Novel Approaches for BAV Aortopathy Prediction—Is There a Need for Cohort Studies and Biomarkers?
Abstract
:1. BAV–The Clinical Perspective
1.1. Controversy of BAV Aortopathy and Current Clinical Diagnostic Tools
1.2. Definition of Aortopathy
1.3. Current Risk Stratification of Bicuspid Aortopathy
1.4. Limitations of the Diameter-Based Risk Stratification
1.5. Phenotype-Based Risk Stratification of Bicuspid Aortopathy
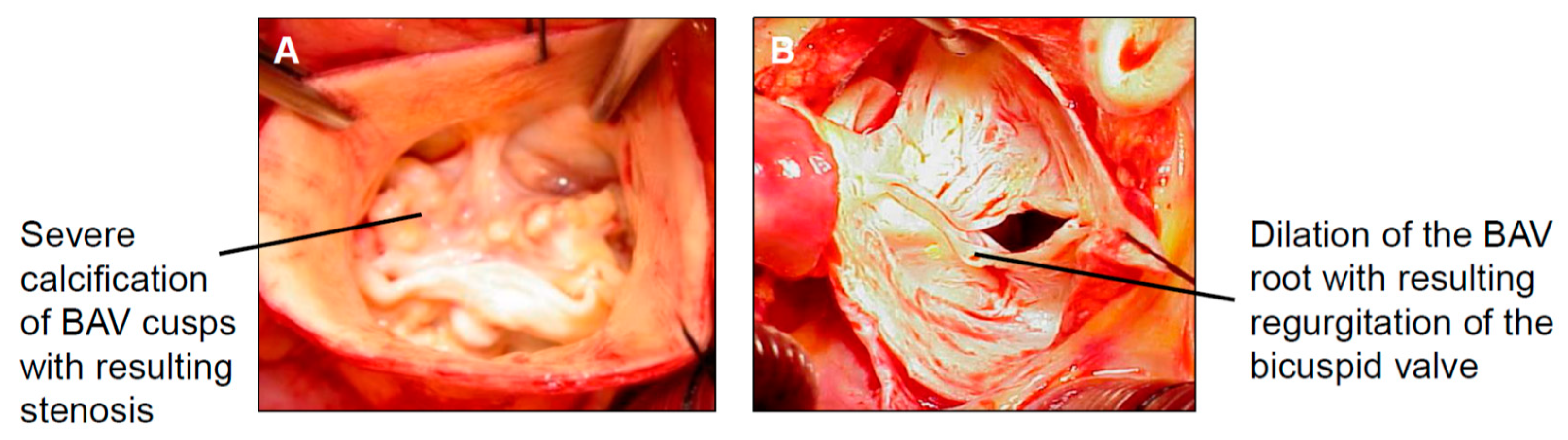
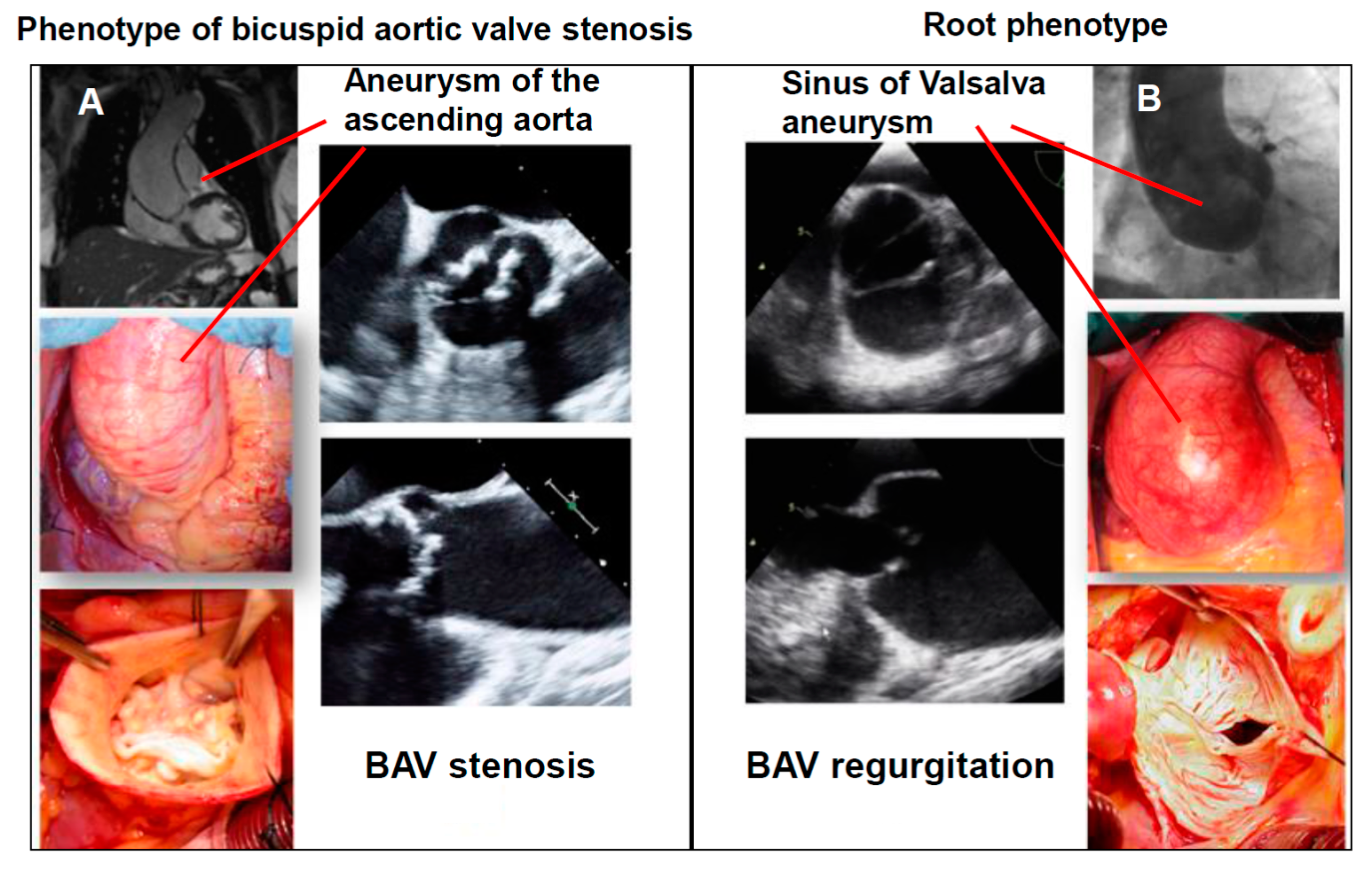
1.5.1. The Phenotype of Bicuspid Aortic Stenosis with Dilatation of the Tubular Ascending Aorta
1.5.2. Root Phenotype (Dilatation of the Sinus of Valsalva in Combination with BAV Regurgitation)
1.6. Current Knowledge on Genetics in BAV
1.7. Limitations of the Phenotype-Based Risk Stratification
2. How to Overcome the Current Shortcomings in the Risk Stratification of BAV Aortopathy
2.1. Example of the Disease Cohort—BAV
2.2. Population-Based Cohorts—Example Hamburg City Health Study (HCHS)
3. BAV and Circulating Biomarkers for the Risk Stratification in Bicuspid Aortopathy: Current Knowledge
3.1. Protein-Based Biomarkers
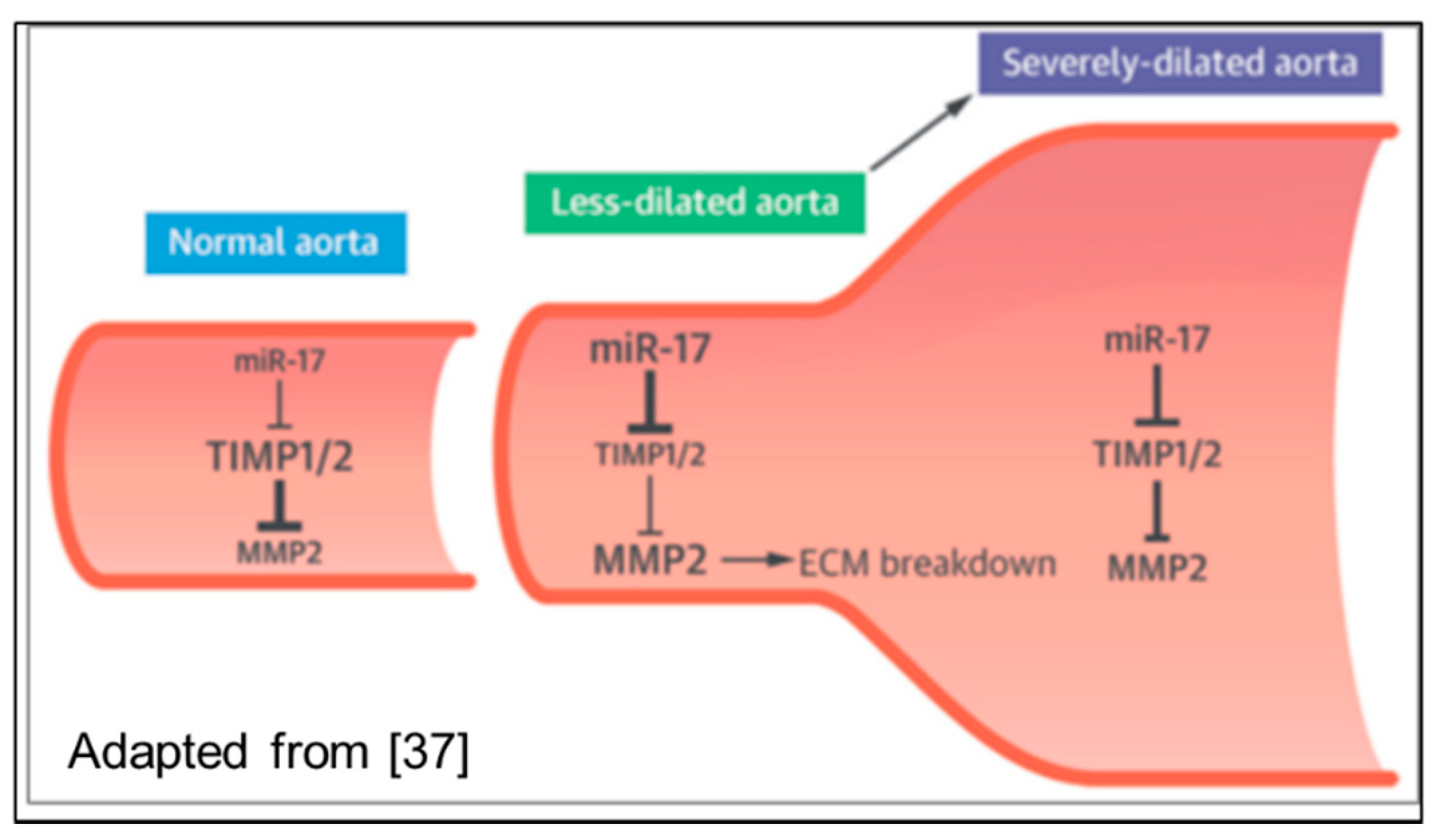
3.2. Non-Coding RNAs
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Ward, C. Clinical significance of the bicuspid aortic valve. Heart 2000, 83, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.S.; Kloesel, B.; Norris, R.A.; Lindsay, M.; Milan, D.; Body, S.C. Embryonic Development of the Bicuspid Aortic Valve. J. Cardiovasc. Dev. Dis. 2015, 2, 248–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, T.; Mani, A.; Elefteriades, J.A. Bicuspid aortic valve: Clinical approach and scientific review of a common clinical entity. Expert Rev. Cardiovasc. Ther. 2008, 6, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C. The congenitally bicuspid aortic valve: A study of 85 autopsy cases. Am. J. Cardiol. 1970, 26, 72–83. [Google Scholar] [CrossRef]
- Fedak, P.W.; Verma, S.; David, T.E.; Leask, R.L.; Weisel, R.D.; Butany, J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002, 106, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Braverman, A.C.; Güven, H.; Beardslee, M.A.; Makan, M.; Kates, A.M.; Moon, M.R. The bicuspid aortic valve. Curr. Probl. Cardiol. 2005, 30, 470–522. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.H.; Sundt, T.M. Bicuspid Aortic Valvulopathy and Associated Aortopathy: A Review of Contemporary Studies Relevant to Clinical Decision-Making. