An Empirical Analysis of the Perceived Challenges and Benefits of Introducing Biosimilars in Bangladesh: A Paradigm Shift
Abstract
:1. Introduction
2. Case Study: Bangladesh Pharmaceutical Industry
2.1. The Adoption of Pharmaceutical Biotechnology in the Pharmaceutical Industry of Bangladesh
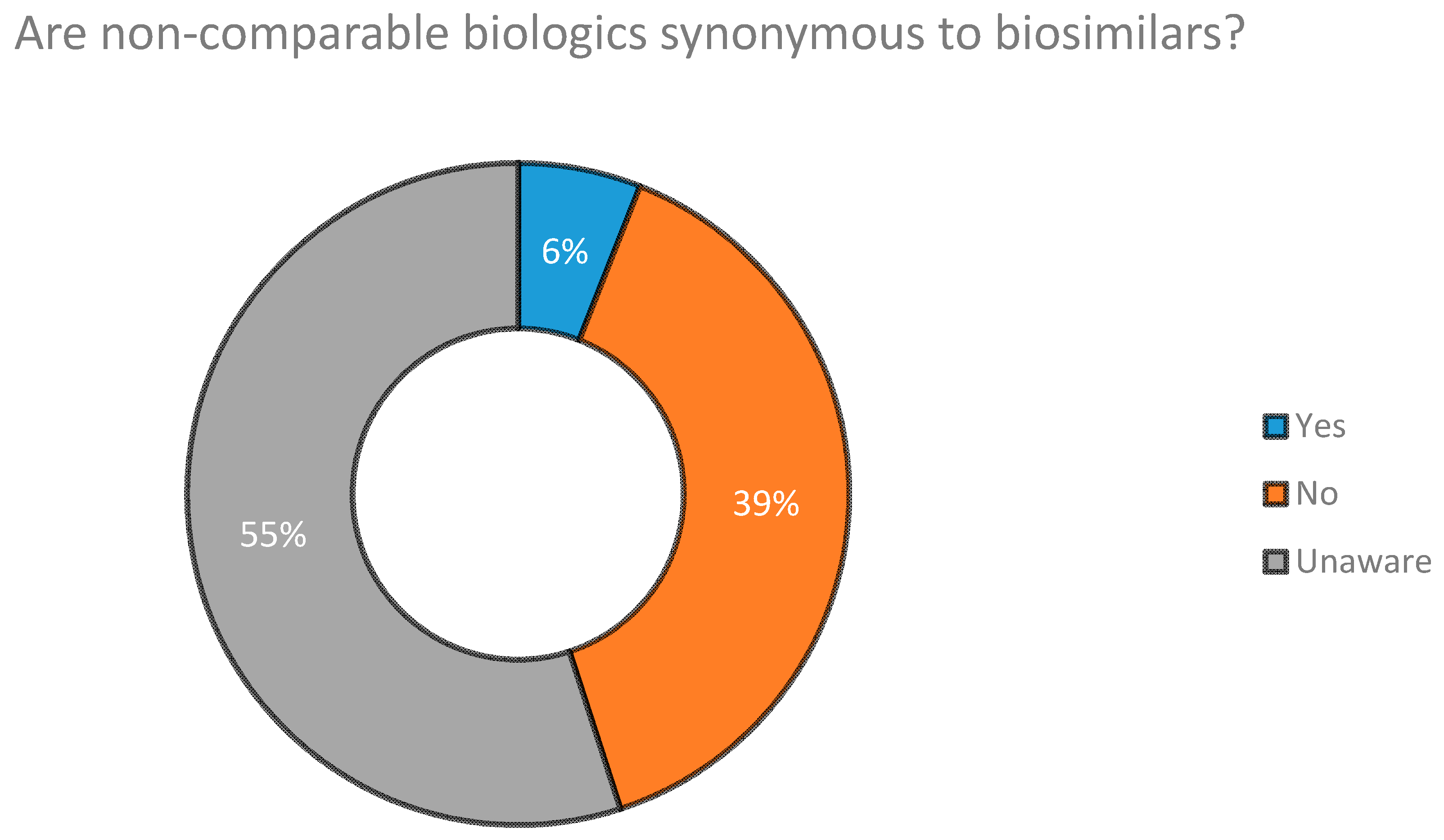
2.2. Concept of Non Comparable Biologics (NCBs) in Bangladesh
2.3. Landscape of Current Regulatory Guidelines
3. Materials and Methods
3.1. Secondary Data
3.2. Primary Data
3.2.1. Identification of Stakeholders
- Which stakeholders are most likely to recognize the specific approved industry standards required for biosimilar manufacture?
- Which stakeholders are most responsible for recognizing the value provided from a company’s biosimilar product in order to prescribe it?
- Which stakeholders hold the responsibility of promoting biosimilar awareness and education?
