem.–Eur. J. 2019, 25, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Pothiraj, K.; Baskaran, T. DNA interaction, antimicrobial, electrochemical and spectroscopic studies of metal(II) complexes with tridentate heterocyclic Schiff base derived from 2′-methylacetoacetanilide. J. Mol. Struct. 2011, 1000, 135–144. [Google Scholar] [CrossRef]
- El-Asmy, H.A.; Butler, I.S.; Mouhri, Z.S.; Jean-Claude, B.J.; Emmam, M.S.; Mostafa, I. Synthesis, characterization and DNA interaction studies of new complexes containing 2-mercaptobenzothiazole and different dinitrogen or phosphorous aromatic donors. Inorg. Chim. Acta 2016, 441, 20–33. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity LWT. Food Sci. Tech. 1995, 28, 25–30. [Google Scholar]
- Floegela, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Kellett, A.; Molphy, Z.; Slator, C.; McKee, V.; Farrell, N.P. Molecular methods for assessment of non-covalent metallodrug-DNA interactions. Chem. Soc. Rev. 2019, 48, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Slator, C.; Molphy, Z.; McKee, V.; Kellett, A. Triggering autophagic cell death with a di-manganese(II) developmental therapeutic. Redox Biol. 2017, 12, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Slator, C.; Molphy, Z.; McKee, V.; Long, C.; Brown, T.; Kellett, A. Di-copper metallodrugs promote NCI-60 chemotherapy via singlet oxygen and superoxide production with tandem TA/TA and AT/AT oligonucleotide discrimination. Nucleic Acids Res. 2018, 46, 2733–2750. [Google Scholar] [CrossRef]
- Shahabadi, N.; Mohammadi, S.; Alizadeh, R. DNA interaction studies of a new platinum(II) complex containing different aromatic dinitrogen ligands. Bioinorg. Chem. Appl. 2011, 2011, 429241. [Google Scholar] [CrossRef]
- Waring, M.J. Complex formation between ethidium bromide and nucleic acids. J. Mol. Biol. 1965, 13, 269–282. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Velena, A.; Zarkovic, N.; Troselj, G.K.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1,4-Dihydropyridine derivatives: Dihydronicotinamide analogues-model compounds targeting oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 1892412. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Sugihara, T.; Rao, G.; Hebbel, R.P. Diphenylamine: An unusual antioxidant. Free Radic. Biol. Med. 1993, 14, 381–387. [Google Scholar] [CrossRef]
- Mulder, P.; Litwinienko, G.; Lin, S.; MacLean, P.D.; Barclay, L.R.; Ingold, K.U. The L-type calcium channel blockers, hantzsch 1,4-dihydropyridines, are not peroxyl radical-trapping, chain-breaking antioxidants. Chem. Res. Toxicol. 2006, 19, 79–85. [Google Scholar] [CrossRef]
- Cory, G. Scratch-wound assay. Methods Mol. Biol. 2011, 769, 25–30. [Google Scholar] [PubMed]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, X.; Li, B.; Gao, C.; Jiang, Y. Acridine and its derivatives: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 647–664. [Google Scholar] [CrossRef] [PubMed]


| Compound | λmax (nm) | Change in Absorbance | Kb × 106 (M−1) Incubation 24 h | Kb × 106 (M−1) Incubation 48 h |
|---|---|---|---|---|
| 2 | 255.5 | Hyperchromicity | 4.0 | 2.0 |
| 9 | 258.5 | Hypochromicity | 66.7 | 22.2 |
| Analog | Radical Scavenging Activity (μM) | |
|---|---|---|
| DPPH-Assay 1 IC50 ± sd (TEAC) | ABTS-Assay 1 IC50 ± sd (TEAC) | |
| 7 | 16.98 ± 0.88 (1.03) | 32.32 ± 1.90 (3.68) |
| 8 | 33.7 ± 2.1 (2.00) | 9.33 ± 0.42 (1.07) |
| 9 | 30.2 ± 1.17 (1.44) | 21.45 ± 1.15 (1.14) |
| 10 | 28.350 ± 3.1 (1.69) | 16.88 ± 1.25 (1.92) |
| 11 | 17.73 ± 0.53 (1.05) | 12.43 ± 0.86 (1.42) |
| 12 | 16.02 ± 0.56 (0.96) | 24.1 ± 1.11 (1.52) |
| Caffeic acid | 6.95 ± 0.46 (0.42) | 8.67 ± 0.36 (0.99) |
| Trolox | 16.76 ± 0.54 (1.0) | 8.78 ± 0.29 (1.0) |
| Analog | 24 h | 48 h | 72 h |
|---|---|---|---|
| 1 | 6.75 | 1.77 | 0.02 |
| 2 | 10.01 | 4.07 | 1.89 |
| 3 | 11.67 | 5.30 | 3.32 |
| 4 | 13.80 | 8.07 | 3.78 |
| 5 | 14.81 | 7.74 | 4.27 |
| 6 | 14.13 | 8.74 | 5.16 |
| 7 | 19.13 | 11.75 | 9.57 |
| 8 | 15.65 | 8.78 | 6.70 |
| 9 | 28.40 | 18.45 | 10.60 |
| 10 | 30.95 | 17.29 | 14.05 |
| 11 | 34.54 | 23.10 | 14.64 |
| 12 | 33.95 | 23.40 | 14.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krokidis, M.G.; Molphy, Z.; Efthimiadou, E.K.; Kokoli, M.; Argyri, S.-M.; Dousi, I.; Masi, A.; Papadopoulos, K.; Kellett, A.; Chatgilialoglu, C. Assessment of DNA Topoisomerase I Unwinding Activity, Radical Scavenging Capacity, and Inhibition of Breast Cancer Cell Viability of N-alkyl-acridones and N,N′-dialkyl-9,9′-biacridylidenes. Biomolecules 2019, 9, 177. https://doi.org/10.3390/biom9050177
Krokidis MG, Molphy Z, Efthimiadou EK, Kokoli M, Argyri S-M, Dousi I, Masi A, Papadopoulos K, Kellett A, Chatgilialoglu C. Assessment of DNA Topoisomerase I Unwinding Activity, Radical Scavenging Capacity, and Inhibition of Breast Cancer Cell Viability of N-alkyl-acridones and N,N′-dialkyl-9,9′-biacridylidenes. Biomolecules. 2019; 9(5):177. https://doi.org/10.3390/biom9050177
Chicago/Turabian StyleKrokidis, Marios G., Zara Molphy, Eleni K. Efthimiadou, Marianna Kokoli, Smaragda-Maria Argyri, Irini Dousi, Annalisa Masi, Kyriakos Papadopoulos, Andrew Kellett, and Chryssostomos Chatgilialoglu. 2019. "Assessment of DNA Topoisomerase I Unwinding Activity, Radical Scavenging Capacity, and Inhibition of Breast Cancer Cell Viability of N-alkyl-acridones and N,N′-dialkyl-9,9′-biacridylidenes" Biomolecules 9, no. 5: 177. https://doi.org/10.3390/biom9050177