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Kalahasti, V.; Svensson, L.G.; Alashi, A.; Schoenhagen, P.; Roselli, E.E.; Johnston, D.R.; Rodriguez, L.L.; Griffin, B.P.; Desai, M.Y. Aortic Cross-Sectional Area/Height Ratio and Outcomes in Patients With Bicuspid Aortic Valve and a Dilated Ascending Aorta. Circ. Cardiovasc. Imaging 2017, 10, e006249. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.M.; Klein, M.D.; Shapira, O.M. Ascending aortic dilatation associated with bicuspid aortic valve. Pathophysiology, molecular biology and clinical implications. Circulation 2009, 119, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; von Kodolitsch, Y.; Detter, C.; Reichenspurner, H. Therapie der erweiterten Aorta ascendens: Bikuspide vs. trikuspide Aortopathie. Zeitschrift für Herz- Thorax- und Gefäßchirurgie 2016. [Google Scholar] [CrossRef]
- Edwards, W.D.; Leaf, D.S.; Edwards, J.E. Dissecting aortic aneurysm associated with congenital bicuspid aortic valve. Circulation 1978, 57, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.W.; Edwards, W.D. Risk factors for aortic dissection: A necropsy study of 161 cases. Am. J. Cardiol. 1984, 53, 849–855. [Google Scholar] [CrossRef]
- Michelena, H.I.; Desjardins, V.A.; Avierinos, J.F.; Russo, A.; Nkomo, V.T.; Sundt, T.M.; Pellikka, P.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008, 117, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Padang, R.; Bannon, P.G.; Jeremy, R.; Richmond, D.R.; Semsarian, C.; Vallely, M.; Wilson, M.; Yan, T.D. The genetic and molecular basis of bicuspid aortic valve associated thoracic aortopathy: A link to phenotype heterogeneity. Ann. Cardiothorac. Surg. 2013, 2, 83–91. [Google Scholar] [PubMed]
- Davies, R.R.; Goldstein, L.J.; Coady, M.A.; Tittle, S.L.; Rizzo, J.A.; Kopf, G.S.; Elefteriades, J.A. Yearly rupture or dissection rates for thoracic aortic aneurysms: Simple prediction based on size. Ann. Thorac. Surg. 2002, 73, 17–27. [Google Scholar] [CrossRef]
- Davies, R.R.; Gallo, A.; Coady, M.A.; Tellides, G.; Botta, D.M.; Burke, B.; Coe, M.P.; Kopf, G.S.; Elefteriades, J.A. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann. Thorac. Surg. 2006, 81, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Creager, M.A.; Isselbacher, E.M.; Svensson, L.G.; Nishimura, R.A.; Bonow, R.O.; Guyton, R.A.; Sundt, T.M., III. Surgery for Aortic Dilatation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 67, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Rylski, B.; Branchetti, E.; Bavaria, J.E.; Vallabhajosyula, P.; Szeto, W.Y.; Milewski, R.K.; Desai, N.D. Modeling