The Effect of Arabic Gum on Renal Function in Reversible Unilateral Ureteric Obstruction
Abstract
:1. Introduction
2. Methods
2.1. Ureteric Occlusion Operation and Reversal
2.2. Arabic Gum Administration and Experimental Groups
- AG-1 (n = 8) received AG starting 7 days prior to and continuing throughout the period of UUO but was sacrificed at the end of the 3 day period of obstruction to measure the tissue level of oxidative stress markers and gene expression of cytokines.
- AG-2 (n = 12) received AG starting 7 days prior to and continuing throughout the period of obstruction until the terminal experiment six days post-UUO reversal.
- Vx-1 (n = 8) underwent a similar protocol as AG-1 but no AG was added to the drinking water.
- Vx-2 (n = 12) underwent a similar protocol as AG-2 but no AG was added to the drinking water.
2.3. Surgical Procedure in the Terminal Experiment and Measurement of Renal Functions
2.4. Gene Expression Analysis
2.5. Measurement of Oxidative Stress Markers
2.6. Histological Studies
2.7. Statistical Analysis
3. Results
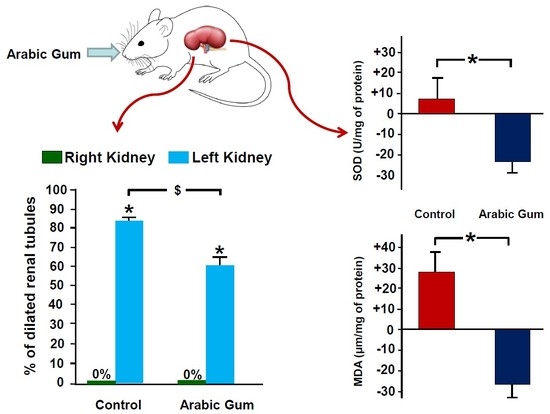
3.1. Oxidative Stress Markers
3.2. Gene Expression Analysis Results
3.3. Glomerular and Tubular Functions in Vx-2 and AG-2
3.4. Histological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klahr, S. Obstructive nephropathy. Intern. Med. 2000, 39, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Wheatley, A.M.; Davis, G. Long-term renal effects of unilateral ureteral obstruction and the role of endothelin. Kidney Int. 2000, 58, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klahr, S. Urinary tract obstruction. Semin. Nephrol. 2001, 21, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Yarger, W.E.; Schocken, D.D.; Harris, R.H. Obstructive nephropathy in the rat: Possible roles for the renin-angiotensin system, prostaglandins, and thromboxanes in postobstructive renal function. J. Clin. Investig. 1980, 65, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Lubbad, L. Does curcumin protect against renal dysfunction following reversible unilateral ureteric obstruction in the rat? Eur. Surg. Res. 2011, 46, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Lubbad, L. The effect of diclofenac sodium on renal function in reversible unilateral ureteric obstruction. Urol. Res. 2011, 39, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Lubbad, L. The effect of aliskiren on the renal dysfunction following unilateral ureteral obstruction in the rat. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 70–77. [Google Scholar]
- Hammad, F.T.; Lubbad, L. The effect of epigallocatechin-3-gallate on the renal dysfunction in the obstructed kidney in the rat. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 119–126. [Google Scholar]
- Klotman, P.E.; Smith, S.R.; Volpp, B.D.; Coffman, T.M.; Yarger, W.E. Thromboxane synthetase inhibition improves function of hydronephrotic rat kidneys. Am. J. Physiol. 1986, 250, F282–F287. [Google Scholar] [CrossRef]
- Ozturk, H.; Ozdemir, E.; Otcu, S.; Buyukbayram, H.; Ihsan Dokucu, A. Renal effects on a solitary kidney of specific inhibition of cyclooxygenease-2 after 24 h of complete ureteric obstruction in rats. Urol. Res. 2002, 30, 223–226. [Google Scholar]
- Hammad, F.T.; Lubbad, L. The effect of thymoquinone on the renal functions following ischemia-reperfusion injury in the rat. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 152–159. [Google Scholar] [PubMed]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices–A mini review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S. Dietary Plants for the Prevention and Management of Kidney Stones: Preclinical and Clinical Evidence and Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef] [PubMed]
- Zeni, A.L.B.; Moreira, T.D.; Dalmagro, A.P.; Camargo, A.; Bini, L.A.; Simionatto, E.L.; Scharf, D.R. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An Acad. Bras. Cienc. 2017, 89, 2805–2815. [Google Scholar] [CrossRef]
- Vuorelaa, P.; Leinonenb, M.; Saikkuc, P.; Tammelaa, P.; Rauhad, J.P.; Wennberge, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Gum arabic. In Handbook of Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2000; pp. 155–168. [Google Scholar]