3.2.2. Research Approval
3.2.3. Design of the Questionnaire
3.2.4. Questionnaire Pretesting
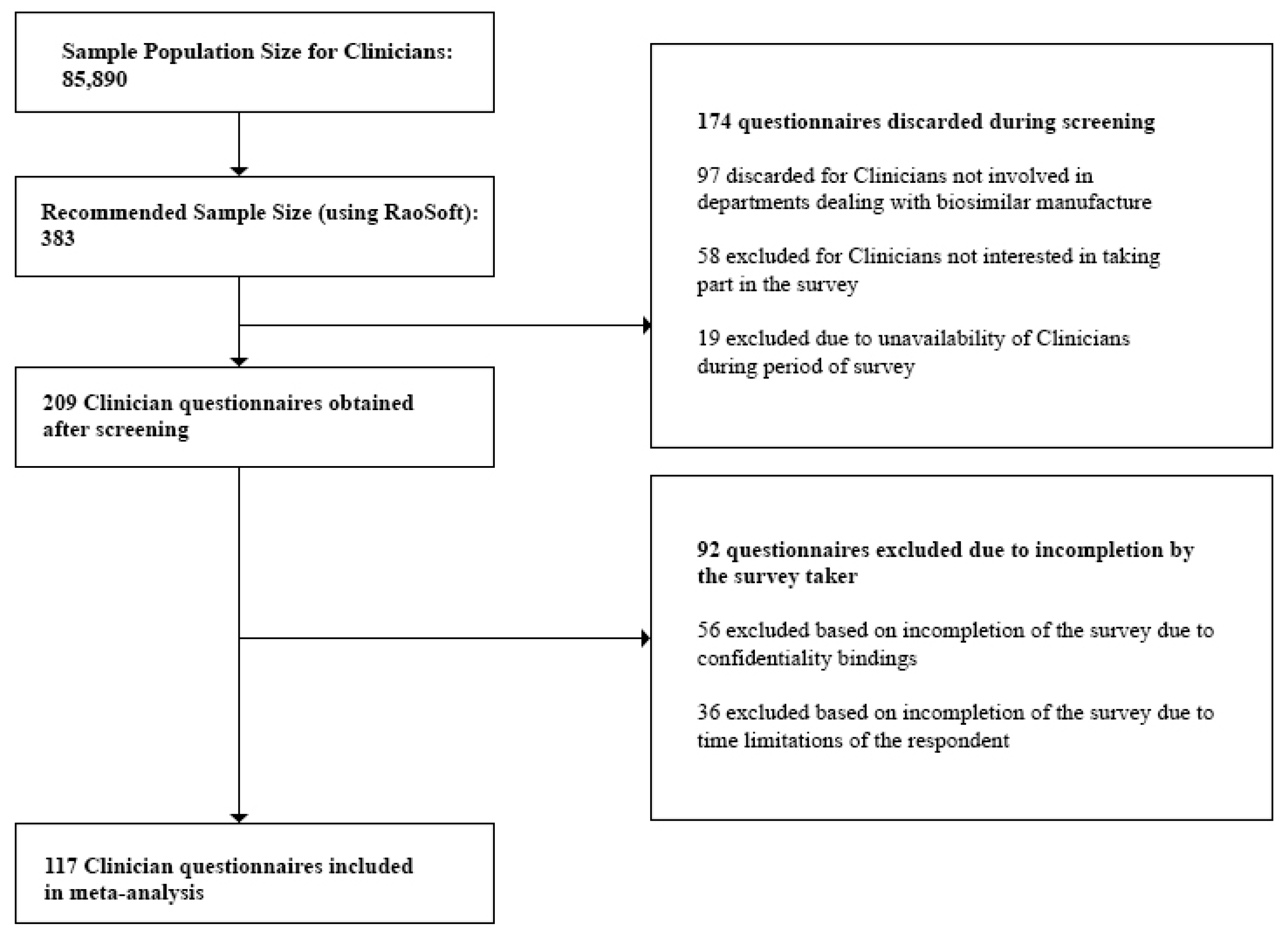
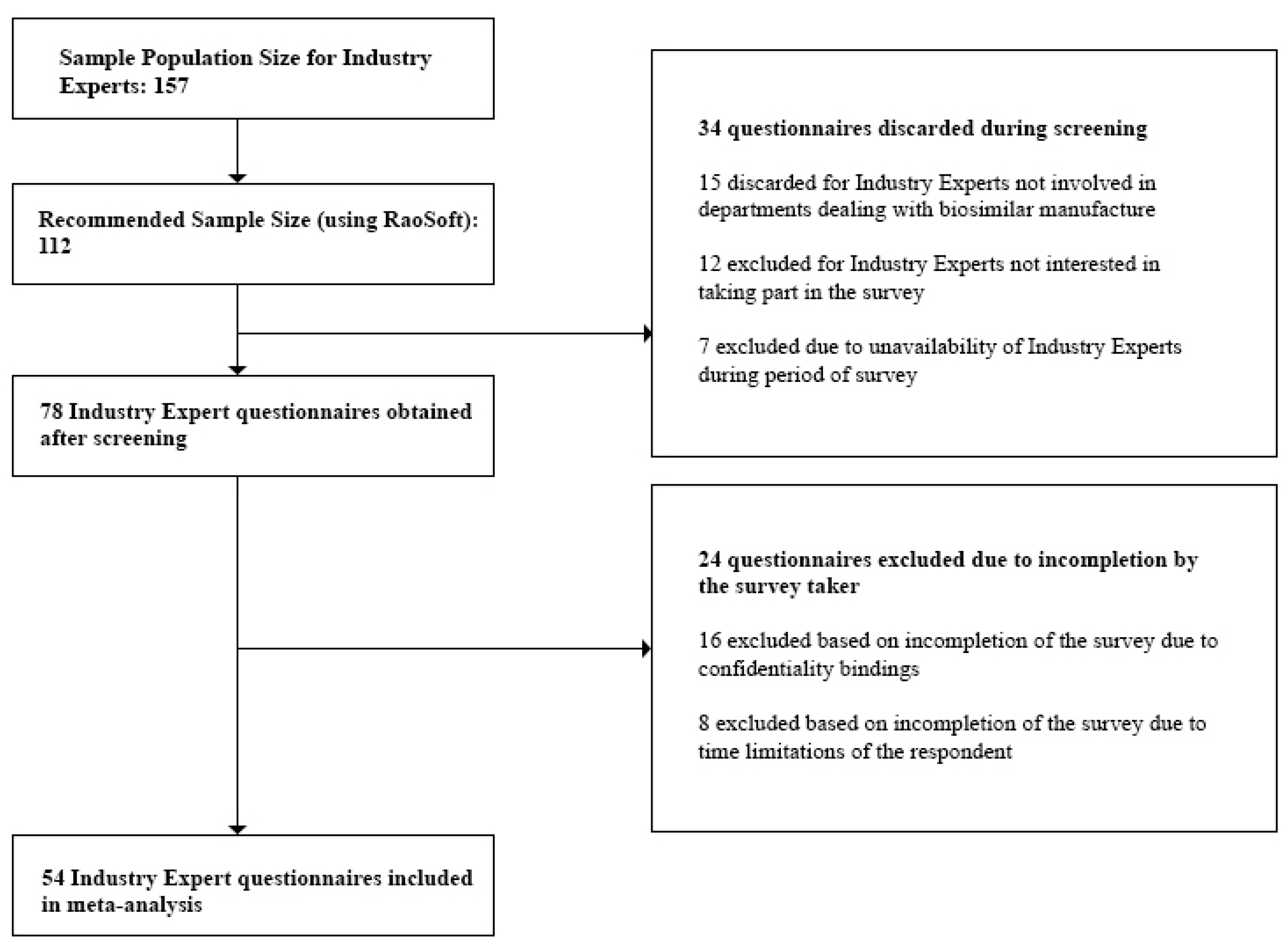
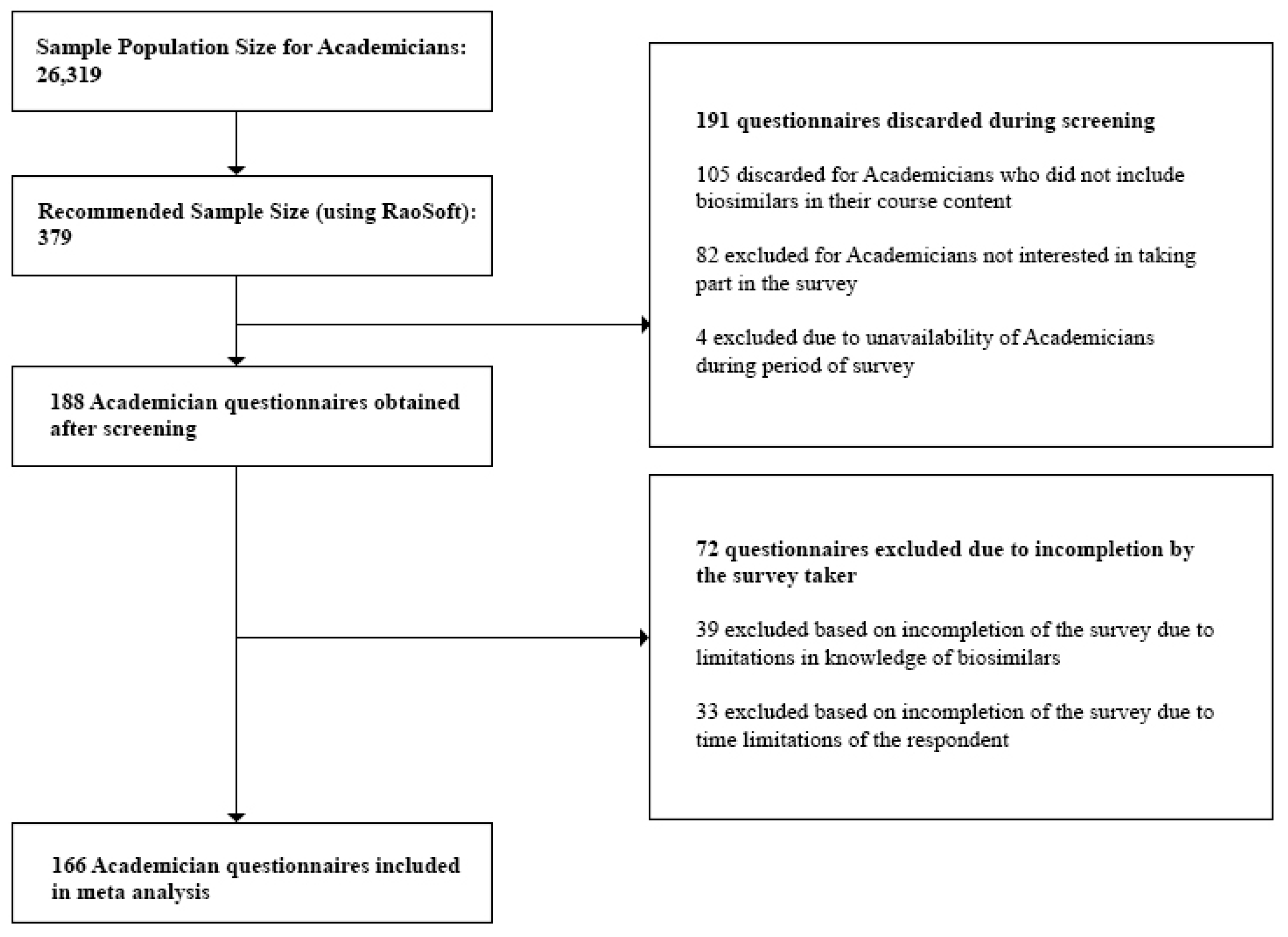
3.2.5. Sample Size
- (a)
- Clinicians: At the time of the study, the number of registered Clinicians was 85,890 [27]. This number was also validated during the Focus Group Discussions (FGDs). Questionnaires discarded during the screening process included those from Clinicians not currently prescribing biosimilars, and Clinicians who were unavailable during the survey period. Incomplete questionnaires due to confidentiality bindings and time limitations of the respondent were excluded during the post screening analysis. Also, questionnaires which did not provide quality information were excluded from the analysis.
- (b)
- Industry Experts: For the study, we targeted only the top 30 pharmaceutical companies in Bangladesh. These are the companies which held 94.76% of the total market share [28]. We individually approached the Heads of each department within each company and were referred to the respondents, 157 of which were eligible for our study. This number was also validated during the Focus Group Discussions (FGDs). Questionnaires discarded during the screening process involved those returned from Industry Experts not currently involved in departments dealing with biosimilar manufacture and experts who were unavailable during the survey period. Incomplete questionnaires which did not provide quality information were excluded during the post screening analysis.
- (c)
- Academicians: At the time of the study, we obtained the sample population size for Academicians, 26,319, from the University Grants Commission Annual Report [29]. This number was also validated during the Focus Group Discussions (FGDs). Questionnaires discarded during the screening process included those returned from Academicians who did not have biosimilars in their course content and Academicians who were unavailable during the survey period. Incomplete questionnaires which did not provide quality information were excluded during the post screening analysis.