of predissection aortic size in acute type A dissection: More than 90% fail to meet the guidelines for elective ascending replacement. J. Thorac. Cardiovasc. Surg. 2014, 148, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Tsai, T.T.; Isselbacher, E.M.; Oh, J.K.; O’gara, P.T.; Evangelista, A.; Fattori, R.; Meinhardt, G.; Trimarchi, S.; Bossone, E.; et al. International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > 5.5cm is not a good predictor of type A aortic dissection: Observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007, 116, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Disha, K.; Raisin, H.H.; Secknus, M.A.; Borger, M.A.; Kuntze, T. Risk of late aortic events after isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur. J. Cardiothorac. Surg. 2012, 42, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Disha, K.; Rouman, M.; Espinoza, A.; Borger, M.A.; Kuntze, T. Aortic events after isolated aortic valve replacement for bicuspid aortic valve root phenotype: Echocardiographic follow-up study. Eur. J. Cardiothorac. Surg. 2015, 48, e71–e76. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A.; Bancone, C.; Dialetto, G.; Covino, F.E.; Manduca, S.; Montibello, M.V.; De Feo, M.; Buonocore, M.; Nappi, G. The ascending aorta with bicuspid aortic valve: A phenotypic classification with potential prognostic significance. Eur. J. Cardiothorac. Surg. 2014, 46, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Rouman, M.; Disha, K.; Fey, B.; Dubslaff, G.; von Kodolitsch, Y.; Reichenspurner, H.; Borger, M.A.; Kuntze, T. Morphologic and Functional Markers of Aortopathy in Patients With Bicuspid Aortic Valve Insufficiency Versus Stenosis. Ann. Thorac. Surg. 2017, 103, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, S.A.; Martin-Doyle, W.; Shaikh, A.Y.; Pape, L.A. Predictors of ascending aortic dilation in bicuspid aortic valve disease: A five-year prospective study. Am. J. Med. 2015, 128, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Peeters, F.E.; Van der Linden, N.; Thomassen, A.L.; Crijns, H.J.; Meex, S.J.; Kietselaer, B.L. Clinical and echocardiographic determinants in bicuspid aortic dilatation: Results from a longitudinal observational study. Medicine 2016, 95, e5699. [Google Scholar] [CrossRef] [PubMed]
