A Novel Class of tRNA-Derived Small Non-Coding RNAs Respond to Myocardial Hypertrophy and Contribute to Intergenerational Inheritance
Abstract
:1. Introduction
2. Results
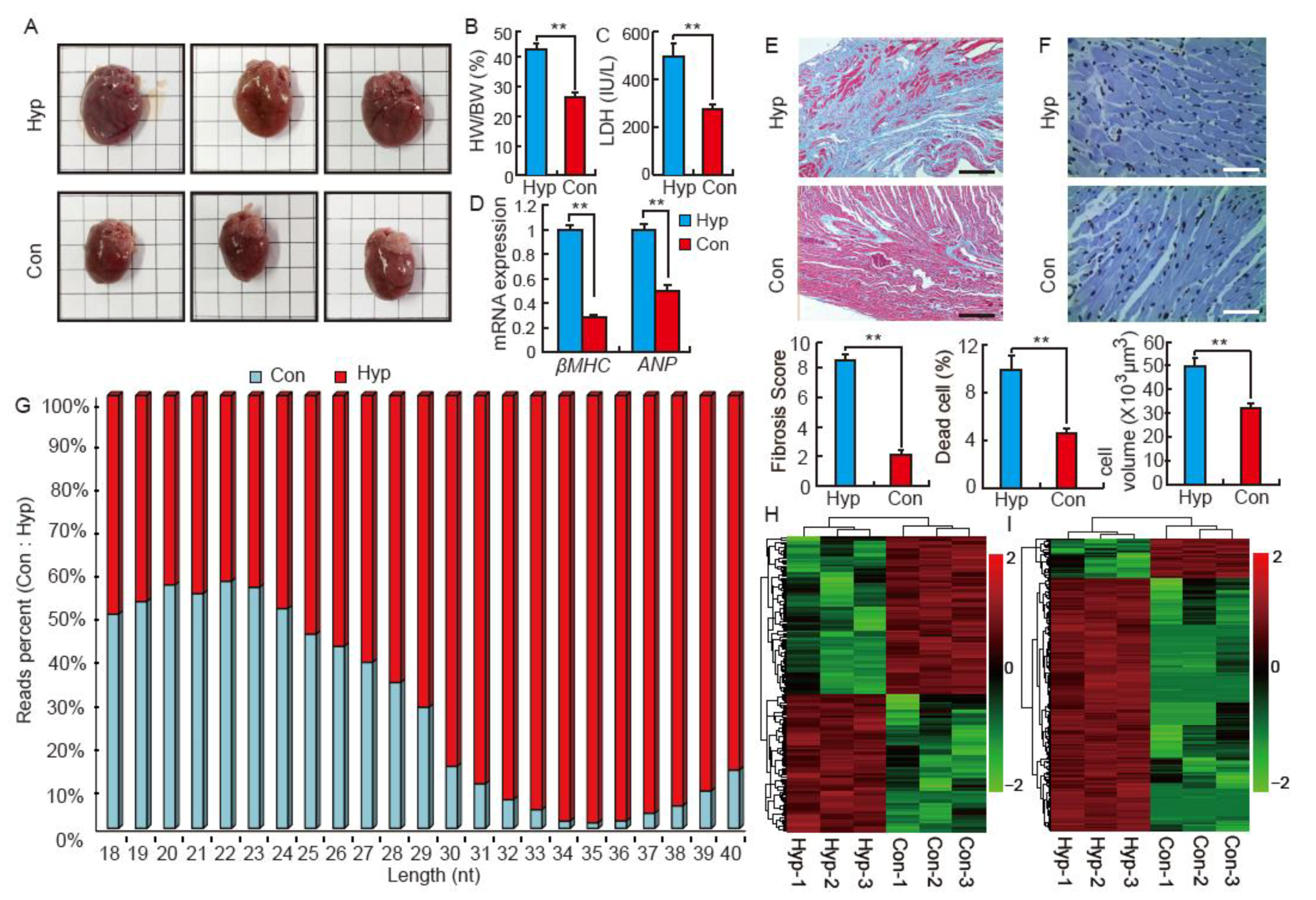
2.1. Isoproterenol Induces Cardiac Hypertrophy and Influences Small RNA Distribution in SD Rats
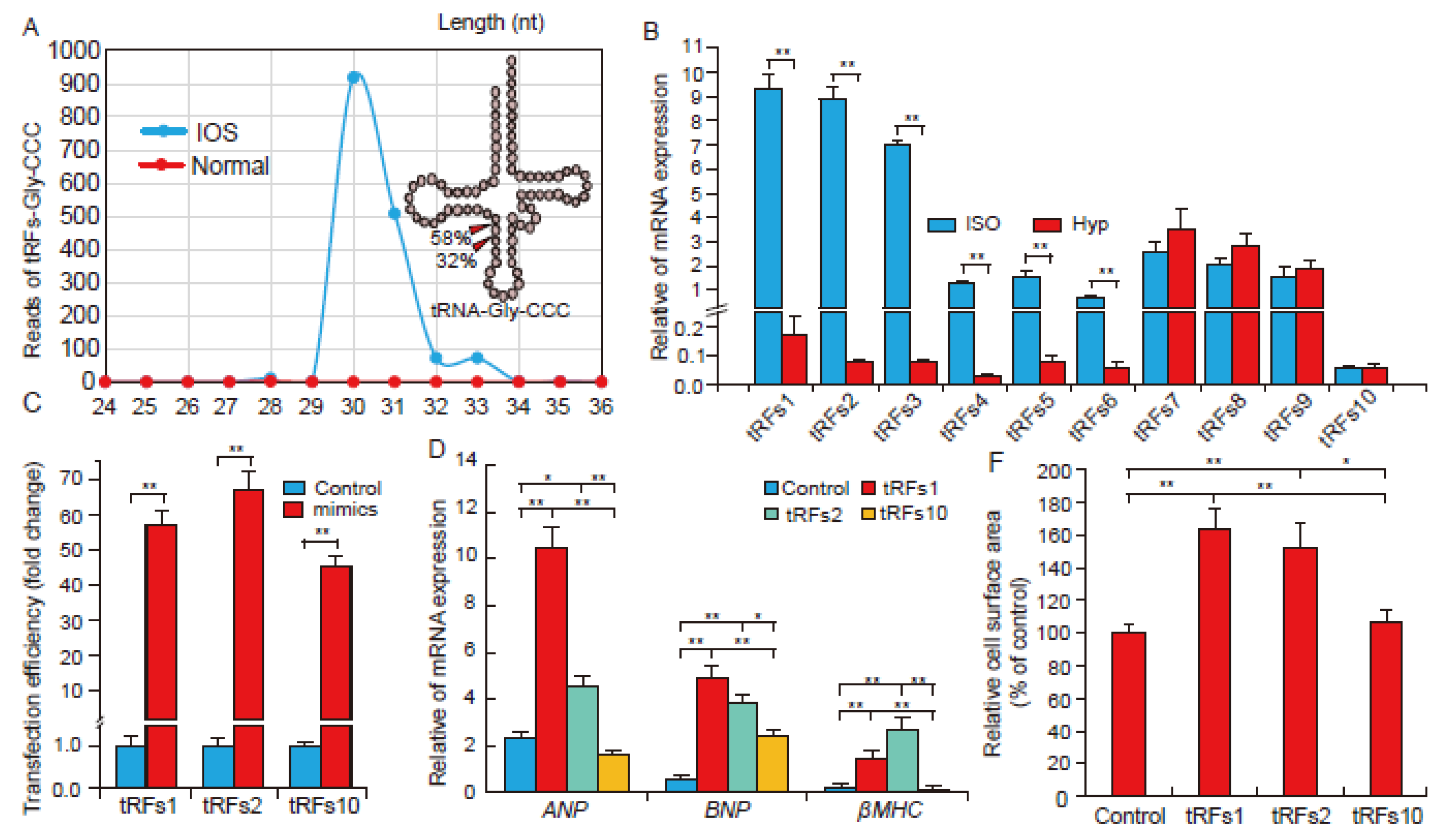
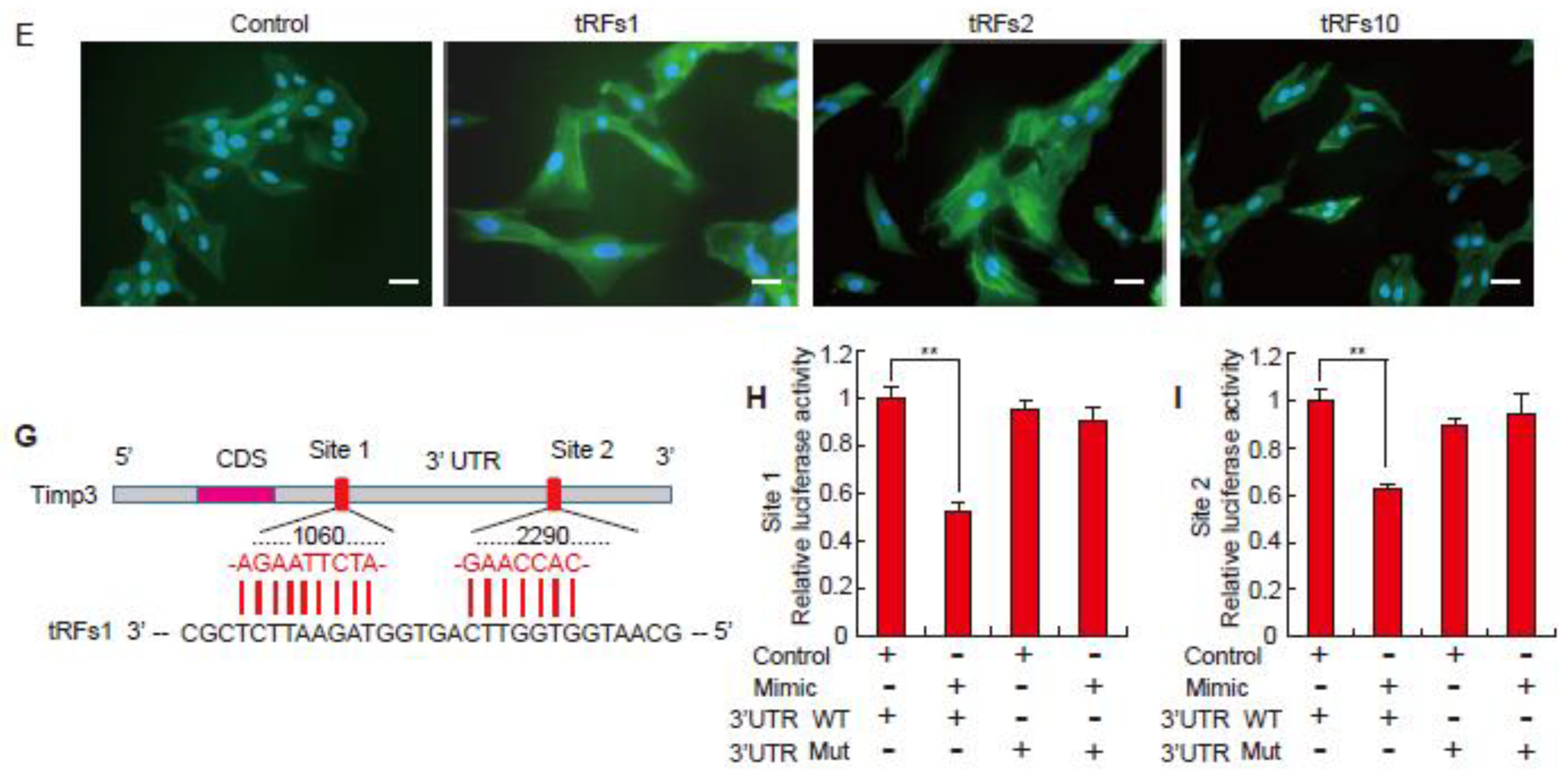
2.2. tRFs Promote Cardiac Hypertrophy in Cardiomyocytes
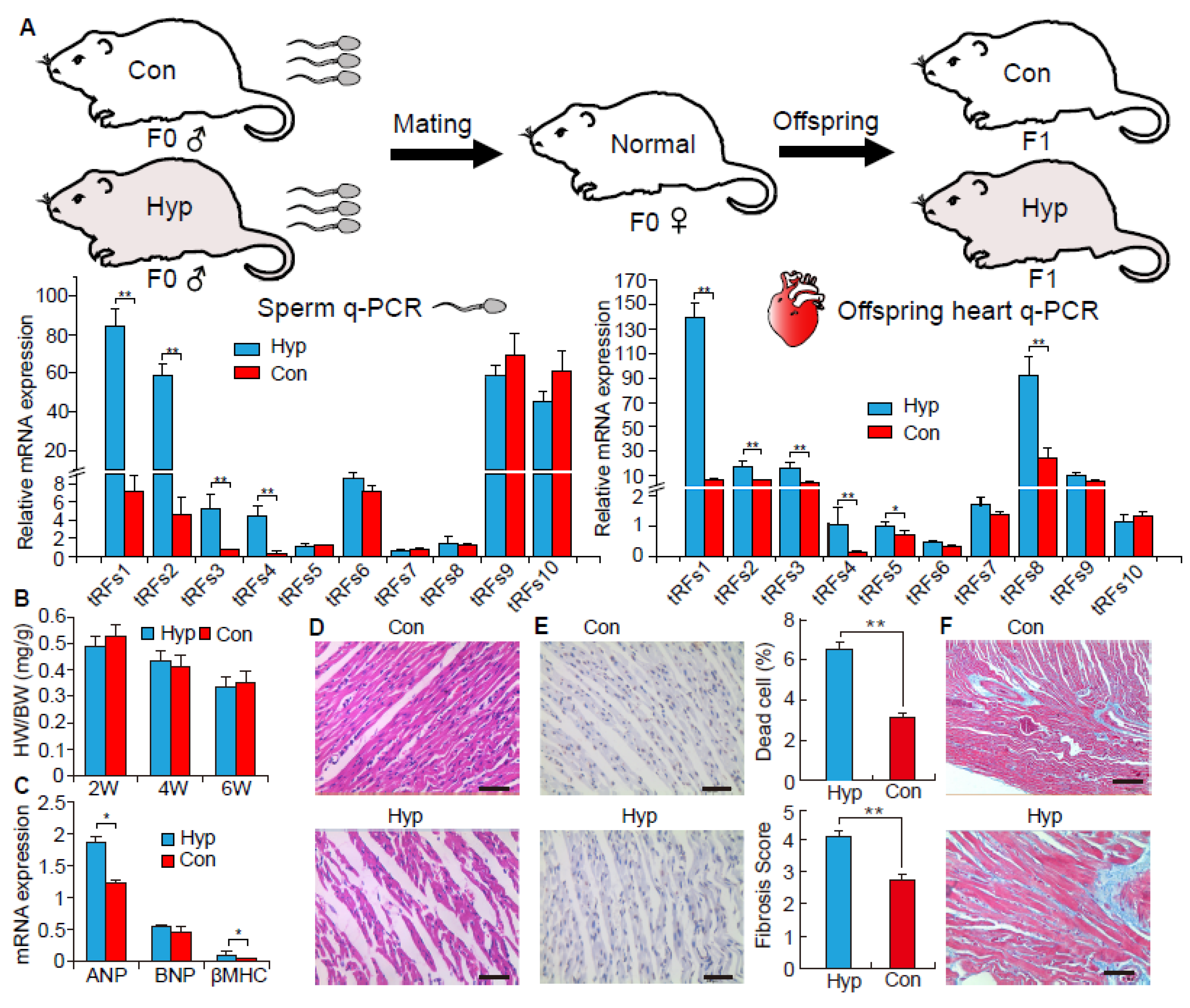
2.3. The Function of tRNA-Derived Fragments on the Intergenerational Inheritance of Cardiac Hypertrophy
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.-T. Overview of microRNAs in cardiac hypertrophy, fibrosis, and apoptosis. Int. J. Mol. Sci. 2016, 17, 749. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gao, Q.; Cao, F. Long noncoding RNAs (LncRNAs)—The dawning of a new treatment for cardiac hypertrophy and heart failure. BBA-Mol. Basis Dis. 2017, 1863, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Devaux, Y.; Creemers, E.E.; Boon, R.A.; Werfel, S.; Thum, T.; Engelhardt, S.; Dimmeler, S.; Squire, I. Circular RNAs in heart