- Grein, A.; da Silva, B.C.; Wendel, C.F.; Tischer, C.A.; Sierakowski, M.R.; Moura, A.B.; Iacomini, M.; Gorin, P.A.; Simas-Tosin, F.F.; Riegel-Vidotti, I.C. Structural characterization and emulsifying properties of polysaccharides of Acacia mearnsii de Wild gum. Carbohydr. Polym. 2013, 92, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Diaai, A.A.; Ahmed, F. Evaluation of the efficacy of ginger, Arabic gum, and Boswellia in acute and chronic renal failure. Ren. Fail. 2012, 34, 73–82. [Google Scholar] [CrossRef]
- Gamal el-din, A.M.; Mostafa, A.M.; Al-Shabanah, O.A.; Al-Bekairi, A.M.; Nagi, M.N. Protective effect of arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol. Res. 2003, 48, 631–635. [Google Scholar] [CrossRef] [Green Version]
- Abd-Allah, A.R.; Al-Majed, A.A.; Mostafa, A.M.; Al-Shabanah, O.A.; Din, A.G.; Nagi, M.N. Protective effect of arabic gum against cardiotoxicity induced by doxorubicin in mice: A possible mechanism of protection. J. Biochem. Mol. Toxicol. 2002, 16, 254–259. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Husseni, I.; Beegam, S.; Al-Shukaili, A.; Nemmar, A.; Schierling, S.; Queisser, N.; Schupp, N. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLoS ONE 2013, 8, e55242. [Google Scholar] [CrossRef] [PubMed]
- Wadood, A.; Wadood, N.; Shah, S.A. Effects of Acacia arabica and Caralluma edulis on blood glucose levels of normal and alloxan diabetic rabbits. J. Pak. Med. Assoc. 1989, 39, 208–212. [Google Scholar] [PubMed]
- Kaddam, L.; Fadl-Elmula, I.; Eisawi, O.A.; Abdelrazig, H.A.; Salih, M.A.; Lang, F.; Saeed, A.M. Gum Arabic as novel anti-oxidant agent in sickle cell anemia, phase II trial. BMC Hematol. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Gado, A.M.; Aldahmash, B.A. Antioxidant effect of Arabic gum against mercuric chloride-induced nephrotoxicity. Drug Des. Dev. Ther. 2013, 7, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Al-Salam, S.; Yuvaraju, P.; Lubbad, L. Alda-1, an aldehyde dehydrogenase-2 agonist, causes deterioration in renal functions following ischemia-reperfusion injury due to crystalline nephropathy. Drug Dev. Res. 2018, 79, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar]
- Hosseinian, S.; Rad, A.K.; Bideskan, A.E.; Soukhtanloo, M.; Sadeghnia, H.; Shafei, M.N.; Motejadded, F.; Mohebbati, R.; Shahraki, S.; Beheshti, F. Thymoquinone ameliorates renal damage in unilateral ureteral obstruction in rats. Pharmacol. Rep. 2017, 69, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Otunctemur, A.; Ozbek, E.; Cakir, S.S.; Polat, E.C.; Dursun, M.; Cekmen, M.; Somay, A.; Ozbay, N. Pomegranate extract attenuates unilateral ureteral obstruction-induced renal damage by reducing oxidative stress. Urol. Ann. 2015, 7, 166–171. [Google Scholar] [CrossRef]
- Nemmar, A.; Al Hemeiri, A.; Al Hammadi, N.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Elwasila, M.; Ali, B.H.; Adeghate, E. Early pulmonary events of nose-only water pipe (shisha) smoking exposure in mice. Physiol. Rep. 2015, 3, e12258. [Google Scholar] [CrossRef]
- Guo, G.; Morrissey, J.; McCracken, R.; Tolley, T.; Klahr, S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am. J. Physiol. 1999, 277, F766–F772. [Google Scholar] [CrossRef]
- Meldrum, K.K.; Misseri, R.; Metcalfe, P.; Dinarello, C.A.; Hile, K.L.; Meldrum, D.R. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1456–R1464. [Google Scholar] [CrossRef] [PubMed]
- Misseri, R.; Meldrum, K.K. Mediators of fibrosis and apoptosis in obstructive uropathies. Curr. Urol. Rep. 2005, 6, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, E.S.; Cha, S.H.; Park, J.H.; Park, J.S.; Chang, Y.C.; Park, K.K. Transcriptional regulation of NF-kappaB by ring type decoy oligodeoxynucleotide in an animal model of nephropathy. Exp. Mol. Pathol. 2009, 86, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Morrissey, J.; McCracken, R.; Reyes, A.; Ishidoya, S.; Klahr, S. The expression of mRNA for tumor necrosis factor-α increases in the obstructed kidney of rats soon after unilateral ureteral ligation. Nephrology 1996, 2, 161–166. [Google Scholar] [CrossRef]
