Emerging Roles for VEGF-D in Human Disease
Abstract
:1. Introduction
2. Cloning, Gene Regulation and Biosynthesis of VEGF-D
3. Receptor Signaling and Biological Function
3.1. Receptor Signaling
3.2. Biological Function
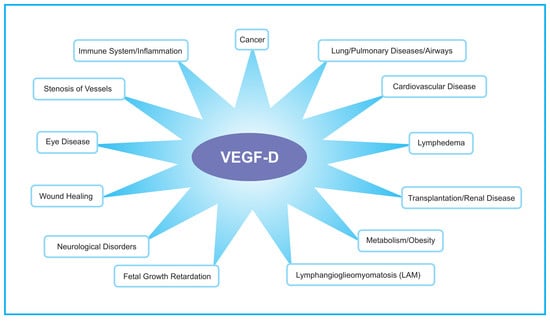
4. Disease Involvement: Humans and Model Systems
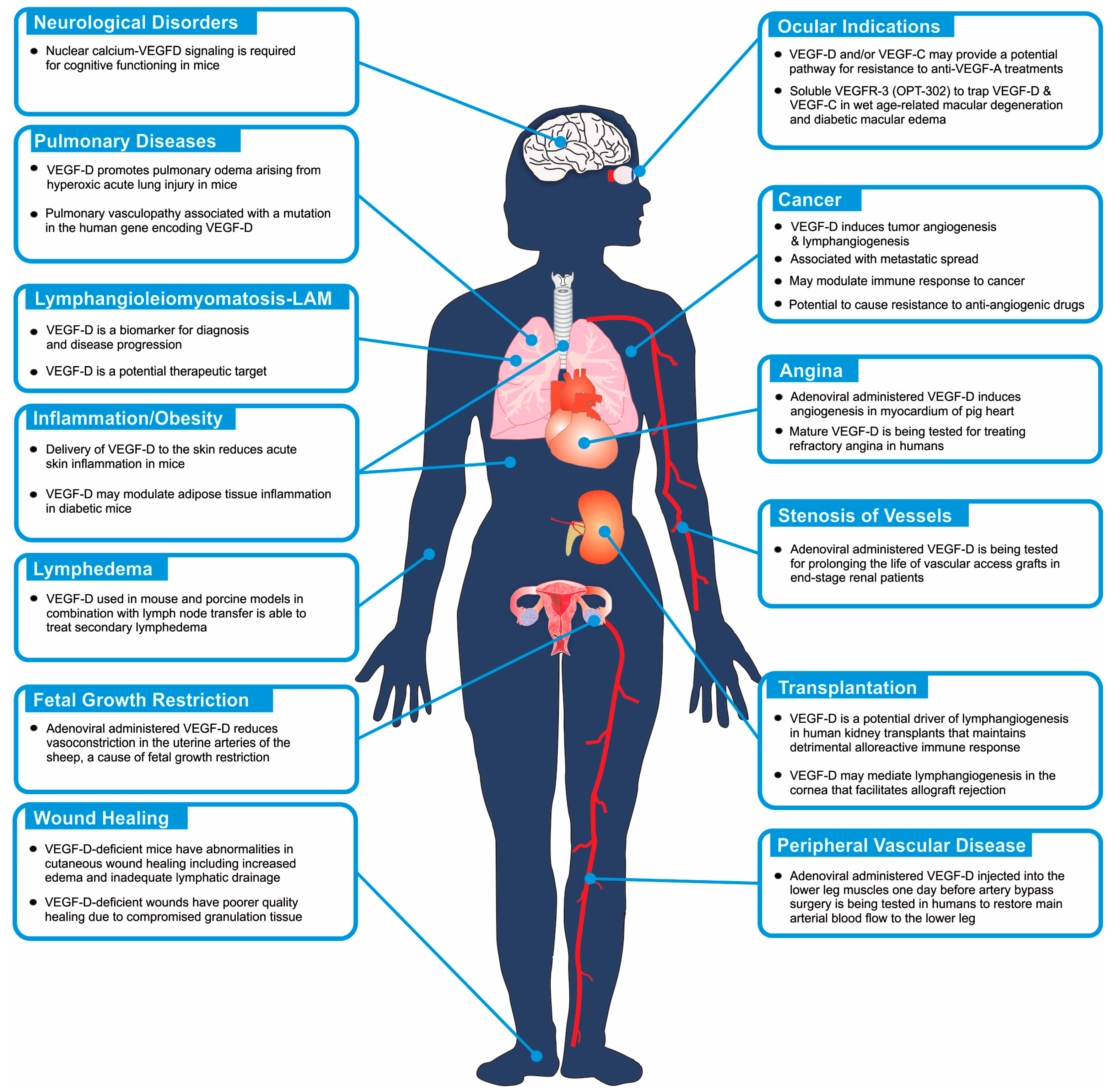
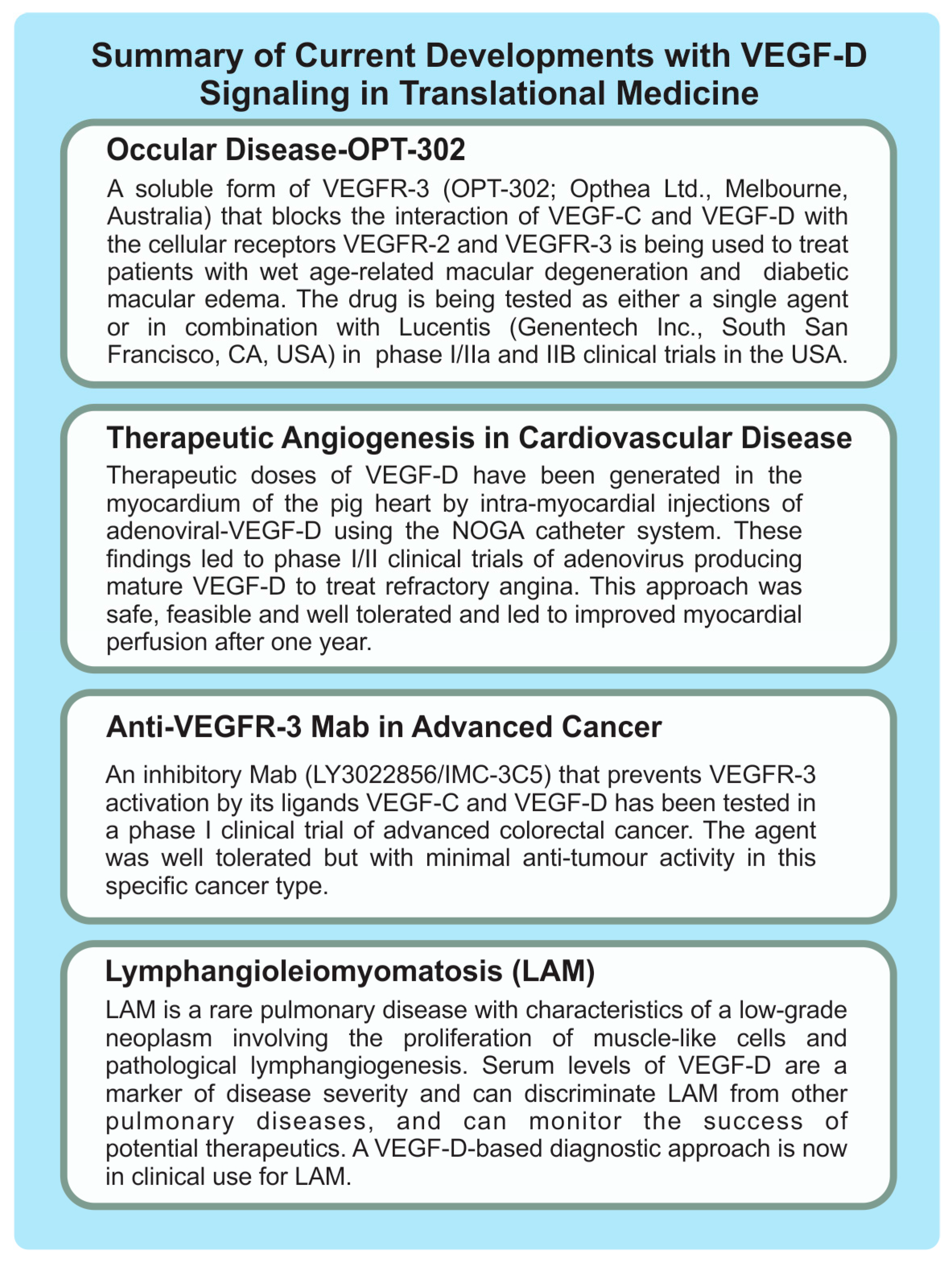
4.1. Lymphangioleiomyomatosis
4.2. Pulmonary Diseases
4.3. Cardiovascular Diseases
4.4. Ocular Indications
4.5. Cancer
4.6. Inflammation and Obesity
4.7. Lymphedema
4.8. Other Clinical Settings
4.8.1. Wound Healing
4.8.2. Transplantation
4.8.3. Neurological Disorders
5. Concluding Comments
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R.; Jackson, D.G.; Nishikawa, S.; Kubo, H.; Achen, M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001, 7, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Makinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Young, L.R.; Vandyke, R.; Gulleman, P.M.; Inoue, Y.; Brown, K.K.; Schmidt, L.S.; Linehan, W.M.; Hajjar, F.; Kinder, B.W.; Trapnell, B.C.; et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest 2010, 138, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Young, L.R.; Lee, H.S.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; et al. Serum VEGF-D concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: A prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir. Med. 2013, 1, 445–452. [Google Scholar] [CrossRef]
- Williams, S.P.; Odell, A.F.; Karnezis, T.; Farnsworth, R.H.; Gould, C.M.; Li, J.; Paquet-Fifield, S.; Harris, N.C.; Walter, A.; Gregory, J.L.; et al. Genome-wide functional analysis reveals central signaling regulators of lymphatic endothelial cell migration and remodeling. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.P.; Gould, C.M.; Nowell, C.J.; Karnezis, T.; Achen, M.G.; Simpson, K.J.; Stacker, S.A. Systematic high-content genome-wide RNAi screens of endothelial cell migration and morphology. Sci. Data 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, M.; Marconcini, L.; Ferruzzi, R.; Oliviero, S. Identification of a c-fos-induced gene that is related to the platelet-derived growth factor/vascular endothelial growth factor family. Proc. Natl. Acad. Sci. USA 1996, 93, 11675–11680. [Google Scholar] [CrossRef] [PubMed]
- Davydova, N.; Harris, N.C.; Roufail, S.; Paquet-Fifield, S.; Ishaq, M.; Streltsov, V.A.; Williams, S.P.; Karnezis, T.; Stacker, S.A.; Achen, M.G. Differential receptor binding and regulatory mechanisms for the lymphangiogenic growth factors vascular endothelial growth factor (VEGF)-C and -D. J. Biol. Chem. 2016, 291, 27265–27278. [Google Scholar] [CrossRef] [PubMed]