failure. Eur. J. Heart Fail. 2017, 19, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, L.; da Costa Martins, P.A. Non-coding RNAs in cardiac hypertrophy. J. Physiol. 2017, 595, 4037–4050. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A novel class of small RNAs: TRNA-derived RNA fragments (tRFs). Genes Dev. 2009, 23, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Selitsky, S.R.; Baran-Gale, J.; Honda, M.; Yamane, D.; Masaki, T.; Fannin, E.E.; Guerra, B.; Shirasaki, T.; Shimakami, T.; Kaneko, S.; et al. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci. Rep. 2015, 5, 7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.-H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Sobala, A.; Hutvagner, G. Small RNAs derived from the 5′ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013, 10, 553–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Couvillion, M.T.; Bounova, G.; Purdom, E.; Speed, T.P.; Collins, K. A Tetrahymena Piwi bound to mature tRNA 3′ fragments activates the exonuclease Xrn2 for RNA processing in the nucleus. Mol. Cell 2012, 48, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M.; Lu, C.; Green, P.J.; Parker, R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 2008, 14, 2095–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Hu, B.; Hu, G.-W.; Chen, C.-Y.; Niu, X.; Liu, J.; Zhou, S.-M.; Zhang, C.-Q.; Wang, Y.; Deng, Z.-F. tRNA-derived small non-coding RNAs in response to ischemia inhibit angiogenesis. Sci. Rep. 2016, 6, 20850. [Google Scholar] [CrossRef] [PubMed]
- Kassiri, Z.; Defamie, V.; Hariri, M.; Oudit, G.Y.; Anthwal, S.; Dawood, F.; Liu, P.; Khokha, R. Simultaneous transforming growth factor β-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart. J. Biol. Chem. 2009, 284, 29893–29904. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.P.; Hutvagner, G. tRNA-derived fragments (tRFs): Emerging new roles for an ancient RNA in the regulation of gene expression. Life 2015, 5, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef] [PubMed]
- Noordhuizen-Stassen, E.N.; Beijer, H.; Charbon, G.; Wensing, C. The effect of norepinephrine, isoprenaline and acetylcholine on the testicular and epididymal circulation in the pig. Int. J. Androl. 