- (i)
- Clinicians—117
- (ii)
- Industry Experts—54
- (iii)
- Academicians—116
- (i)
- Clinicians—100
- (ii)
- Industry Experts—50
- (iii)
- Academicians—100
3.3. Purpose of the Study
4. Results
Survey Feedback
5. Discussion
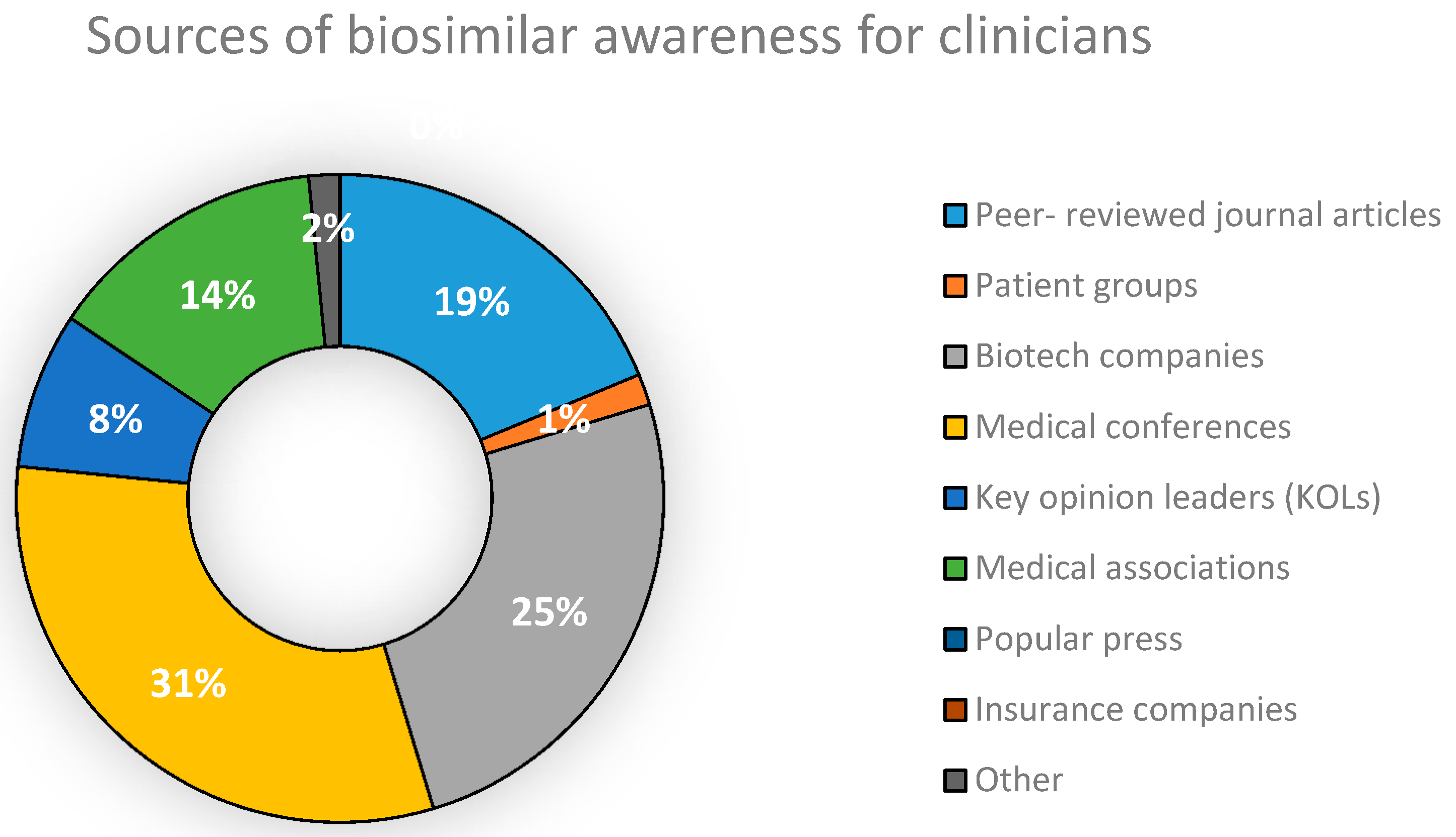
5.1. Concept and Awareness
5.2. Challenges in Biosimilar Introduction
5.3. Benefits of Biosimilar Therapy in Bangladesh
6. Recommendations
Limitations
- (a)
- only respondents who were experts in the targeted field of study were approached
- (b)
- certain respondents were unable to provide feedback due to confidentiality bindings
- (c)
- respondents were unavailable during the survey period
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. (Terminologies)
Appendix B. (Additional Tables)
| Name of Biosimilar Product | Approved By | Date of Approval | Manufacturer |
|---|---|---|---|
| Omnitrope | EMA | April 2006 | Sandoz |
| Binocrit | EMA | August 2007 | Sandoz |
| Epoetin alfa Hexal | EMA | August 2007 | Hexal |
| Abseamed | EMA | August 2007 | Medice |
| Silapo | EMA | December 2007 | Stada |
| Retacrit | EMA | December 2007 | Hospira UK |
| Ratiograstim | EMA | September 2008 | Ratiopharm |
| Tevagrastim | EMA | September 2008 | Teva |
| Biograstim | EMA | September 2008 | CT Arzneimittel |
| Filgrastim ratiopharm | EMA | September 2008 | Ratiopharm |
| Filgrastim hexal | EMA | February 2009 | Hexal |
| Zarzio | EMA | February 2009 | Sandoz |
| Nivestim | EMA | June 2010 | Hospira UK |
| Remsima | EMA | September 2013 | Celltrion |
| Ovaleap | EMA | September 2013 | Teva |
| Grastofil | EMA | October 2013 | Apobiologix |
| Bemfola | EMA | March 2014 | Finox Biotech |
| Accofil | EMA | July 2014 | Accord |
| Abasaglar | EMA | September 2014 | Eli Lilly |
| Zarxio | US FDA | March 2015 | Sandoz |
| Basaglar | US FDA | December 2015 | Eli Lilly |
| Benepali | EMA | January 2016 | Samsung Bioepis |
| Inflectra | US FDA | April 2016 | Celltrion |
| Flixabi | EMA | May 2016 | Samsung Bioepis |
| Inhixa | EMA | July 2016 | Techdow Europe AB |
| Thorinane | EMA | July 2016 | Pharmathen S.A |
| Erelzi | US FDA | August 2016 | Sandoz |
| Amjevita | US FDA | September 2016 | Amgen |
| Movymia | EMA | November 2016 | Stada |
| Terrosa | EMA | November 2016 | Gedeon Richter |
| Amgevita | EMA | January 2017 | Amgen |
| Solymbic | EMA | March 2017 | Amgen |
| Renflexis | US FDA | April 2017 | Merck & Co. Inc. |
| Cyltezo | US FDA | April 2017 | Boehringer Ingelheim |
| Truxima | EMA | August 2017 | Celltrion |
| Mvasi | US FDA | September 2017 | Genentech |
| Ogivri | US FDA | December 2017 | Mylan |
| Retacrit | US FDA | May 2018 | Pfizer Hospira |
| Fulphila | US FDA | June 2018 | Mylan NV |
| Challenges | Solutions |
|---|---|
| Reluctance of drug substitution | Promotion of Clinician and patient education of biosimilars, provision of sufficient clinical data to raise assurance and improved Clinician acceptance |
| Complex manufacturing process | Investment in advanced production techniques as well as outsourcing of biosimilar technology. Approval of related regulatory authority in even minor variations in the production process |
| Regulatory complications and lack of clarity | Establishment of proper communication with regulatory authorities and connected stakeholders. Allowance for open platforms of dialogue between all parties |
| Intellectual property rights | Design of strong unified and unitary patent litigation systems |
| Reach of biosimilar manufacturers to patients | Encouragement of manufacturers to differentiate their product from other service offerings and provide sufficient evidence of cost effectiveness, quality, safety, etc |
| Name of Biosimilar Product | Reference Product | Class of Biological Medicine | International Non-Proprietary Name (INN) | Indications |
|---|---|---|---|---|
| Zarxio | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Zarzio | Certain forms of cancer | |||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Remicade | Tumor Necrosis Factor (TNF) alpha blocker | Infliximab | Psoriasis | |
| Rheumatoid arthritis | ||||
| Inflectra | Psoriatic arthritis | |||
| Remsima | Crohn’s disease | |||
| Ulcerative colitis | ||||
| Ankylosing spondilitis | ||||
| Erelzi | Enbrel | Tumor Necrosis Factor (TNF) alpha blocker | Etanercept | Rheumatoid arthritis |
| Polyarticular juvenile idiopathic | ||||
| arthritis | ||||
| Plaque psoriasis | ||||
| Ankylosing spondylitis Psoriatic arthritis | ||||
| Humira | Tumor Necrosis Factor (TNF) alpha blocker | Adalimumab | Rheumatoid arthritis | |
| Psoriatic arthritis | ||||
| Ankylosing spondylitis | ||||
| Amjevita | Crohn’s disease | |||
| Amgevita | Ulcerative colitis | |||
| Juvenile idiopathic arthritis | ||||
| Plaque psoriasis | ||||
| Uveitis | ||||