- McDonald, N.Q.; Hendrickson, W.A. A structural superfamily of growth factors containing a cystine knot motif. Cell 1993, 73, 421–424. [Google Scholar] [CrossRef]
- Stacker, S.A.; Stenvers, K.; Caesar, C.; Vitali, A.; Domagala, T.; Nice, E.; Roufail, S.; Simpson, R.J.; Moritz, R.; Karpanen, T.; et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 1999, 274, 32127–32136. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, V.M.; Jeltsch, M.; Anisimov, A.; Tvorogov, D.; Aho, K.; Kalkkinen, N.; Toivanen, P.; Yla-Herttuala, S.; Ballmer-Hofer, K.; Alitalo, K. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood 2011, 117, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, M.; Oliviero, S. In fibroblasts VEGF-D expression is induced by cell-cell contact mediated by cadherin-11. J. Biol. Chem. 2001, 276, 6576–6581. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Zhang, Q.; Qiu, X.; Wang, E. Interleukin 7/interleukin 7 receptor induce c-fos/c-jun-dependent vascular endothelial growth factor-D up-regulation: A mechanism of lymphangiogenesis in lung cancer. Eur. J. Cancer 2009, 45, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, M.A.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Interleukin 7 upregulates vascular endothelial growth factor D in breast cancer cells and induces lymphangiogenesis in vivo. Br. J. Surg. 2005, 92, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Osorio, J.C.; Risquez, C.; Wang, H.; Shi, Y.; Gochuico, B.R.; Morse, D.; Rosas, I.O.; El-Chemaly, S. Transforming growth factor-β1 downregulates vascular endothelial growth factor-D expression in human lung fibroblasts via the Jun NH2-terminal kinase signaling pathway. Mol. Med. 2014, 20, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Schafer, G.; Wissmann, C.; Hertel, J.; Lunyak, V.; Hocker, M. Regulation of vascular endothelial growth factor D by orphan receptors hepatocyte nuclear factor-4 α and chicken ovalbumin upstream promoter transcription factors 1 and 2. Cancer Res. 2008, 68, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, M.; Semboloni, S.; Oliviero, S. Beta-catenin inversely regulates vascular endothelial growth factor-D mRNA stability. J. Biol. Chem. 2003, 278, 44650–44656. [Google Scholar] [CrossRef] [PubMed]
- McColl, B.K.; Paavonen, K.; Karnezis, T.; Harris, N.C.; Davydova, N.; Rothacker, J.; Nice, E.C.; Harder, K.W.; Roufail, S.; Hibbs, M.L.; et al. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007, 21, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- McColl, B.K.; Baldwin, M.E.; Roufail, S.; Freeman, C.; Moritz, R.L.; Simpson, R.J.; Alitalo, K.; Stacker, S.A.; Achen, M.G. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J. Exp. Med. 2003, 198, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Fifield, S.; Roufail, S.; Zhang, Y.F.; Sofian, T.; Byrne, D.J.; Coughlin, P.B.; Fox, S.B.; Stacker, S.A.; Achen, M.G. The fibrinolysis inhibitor α2-antiplasmin restricts lymphatic remodelling and metastasis in a mouse model of cancer. Growth Factors 2017, 35, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E.; Roufail, S.; Halford, M.M.; Alitalo, K.; Stacker, S.A.; Achen, M.G. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J. Biol. Chem. 2001, 276, 44307–44314. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signaling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Zarkada, G.; Nurmi, H.; Jakobsson, L.; Heinolainen, K.; Tvorogov, D.; Zheng, W.; Franco, C.A.; Murtomaki, A.; Aranda, E.; et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signaling. Nat. Cell Biol. 2011, 13, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Zarkada, G.; Wallgard, E.; Murtomaki, A.; Suchting, S.; Wirzenius, M.; Waltari, M.; Hellstrom, M.; Schomber, T.; Peltonen, R.; et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008, 454, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Wirzenius, M.; Tammela, T.; Uutela, M.; He, Y.; Odorisio, T.; Zambruno, G.; Nagy, J.A.; Dvorak, H.F.; Yla-Herttuala, S.; Shibuya, M.; et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J. Exp. Med. 2007, 204, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Dixelius, J.; Makinen, T.; Wirzenius, M.; Karkkainen, M.J.; Wernstedt, C.; Alitalo, K.; Claesson-Welsh, L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J. Biol. Chem. 2003, 278, 40973–40979. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, T.; Shayan, R.; Caesar, C.; Roufail, S.; Harris, N.C.; Ardipradja, K.; Zhang, Y.F.; Williams, S.P.; Farnsworth, R.H.; Chai, M.G.; et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 2012, 21, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, T.; Shayan, R.; Fox, S.; Achen, M.G.; Stacker, S.A. The connection between lymphangiogenic signaling and prostaglandin biology: A missing link in the metastatic pathway. Oncotarget 2012, 3, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E.; Halford, M.M.; Roufail, S.; Williams, R.A.; Hibbs, M.L.; Grail, D.; Kubo, H.; Stacker, S.A.; Achen, M.G. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol. Cell. Biol. 2005, 25, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Dettori, D.; Van Nuffelen, A.; Souffreau, J.; Marconcini, L.; Wallays, G.; Moons, L.; Bruyere, F.; Oliviero, S.; Noel, A.; et al. VEGF-D deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis. J. Pathol. 