1983, 6, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-Q.; Chao, M.; Lu, Q.-H.; Chai, W.-L.; Zhang, W.; Chen, X.-Y.; Liang, E.-S.; Wang, L.-B.; Tian, H.-L.; Chen, Y.-G.; et al. Prohibitin overexpression improves myocardial function in diabetic cardiomyopathy. Oncotarget 2016, 7, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gan, M.; Li, Q.; Wang, J.; Li, X.; Zhang, S.; Zhu, L. MicroRNA-200b regulates preadipocyte proliferation and differentiation by targeting KLF4. Biomed. Pharmacother. 2018, 103, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence | Length | tRNA Mapped | Fold Change |
|---|---|---|---|---|
| tRFs1 | GCAATGGTGGTTCAGTGGTAGAATTCTCGC | 30 | tRNA-Gly-GCC | 867.53 |
| tRFs2 | TCCCATATGGTCTAGCGGTTAGGATTCCTGGTTT | 34 | tRNA-Glu-TTC | 415.64 |
| tRFs3 | TCCATGGTGGTCTAGTGGTTAGGATTCGGC | 30 | tRNA-Glu-CTC | 352.76 |
| tRFs4 | GGTTCCATGGTGTAATGGTTAGCACTCTGGACTC | 34 | tRNA-Gln-CTG | 137.83 |
| tRFs5 | GCACTGGTGGTTCAGTGGTAGAATTCTCGC | 30 | tRNA-Gly-CCC | 124.92 |
| tRFs6 | GTTTCCGTAGTGTAGTGGTTATCACGTTCGCCTC | 34 | tRNA-Val-CAC | 96.76 |
| tRFs7 | ATTAGGGTGGCAGAGCCAGGTAATT | 25 | tRNA-Leu-TAA | 0.94 |