| Remicade | Tumor Necrosis Factor (TNF) alpha blocker | Infliximab | Psoriasis | |
| Rheumatoid arthritis | ||||
| Renflexis | Ankylosing spondilitis | |||
| Flixabi | Psoriatic arthritis | |||
| Crohn’s disease | ||||
| Ulcerative colitis | ||||
| Cyltezo | Humira | Tumor Necrosis Factor (TNF) alpha blocker | Adalimumab | Rheumatoid arthritis |
| Psoriatic arthritis | ||||
| Ankylosing spondylitis | ||||
| Crohn’s disease | ||||
| Ulcerative colitis | ||||
| Juvenile idiopathic arthritis | ||||
| Plaque psoriasis | ||||
| Uveitis | ||||
| Mvasi | Avastin | Monoclonal Antibody (MAb) | Bevacizumab | Metastatic Colorectal cancer |
| Metastatic HER2-negative Breast cancer | ||||
| Metastatic Renal cell carcinoma | ||||
| Second line treatment of Glioblastoma | ||||
| Second line treatment of Metastatic | ||||
| Colorectal cancer | ||||
| First line treatment of Non-Small Cell | ||||
| Lung Cancer (NSCLC) | ||||
| Omnitrope | Genotropin | Growth Hormone (GH) | Somatotropin | Inadequate endogenous GH secretion |
| Prader—Willi Syndrome | ||||
| Turner Syndrome Childhood/adult onset | ||||
| GH deficiency | ||||
| Pituitary dwarfism | ||||
| Binocrit | Eprex | Erythropoietin (EPO) | Epoetin alpha | Anemia due to Chronic Kidney Disease (CKD) |
| Anemia due to Zidovudine in patients with HIV infection | ||||
| Anemia due to chemotherapy in | ||||
| patients with cancer | ||||
| Reduction of allogenic red blood cell transfusions | ||||
| Epoetin alfa Hexal | Eprex | Erythropoietin (EPO) | Epoetin alpha | Anemia due to Chronic Kidney Disease (CKD) |
| Anemia due to Zidovudine in patients with HIV infection | ||||
| Anemia due to chemotherapy in patients with cancer | ||||
| Reduction of allogenic red blood cell transfusions | ||||
| Abseamed | Eprex | Erythropoietin (EPO) | Epoetin alpha | Anemia due to Chronic Kidney Disease (CKD) |
| Anemia due to Zidovudine in patients with HIV infection | ||||
| Anemia due to chemotherapy in patients with cancer | ||||
| Reduction of allogenic red blood cell transfusions | ||||
| Silapo | Eprex | Erythropoietin (EPO) | Epoetin zeta | Anemia due to Chronic Kidney Disease (CKD) |
| Anemia of renal origin accompanied by clinical symptoms in patients with renal insufficiency | ||||
| Reduction of transfusion requirements in patients receiving chemotherapy for tumours, malignant lymphoma or | ||||
| multiple myeloma | ||||
| Retacrit | Eprex | Erythropoietin (EPO) | Epoetin zeta | Anemia due to Chronic Kidney Disease (CKD) |
| Anemia of renal origin accompanied by clinical symptoms in patients with renal insufficiency | ||||
| Reduction of transfusion requirements in | ||||
| patients receiving chemotherapy for | ||||
| tumours, malignant lymphoma or multiple myeloma | ||||
| Ratiograstim | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Tevagrastim | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Biograstim | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Filgrastim ratiopharm | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Filgrastim hexal | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Accofil | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Nivestim | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Ovaleap | Gonal-f | Follicle Stimulating Hormone (FSH) | Follitropin alpha | Polycystic ovary syndrome |
| Grastofil | Neupogen | Granulocyte Colony Stimulating Factor (G-CSF) | Filgrastim | HIV or drug therapy induced neutropenia |
| Certain forms of cancer | ||||
| Severe chronic congenital neutropenia | ||||
| Hematopoietic stem cell transplantation | ||||
| Bemfola | Gonal-f | Follicle Stimulating Hormone (FSH) | Follitropin alpha | Induction of ovulation in anovulatory |
| infertile patient | ||||
| Induction of pregnancy in anovulatory infertile patient | ||||
| Abasaglar Basaglar | Lantus | Human Insulin Hormone | Insulin glargine | Type 1 diabetes (in adults and children) |
| Type 2 diabetes (in adults when basal | ||||
| insulin is needed) | ||||
| Benepali | Enbrel | Tumor Necrosis Factor (TNF) alpha blocker | Etanercept | Rheumatoid arthritis |
| Polyarticular juvenile idiopathic arthritis | ||||
| Plaque psoriasis | ||||
| Ankylosing spondylitis Psoriatic arthritis | ||||
| Lusduna | Lantus | Human Insulin Hormone | Insulin glargine | Type 1 diabetes (in adults and children) |
| Type 2 diabetes (in adults when basal | ||||
| insulin is needed) | ||||
| Inhixa | Clexane | Low molecular weight heparin (Anticoagulant) | Enoxaparin Sodium | Prophylaxis of deep vein thrombosis (DVT) |
| Prophylaxis of thromboembolic complications | ||||
| Thorinane | Clexane | Low molecular weight heparin (Anticoagulant) | Enoxaparin Sodium | Prophylaxis of deep vein thrombosis (DVT) |
| Prophylaxis of thromboembolic complications | ||||