- Affres, H.; Perez, J.; Hagege, J.; Fouqueray, B.; Kornprobst, M.; Ardaillou, R.; Baud, L. Desferrioxamine regulates tumor necrosis factor release in mesangial cells. Kidney Int. 1991, 39, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, G.; Brian Reeves, W. Cisplatin increases TNF-α mRNA stability in kidney proximal tubule cells. Ren. Fail. 2006, 28, 583–592. [Google Scholar] [CrossRef]
- Tipping, P.G.; Leong, T.W.; Holdsworth, S.R. Tumor necrosis factor production by glomerular macrophages in anti-glomerular basement membrane glomerulonephritis in rabbits. Lab. Investing. J. Tech. Methods Pathol. 1991, 65, 272–279. [Google Scholar]
- Bottinger, E.P. TGF-β in renal injury and disease. Semin Nephrol. 2007, 27, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Muragaki, Y.; Saika, S.; Roberts, A.B.; Ooshima, A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Investig. 2003, 112, 1486–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneto, H.; Morrissey, J.; Klahr, S. Increased expression of TGF-β1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int. 1993, 44, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuruskan, H.; Caliskan, Z.; Kordan, Y.; Ozakin, C.; Yavascaoglu, I.; Oktay, B. Elevated plasma concentrations of transforming growth factor-β1 in patients with unilateral ureteral obstruction. Urol. Res. 2005, 33, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Mendoza, L.; Rha, S.J.; Sheikh-Hamad, D.; Baranowska-Daca, E.; Nguyen, V.; Smith, C.W.; Nassar, G.; Suki, W.N.; Truong, L.D. Role of p53-dependent activation of caspases in chronic obstructive uropathy: Evidence from p53 null mutant mice. J. Am. Soc. Nephrol. 2001, 12, 983–992. [Google Scholar] [PubMed]
- Brady, C.A.; Attardi, L.D. p53 at a glance. J. Cell Sci. 2010, 123, 2527–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasir, O.; Umbach, A.T.; Rexhepaj, R.; Ackermann, T.F.; Bhandaru, M.; Ebrahim, A.; Artunc, F.; Kempe, D.S.; Puchchakayala, G.; Siraskar, B.; et al. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice. Kidney Blood Press Res. 2012, 35, 365–372. [Google Scholar] [CrossRef]
- Ali, B.H.; Inuwa, I.; Al Za’abi, M.; Al Bahlani, S.; Al Issaei, H.; Ramkumar, A.; Madanagopal, T.; Nemmar, A.; Malheiros, D.M.; Zatz, R. Renal and myocardial histopathology and morphometry in rats with adenine—induced chronic renal failure: Influence of gum acacia. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 34, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Al Suleimani, Y.M.; Al Za’abi, M.; Ramkumar, A.; Al Mahruqi, A.S.; Tageldin, M.H.; Nemmar, A.; Ali, B.H. Influence of treatment with gum acacia on renal vascular responses in a rat model of chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 498–506. [Google Scholar]
- Bander, S.J.; Buerkert, J.E.; Martin, D.; Klahr, S. Long-term effects of 24-h unilateral ureteral obstruction on renal function in the rat. Kidney Int. 1985, 28, 614–620. [Google Scholar] [CrossRef]








| Gene | Gene Bank Reference | 5′–3′ Sequence | |
|---|---|---|---|
| TNF-α | NM_012675.3 | Forward | GGCTCCCTCTCATCAGTTCCAT |
| Reverse | CGCTTGGTGGTTTGCTACG | ||
| Probe | dFAM-CCCAGACCCTCACACTCAGATCATC-BHQ-1 | ||
| TGF-β1 | NM_021578.2 | Forward | GTGGCTGAACCAAGGAGACG |
| Reverse | CGTGGAGTACATTATCTTTGCTGTC | ||
| Probe | dFAM-ACAGGGCTTTCGCTTCAGTGCTC-BHQ-1 | ||
| p53 | NM_030989.3 | Forward | CGAGATGTTCCGAGAGCTGAATG |
| Reverse | GTCTTCGGGTAGCTGGAGTG | ||
| Probe | dFAM-CCTTGGAATTAAAGGATGCCCGTGC-BHQ-1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, F.T.; Al Salam, S.; Nemmar, A.; Ali, M.; Lubbad, L. The Effect of Arabic Gum on Renal Function in Reversible Unilateral Ureteric Obstruction. Biomolecules 2019, 9, 25. https://doi.org/10.3390/biom9010025
Hammad FT, Al Salam S, Nemmar A, Ali M, Lubbad L. The Effect of Arabic Gum on Renal Function in Reversible Unilateral Ureteric Obstruction. Biomolecules. 2019; 9(1):25. https://doi.org/10.3390/biom9010025
Chicago/Turabian StyleHammad, Fayez T., Suhail Al Salam, Abderrahim Nemmar, Mahmoud Ali, and Loay Lubbad. 2019. "The Effect of Arabic Gum on Renal Function in Reversible Unilateral Ureteric Obstruction" Biomolecules 9, no. 1: 25. https://doi.org/10.3390/biom9010025