| tRFs8 | GTAGTCGTGGCCGAGTGGTTAAG | 23 | tRNA-Ser-AGA | 0.98 |
| tRFs9 | TAGGATAGGGTGTATTGGTAGCAC | 24 | tRNA-Gln-TTG | 1.04 |
| tRFs10 | TTGGGGTGCGAGAGGTCCCGGGTT | 24 | tRNA-Pro-AGG | 1.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Gan, M.; Tan, Z.; Jiang, D.; Jiang, Y.; Li, M.; Wang, J.; Li, X.; Zhang, S.; Zhu, L. A Novel Class of tRNA-Derived Small Non-Coding RNAs Respond to Myocardial Hypertrophy and Contribute to Intergenerational Inheritance. Biomolecules 2018, 8, 54. https://doi.org/10.3390/biom8030054
Shen L, Gan M, Tan Z, Jiang D, Jiang Y, Li M, Wang J, Li X, Zhang S, Zhu L. A Novel Class of tRNA-Derived Small Non-Coding RNAs Respond to Myocardial Hypertrophy and Contribute to Intergenerational Inheritance. Biomolecules. 2018; 8(3):54. https://doi.org/10.3390/biom8030054
Chicago/Turabian StyleShen, Linyuan, Mailin Gan, Zhengdong Tan, Dongmei Jiang, Yanzhi Jiang, Mingzhou Li, Jinyong Wang, Xuewei Li, Shunhua Zhang, and Li Zhu. 2018. "A Novel Class of tRNA-Derived Small Non-Coding RNAs Respond to Myocardial Hypertrophy and Contribute to Intergenerational Inheritance" Biomolecules 8, no. 3: 54. https://doi.org/10.3390/biom8030054