| Solymbic | Humira | Tumor Necrosis Factor (TNF) alpha blocker | Adalimumab | Rheumatoid arthritis |
| Psoriatic arthritis | ||||
| Ankylosing spondylitis | ||||
| Crohn’s disease | ||||
| Ulcerative colitis | ||||
| Juvenile idiopathic arthritis | ||||
| Plaque psoriasis | ||||
| Uveitis | ||||
| Truxima | MabThera | Monoclonal Antibody (MAb) | Rituximab | Chronic lymphocytic leukemia |
| Rheumatoid arthritis | ||||
| Non-Hodgkin’s lymphoma | ||||
| Granulomatos with Polyangiitis (GPA) and microscopic Polyangiitis (MPA) | ||||
| Movymia | Forsteo | Para-Thyroid Hormone (PTH) | Teriparatide | Post-menopausal osteoporosis |
| Idiopathic osteoporosis in men | ||||
| Hypogonodal osteoporosis in men | ||||
| Glucocorticoid induced osteoporosis | ||||
| Terrosa | Forsteo | Para-Thyroid Hormone (PTH) | Teriparatide | Post-menopausal osteoporosis |
| Idiopathic osteoporosis in men Hypogonodal osteoporosis in men | ||||
| Glucocorticoid induced osteoporosis |
| Characteristics | Small Molecule Drugs | Large Molecular Drugs |
|---|---|---|
| Size | Single molecule, hence small | Combination of closely |
| Low molecular weight | related molecules, hence large | |
| Large molecular weight | ||
| Structure | Simple, well defined, regardless of manufacturing process | Complex (heterogeneous), defined by exact manufacturing process |
| Modification | Well defined | Wider range of options |
| Stability | Stable | Unstable, sensitive to external conditions |
| Characterisation | Can be characterized completely | Cannot be characterised completely because of molecular composition and heterogeneity |
| Immunogenicity | Usually non-immunogenic | Immunogenic |
| Manufacturing | Produced by chemical synthesis | Produced in living cell culture |
| Foreseeable chemical process | Difficult to control process from starting material to final API | |
| Duplicate copy can be made | Impossible to ensure duplicate copy | |
| Susceptibility to contamination during manufacture | Low | High |
| Sensitivity to physical factors (heat, light) | Low | High |
| Clinical behaviour | Well defined mode of action | Complex modes of action |
| Species | Interdependent | Specific |
| Absorption | More rapid | Slower |
| Distribution | High | Limited |
| Metabolism | Metabolized to active and non-active metabolites | Broken down to endogenous amino acids |
| Disposition | Rarely target mediated | Mostly target mediated |
| Half-life | Shorter | Longer |
| Pharmaceutical Industry | Biopharmaceutical Industry | Biosimilar Industry | ||
|---|---|---|---|---|
| Parameter | Small Molecule Drug | Generic Drug | Biological Drug | Biosimilar Drug |
| Synthesis | Production of | Copy from the | Manufactured in a living system, usually through recombinant DNA technology | Development derived from |
| original chemical | original chemical | the original biological | ||
| formula | formula | molecule | ||
| Size | 100–1000 Da | 100–1000 Da | 10,000–300,000 Da | 10,000–300,000 Da |
| Glycosylation process | Zero | Zero | Several | Several |
| Molecular structure | Simple | Simple | Complex | Complex |
| Ability to generate immunity | Low | Low | Medium–High | Medium–High |
| Drug development time | 7–10 years | 1–3 years | 10–15 years | 6–9 years |
| Characterisation via analysis in laboratory | N/A | There are techniques to identify similarity to the original drug | N/A | No identification technique for equality of the molecule |
| Clinical studies | ||||
| are needed | ||||
References
- Chopra, I.; Arkells, N.; Chopra, A. Biosimilars: Barriers and Opportunities to Market Access in the United States. Value Health 2018, 21, S93. [Google Scholar] [CrossRef]
- Zalcberg, J. Biosimilars are coming: Ready or not. Intern. Med. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs). 2009. Retrieved 17 April 2017. Available online: http://apps.who.int/medicinedocs/en/d/Js19941en (accessed on 17 April 2017).
- Markus, R.; Liu, J.; Ramchandani, M.; Landa, D.; Born, T.; Kaur, P. Developing the Totality of Evidence for Biosimilars: Regulatory Considerations and Building Confidence for the Healthcare Community. BioDrugs 2017, 31, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Danne, T.; Heinemann, L.; Bolinder, J. New Insulins, Biosimilars, and Insulin Therapy. Diabetes Technol. Ther. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Alam, M.U.; Amin, M. A Global Perspective of the Prospects and Challenges of an Awaiting Revolution of Biosimilars. Bioj. Sci. Technol. 2015, 2, 7–16. [Google Scholar]
- Rader, R.A. Biosimilars Pipeline Analysis: Many Products, More Competition Coming. 2016. Retrieved 20 April 2017. Available online: https://www.bioprocessonline.com/doc/biosimilars-pipeline-analysis-many-products-more-competition-coming-0001 (accessed on 23 April 2017).