- Detaint, D.; Michelena, H.I.; Nkomo, V.T.; Vahanian, A.; Jondeau, G.; Sarano, M.E. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: A comparative study with Marfan syndrome and degenerative aortopathy. Heart 2014, 100, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Rouman, M.; Disha, K.; Dubslaff, G.; Fey, B.; Misfeld, M.; Mashayekhi, K.; Borger, M.A.; Kuntze, T. The fate of mild-to-moderate proximal aortic dilatation after isolated aortic valve replacement for bicuspid aortic valve stenosis: A magnetic resonance imaging follow-up study. Eur. J. Cardiothorac. Surg. 2016, 49, e80–e86. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Rouman, M.; Disha, K.; Espinoza, A.; Dubslaff, G.; Fey, B.; Theis, B.; Petersen, I.; Borger, M.A.; Kuntze, T. Aortopathy in patients with bicuspid aortic valve stenosis: Role of aortic root functional parameters. Eur. J. Cardiothorac. Surg. 2016, 49, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Geist, L.; Disha, K.; Kazakbaev, I.; Groß, T.; Schulz, S.; Ungelenk, M.; Kuntze, T.; Reichenspurner, H.; Kurth, I. Genetic abnormalities in bicuspid aortic valve root phenotype: Preliminary results. Eur. J. Cardiothorac. Surg. 2017, 52, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Rouman, M.; Disha, K.; Espinoza, A.; Misfeld, M.; Borger, M.A.; Kuntze, T. Aortic Dissection after Previous Aortic Valve Replacement for Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2015, 66, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Li, R.G.; Xu, Y.J.; Wang, J.; Liu, X.Y.; Yuan, F.; Huang, R.T.; Xue, S.; Li, L.; Liu, H.; Li, Y.J.; et al. GATA4 Loss-of-Function Mutation and the Congenitally Bicuspid Aortic Valve. Am. J. Cardiol. 2018, 121, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.K.; Qiu, X.B.; Yuan, F.; Wang, J.; Zhao, C.M.; Liu, X.Y.; Zhang, X.L.; Li, R.G.; Xu, Y.J.; Hou, X.M.; et al. A novel NKX2.5 loss-of-function mutation associated with congenital bicuspid aortic valve. Am. J. Cardiol. 2014, 114, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Di, R.M.; Qiao, Q.; Li, X.M.; Huang, R.T.; Xue, S.; Liu, X.Y.; Wang, J.; Yang, Y.Q. GATA6 loss-of-function mutation contributes to congenital bicuspid aortic valve. Gene 2018, 663, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Robinson, P.N.; von Kodolitsch, Y. Interpreting Phenotypic Features of Bicuspid Aortic Valve Disease: From Simplification to Complexity to Simplicity? Am. J. Med. 2017, 130, e315–e316. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Rouman, M.; Disha, K.; Fey, B.; Dubslaff, G.; Theis, B.; Petersen, I.; Gutberlet, M.; Borger, M.A.; Kuntze, T. Functional Aortic Root Parameters and Expression of Aortopathy in Bicuspid Versus Tricuspid Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2016, 67, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, J.S.; Ivey, C.R.; Wheeler, J.B.; Akerman, A.W.; Rice, A.; Patel, R.K.; Stroud, R.E.; Shah, A.A.; Hughes, C.G.; Ferrari, G.; et al. Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J. Thorac. Cardiovasc. Surg. 2013, 145, 1326–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, S.S.; Parks, W.C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015, 44–46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, M.; Millot, N.; Sheikhzadeh, S.; Rybczynski, M.; Gerth, S.; Kölbel, T.; Keyser, B.; Kutsche, K.; Robinson, P.N.; Berger, J.; et al. Total serum transforming growth factor-β1 is elevated in the entire spectrum of genetic aortic syndromes. Clin. Cardiol. 2014, 37, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kilickesmez, K.O.; Abaci, O.; Kocas, C.; Yildiz, A.; Kaya, A.; Okcun, B.; Kucukoglu, S. Dilatation of the ascending aorta and serum alpha 1-antitrypsin level in patients with bicuspid aortic valve. Heart Vessel. 2012, 27, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The RAGE axis: A fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 2010, 106, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Dimmeler, S. MicroRNAs and aneurysm formation. Trends Cardiovasc. Med. 2011, 21, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, B.; Dong, L.; Wang, C.; Wang, X.; Shu, X. Circulating matrix metalloproteinase patterns in association with aortic dilatation in bicuspid aortic valve patients with isolated severe aortic stenosis. Heart Vessel. 2016, 31, 189–197. [Google Scholar] [CrossRef] [PubMed]
- LeMaire, S.A.; Wang, X.; Wilks, J.A.; Carter, S.A.; Wen, S.; Won, T.; Leonardelli, D.; Anand, G.; Conklin, L.D.; Wang, X.L.; et al. Matrix metalloproteinases in ascending aortic aneurysms: Bicuspid versus trileaflet aortic valves. J. Surg. Res. 2005, 123, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Schachner, T.; Golderer, G.; Sarg, B.; Lindner, H.H.; Bonaros, N.; Mikuz, G.; Laufer, G.; Werner, E.R. The amounts of alpha 1 antitrypsin protein are reduced in the vascular wall of the acutely dissected human ascending aorta. Eur. J. Cardiothorac. Surg. 2010, 37, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Branchetti, E.; Bavaria, J.E.; Grau, J.B.; Shaw, R.E.; Poggio, P.; Lai, E.K.; Desai, N.D.; Gorman, J.H.; Gorman, R.C.; Ferrari, G. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Guo, J.; Wu, J.; Sun, Z.; Han, M.; Shan, S.W.; Deng, Z.; Yang, B.B.; Weisel, R.D.; Li, R.K. miR-17 targets tissue inhibitor of metalloproteinase 1 and 2 to modulate cardiac matrix remodeling. FASEB J. 2013, 27, 4254–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Song, H.F.; Li, S.H.; Guo, J.; Tsang, K.; Tumiati, L.; Butany, J.; Yau, T.M.; Ouzounian, M.; Fu, S.; et al. Progressive Aortic Dilation Is Regulated by miR-17-Associated miRNAs. J. Am. Coll. Cardiol. 2016, 67, 2965–2977. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Baiges, I.; Faiges, M.; Alegret, J.M. Specific circulating microRNA signature of bicuspid aortic valve disease. J. Transl. Med. 2017, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Tian, C.; Sun, X.; Qian, X.; Liu, P.; Liu, W.; Chang, Q. Overexpression of microRNA-145 promotes ascending aortic aneurysm media remodeling through TGF-b1. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Foffa, I.; Pulignani, S.; Vecoli, C.; Ait-Ali, L.; Andreassi, M.G. miRNome Profiling in Bicuspid Aortic Valve-Associated Aortopathy by Next-Generation Sequencing. Int. J. Mol. Sci. 2017, 18, E2498. [Google Scholar] [CrossRef] [PubMed]
- Maredia, A.K.; Greenway, S.C.; Verma, S.; Fedak, P.W.M. Bicuspid aortic valve-associated aortopathy: Update on biomarkers. Curr. Opin. Cardiol. 2018, 33, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Petersen, J.; Neumann, N.; Groß, T.; Naito, S.; Hillebrand, M.; Reichenspurner, H.; Blankenberg, S.; Zeller, T. Evaluation of microribonucleic acids as potential biomarkers in the bicuspid aortic valve-associated aortopathy. Interact. Cardiovasc. Thorac. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girdauskas, E.; Petersen, J.; Neumann, N.; Naito, S.; Gross, T.; Jagodzinski, A.; Reichenspurner, H.; Zeller, T. Novel Approaches for BAV Aortopathy Prediction—Is There a Need for Cohort Studies and Biomarkers? Biomolecules 2018, 8, 58. https://doi.org/10.3390/biom8030058
Girdauskas E, Petersen J, Neumann N, Naito S, Gross T, Jagodzinski A, Reichenspurner H, Zeller T. Novel Approaches for BAV Aortopathy Prediction—Is There a Need for Cohort Studies and Biomarkers? Biomolecules. 2018; 8(3):58. https://doi.org/10.3390/biom8030058
Chicago/Turabian StyleGirdauskas, Evaldas, Johannes Petersen, Niklas Neumann, Shiho Naito, Tatiana Gross, Annika Jagodzinski, Hermann Reichenspurner, and Tanja Zeller. 2018. "Novel Approaches for BAV Aortopathy Prediction—Is There a Need for Cohort Studies and Biomarkers?" Biomolecules 8, no. 3: 58. https://doi.org/10.3390/biom8030058