2009, 219, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Haiko, P.; Makinen, T.; Keskitalo, S.; Taipale, J.; Karkkainen, M.J.; Baldwin, M.E.; Stacker, S.A.; Achen, M.G.; Alitalo, K. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol. Cell. Biol. 2008, 28, 4843–4850. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E.; Catimel, B.; Nice, E.C.; Roufail, S.; Hall, N.E.; Stenvers, K.L.; Karkkainen, M.J.; Alitalo, K.; Stacker, S.A.; Achen, M.G. The specificity of receptor binding by vascular endothelial growth factor-D is different in mouse and man. J. Biol. Chem. 2001, 276, 19166–19171. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.; Koltowska, K.; Pichol-Thievend, C.; Le Guen, L.; Fontaine, F.; Smith, K.A.; Truong, V.; Skoczylas, R.; Stacker, S.A.; Achen, M.G.; et al. VEGFD regulates blood vascular development by modulating SOX18 activity. Blood 2014, 123, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Ny, A.; Koch, M.; Vandevelde, W.; Schneider, M.; Fischer, C.; Diez-Juan, A.; Neven, E.; Geudens, I.; Maity, S.; Moons, L.; et al. Role of VEGF-D and VEGFR-3 in developmental lymphangiogenesis, a chemicogenetic study in Xenopus tadpoles. Blood 2008, 112, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Bower, N.I.; Vogrin, A.J.; Le Guen, L.; Chen, H.; Stacker, S.A.; Achen, M.G.; Hogan, B.M. Vegfd modulates both angiogenesis and lymphangiogenesis during zebrafish embryonic development. Development 2017, 144, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.W.; Haggerty, M.J.; Okuda, K.S.; Le Guen, L.; Misa, J.P.; Tromp, A.; Hogan, B.M.; Crosier, K.E.; Crosier, P.S. Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development 2014, 141, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Seyama, K.; Kumasaka, T.; Kurihara, M.; Mitani, K.; Sato, T. Lymphangioleiomyomatosis: A disease involving the lymphatic system. Lymphat. Res. Biol. 2010, 8, 21–31. [Google Scholar] [CrossRef] [PubMed]
- McCormack, F.X.; Travis, W.D.; Colby, T.V.; Henske, E.P.; Moss, J. Lymphangioleiomyomatosis: Calling it what it is: A low-grade, destructive, metastasizing neoplasm. Am. J. Respir. Crit. Care Med. 2012, 186, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Carsillo, T.; Astrinidis, A.; Henske, E.P. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2000, 97, 6085–6090. [Google Scholar] [CrossRef] [PubMed]