- Cazap, E.; Jacobs, I.; Mcbride, A.; Popovian, R.; Sikora, K. Global Acceptance of Biosimilars: Importance of Regulatory Consistency, Education, and Trust. Oncologist 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Bria, E.; Conte, P. Biosimilars as a strategy to improve sustainability. ESMO Open 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Jois, R. The brave new world of biosimilars (‘similar biologics’) in India. Postgrad. Med. J. 2017, 93, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Raj Kumar, V.; Ishita, S.; Ayush, G. Biosimilars: A Review of Regulatory Perspective in Indian Context. Bull. Pharm. Res. 2017, 7, 139. [Google Scholar] [CrossRef]
- Grewal, S.; Ramsey, S.; Balu, S.; Carlson, J.J. Cost-savings for biosimilars in the United States: A theoretical framework and budget impact case study application using filgrastim. Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W. Update on Biosimilars in Asia. Curr. Rheumatol. Rep. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Reinivuori, T.; Kurki, P.; Chamberlain, P. Immunogenicity Assessment of Biosimilars. Pharm. Med. 2018, 32, 103–121. [Google Scholar] [CrossRef]
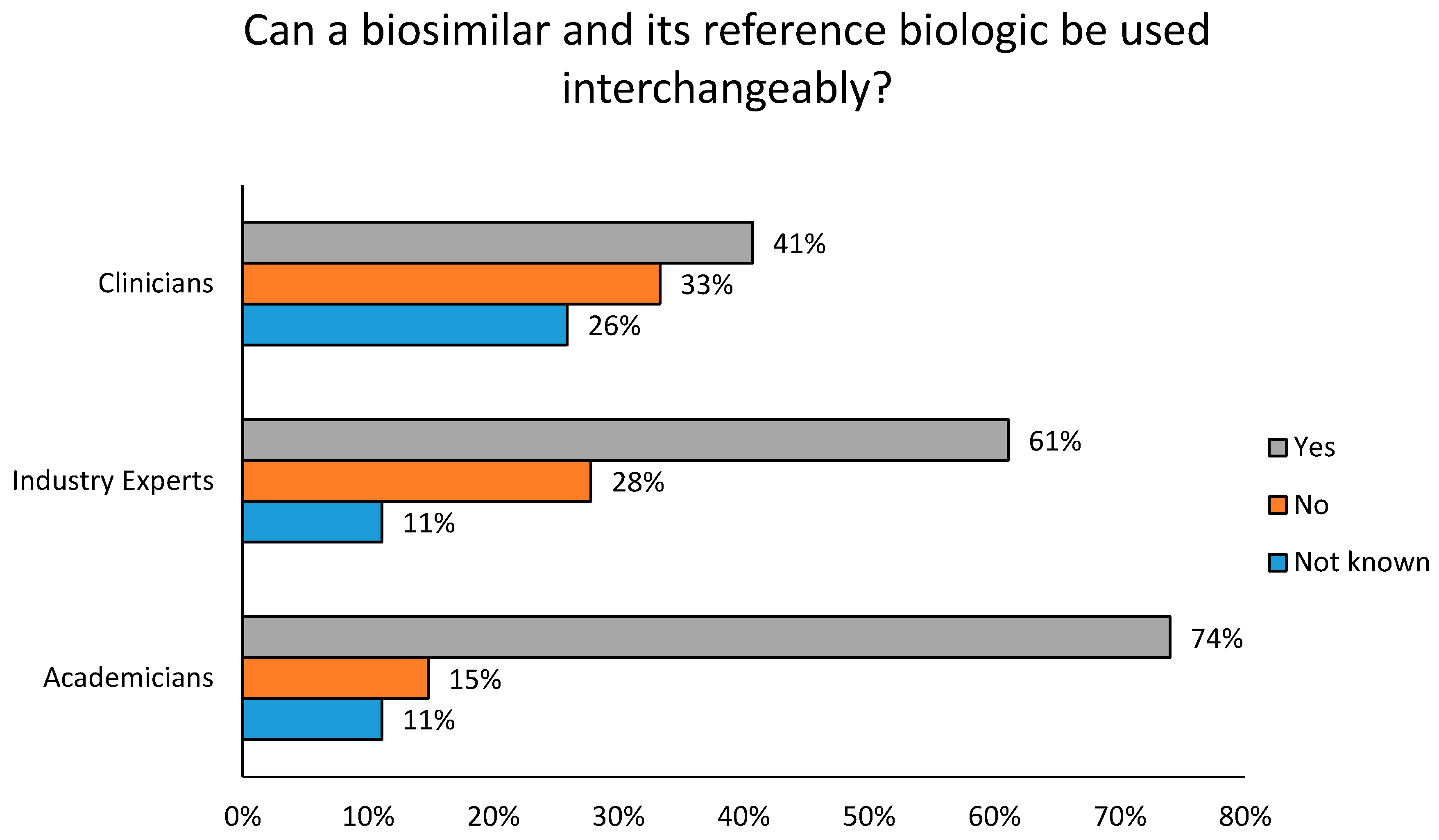
- Trifirò, G.; Marcianò, I.; Ingrasciotta, Y. Interchangeability of biosimilar and biological reference product: Updated regulatory positions and pre- and post-marketing evidence. Expert Opin. Biol. Ther. 2017, 18, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Mysler, E.; Pineda, C.; Horiuchi, T.; Singh, E.; Mahgoub, E.; Coindreau, J.; Jacobs, I. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol. Int. 2016, 36, 613–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abhayawansa, S.; Azim, M. Corporate reporting of intellectual capital: Evidence from the Bangladeshi pharmaceutical sector. Asian Rev. Account. 2014, 22, 98–127. [Google Scholar] [CrossRef]
- Azam, M.M. The impacts of TRIPS on the pharmaceutical regulation and pricing of drugs in Bangladesh. Int. J. Law Manag. 2017, 59, 376–393. [Google Scholar] [CrossRef]
- Sonobe, T.; Mottaleb, K.A.; Amin, M.N. The Miraculous Development of the Garment and Pharmaceutical Industries in Bangladesh. Econ. Soc. Dev. Bangladesh 2017, 27–51. [Google Scholar] [CrossRef]
- Azevedo, V.F.; Mysler, E.; Álvarez, A.A.; Hughes, J.; Flores-Murrieta, F.J.; Castilla, E.M. Recommendations for the regulation of biosimilars and their implementation in Latin America. Generics Biosimilars Initiat. J. 2014, 3, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Farhat, F.; Torres, A.; Park, W.; Lopes, G.D.; Mudad, R.; Ikpeazu, C.; Aad, S.A. The Concept of Biosimilars: From Characterization to Evolution—A Narrative Review. Oncologist 2017, 23, 346–352. [Google Scholar] [CrossRef] [PubMed]
- IFPMA. Considerations for Physicians on Switching Decisions Regarding Biosimilars. 2017. Retrieved 10 April 2017. Available online: https://www.ebe-biopharma.eu/publication/ebe-efpia-and-ifpma-position-paper-considerations-for-physicians-on-switching-decisions-regarding-biosimilars (accessed on 16 April 2017).
- Cassels, A. Why biosimilars should be interchangeable with biologics. Clin. Pharm. 2017, 9. [Google Scholar] [CrossRef]
- Castaneda-Hernandez, G.; Gonzalez-Ramirez, R.; Kay, J.; Scheinberg, M.A. Biosimilars in rheumatology: What the clinician should know. RMD Open 2015, 1, e000010. [Google Scholar] [CrossRef] [PubMed]
- WHO (2017). Similar biotherapeutic products. Retrieved 3 July 2017. Available online: http://www.who.int/biologicals/biotherapeutics/similar_biotherapeutic_products/en/ (accessed on 26 August 2018).
- Sarker, M.M.; Rashid, M.S.; Raju, A.A.; Rana, M.; Karim, M.F.; Akter, R.; Howlader, A.N.; Ming, L.C.; Ismail, N.E. Evaluation of the Pharmaceutical Quality of Different Brands of Ranitidine Tablets Manufactured in Bangladesh: A Pharmaceutical and Public Health Prospective. J. Appl. Pharm. Sci. 2016, 6, 055–061. [Google Scholar] [CrossRef]
- Bangladesh Medical and Dental Council. Retrieved on 12 July 2017. 2016. Available online: http://bmdc.org.bd/doctors-info (accessed on 27 August 2018).
- IMS Health. IMS Health Report 2017 Q1; IMS Health: Danbury, CT, USA, 2016. [Google Scholar]
- University Grants Commission of Bangladesh. UGC 43rd Annual Report. 2016; Retrieved on 13 July 2017. Available online: http://www.ugc.gov.bd/en/home/downloadfile/24 (accessed on 27 August 2018).
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences, 7th ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Calculator Raosoft. Sample Size. Raosoft. 2004. Available online: http://www.raosoft.com/samplesize.html (accessed on 25 July 2018).
- Morton, F.M.; Stern, S. The Impact of the Entry of Biosimilars: Evidence from Europe. SSRN Electron. J. 2016. [Google Scholar] [CrossRef]
- Smith, B.; Huang, S. Novel approaches to address challenges in global drug development. Clin. Pharmacol. Ther. 2015, 97, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Tannoury, M.; Attieh, Z. The Influence of Emerging Markets on the Pharmaceutical Industry. Curr. Ther. Res. 2017, 86, 19–22. [Google Scholar] [CrossRef] [PubMed]
| Stakeholders in the Value Chain of the Pharmaceutical Industry | Which Stakeholders are Most Likely to Recognize the Specific Approved Industry Standards Required for Biosimilar Manufacture? | Which Stakeholders Are Most Responsible for Recognizing the Value Provided from a Company’s Biosimilar Product in Order to Prescribe it? | Which Stakeholders Hold the Responsibility of Promoting Biosimilar Awareness and Education? |
|---|---|---|---|
| Clinicians | - | X | X |
| Patients | - | - | - |
| Industry Experts | X | - | X |
| Academicians | - | - | X |
| A Biosimilar is….. | Industry Experts (NIE = 20%) | Academicians (NA = 40%) | Clinicians (NC = 40%) |
|---|---|---|---|
| a biologic that demonstrates equivalence with the original biodrug and has all the preclinical and clinical trials equal to those already performed with the original biodrug | 36 (72%) | 63 (63%) | 22 (22%) |
| a biologic that demonstrates bioequivalence with an original biodrug and does not need clinical trials to be commercialized | 14 (28%) | 37 (37%) | 78 (78%) |
| Industry Experts (NIE = 20%) | Academicians (NA = 40%) | Clinicians (NC = 40%) | |
|---|---|---|---|
| Need for updated regulatory guidelines | 30.5 (61%) | 68 (68%) | 17% |
| Need for improved pharmacovigilance systems to ensure drug safety | 28 (56%) | 41 (41%) | 56 (56%) |
| Extrapolation of clinical data may be insufficient to determine effectiveness of biosimilar | 25 (50%) | 10 (10%) | 61 (61%) |
| Complications during substitution of drug in patient therapy | 11 (22%) | 56 (56%) | 44 (44%) |
| Economic and societal consequences of biosimilar use | 5.5 (11%) | 0 (0%) | 0 (0%) |
| Drivers for Clinicians to Prescribe Biosimilars | Response of Industry Experts (%) (NIE = 100%) |
|---|---|
| Lower price of the biosimilar in comparison with the innovator biodrug | 28 (56%) |
| Country of origin where the biosimilar has been manufactured | 8.5 (17%) |
| Certified approval for the biosimilar by the relevant authorities | 22 (44%) |
| Good manufacturing practices and high reputation of the manufacturer | 19.5 (39%) |
| Bioefficacy of the biosimilar drug | 22 (44%) |
| Safety of the biosimilar drug | 30.5 (61%) |
| Practice for Demonstrating Reliability of the Product by Manufacturer | Response of Industry Experts (%) (NIE = 100%) |
|---|---|
| Provision of bioequivalent safety and efficacy data | 14 (28%) |
| Provision of data of Phase III clinical trial outcomes within a sample of the local population | 22 (44%) |
| Provision of evidence of strong GMP maintenance | 8.5 (17%) |
| Provision of evidence that WHO guidelines have been followed during product registration | 5.5 (11%) |
| Patient Education Strategy | Response of Industry Experts (%) (NIE = 100%) |
|---|---|
| Public counselling campaigns through various appropriate media | 33.5 (67%) |
| Patient counselling programs sponsored by pharmaceutical companies and hospitals | 25 (50%) |
| Patient education provided by hospital personnel during drug prescription | 8.5 (17%) |
| Patient education through open seminars or symposiums held by Academicians from universities citywide | 14 (28%) |
| Benefits of Biosimilar Medication | Academicians (NA = 40%) | Industry Experts (NIE = 20%) | Clinicians (NC = 40%) |
|---|---|---|---|
| Lower cost | 80 (80%) | 41.5 (83%) | 67 (67%) |
| Commercialization approved with initial indication including all diseases previously approved for the innovator biodrug | 35 (35%) | 22 (44%) | 44 (44%) |
| Administration route different from that of the original biodrug | 14 (14%) | 3 (6%) | 0 (0%) |
| Lower therapeutic dose | 7 (7%) | 0 (0%) | 0 (0%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, E.R.; Moreino, S.S.; Sharif Siam, M.K. An Empirical Analysis of the Perceived Challenges and Benefits of Introducing Biosimilars in Bangladesh: A Paradigm Shift. Biomolecules 2018, 8, 89. https://doi.org/10.3390/biom8030089
Kabir ER, Moreino SS, Sharif Siam MK. An Empirical Analysis of the Perceived Challenges and Benefits of Introducing Biosimilars in Bangladesh: A Paradigm Shift. Biomolecules. 2018; 8(3):89. https://doi.org/10.3390/biom8030089
Chicago/Turabian StyleKabir, Eva Rahman, Shannon Sherwin Moreino, and Mohammad Kawsar Sharif Siam. 2018. "An Empirical Analysis of the Perceived Challenges and Benefits of Introducing Biosimilars in Bangladesh: A Paradigm Shift" Biomolecules 8, no. 3: 89. https://doi.org/10.3390/biom8030089