- Seyama, K.; Kumasaka, T.; Souma, S.; Sato, T.; Kurihara, M.; Mitani, K.; Tominaga, S.; Fukuchi, Y. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat. Res. Biol. 2006, 4, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kumasaka, T.; Seyama, K.; Mitani, K.; Sato, T.; Souma, S.; Kondo, T.; Hayashi, S.; Minami, M.; Uekusa, T.; Fukuchi, Y.; et al. Lymphangiogenesis in lymphangioleiomyomatosis: Its implication in the progression of lymphangioleiomyomatosis. Am. J. Surg. Pathol. 2004, 28, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Kumasaka, T.; Seyama, K.; Mitani, K.; Souma, S.; Kashiwagi, S.; Hebisawa, A.; Sato, T.; Kubo, H.; Gomi, K.; Shibuya, K.; et al. Lymphangiogenesis-mediated shedding of lam cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am. J. Surg. Pathol. 2005, 29, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Mitani, K.; Kumasaka, T.; Takemura, H.; Hayashi, T.; Gunji, Y.; Kunogi, M.; Akiyoshi, T.; Takahashi, K.; Suda, K.; Seyama, K. Cytologic, immunocytochemical and ultrastructural characterization of lymphangioleiomyomatosis cell clusters in chylous effusions of patients with lymphangioleiomyomatosis. Acta Cytol. 2009, 53, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, C.G.; Avila, N.A.; Lin, J.P.; Stylianou, M.P.; Moss, J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest 2009, 135, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- McCormack, F.X.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; Stocks, J.M.; et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011, 364, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- McCormack, F.X.; Gupta, N.; Finlay, G.R.; Young, L.R.; Taveira-DaSilva, A.M.; Glasgow, C.G.; Steagall, W.K.; Johnson, S.R.; Sahn, S.A.; Ryu, J.H.; et al. Official american thoracic society/Japanese respiratory society clinical practice guidelines: Lymphangioleiomyomatosis diagnosis and management. Am. J. Respir. Crit. Care Med. 2016, 194, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Dibble, C.C.; Cantley, L.C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015, 25, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Taveira-DaSilva, A.M.; Jones, A.M.; Julien-Williams, P.; Stylianou, M.; Moss, J. Long-term effect of sirolimus on serum vascular endothelial growth factor D levels in patients with lymphangioleiomyomatosis. Chest 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Parkhitko, A.A.; Henske, E.P. Mammalian target of rapamycin signaling and autophagy: Roles in lymphangioleiomyomatosis therapy. Proc. Am. Thorac. Soc. 2010, 7, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.; Cui, Y.; Casey, A.; Stoler, J.M.; Ai, X.; Ma, D.; Handin, R.; Sliz, P.; Vargas, S.O.; El-Chemaly, S.Y. Pulmonary vasculopathy associated with FIGF gene mutation. Am. J. Pathol. 2017, 187, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Paquet-Fifield, S.; Harris, N.C.; Roufail, S.; Turner, D.J.; Yuan, Y.; Zhang, Y.F.; Fox, S.B.; Hibbs, M.L.; Wilkinson-Berka, J.L.; et al. VEGF-D promotes pulmonary oedema in hyperoxic acute lung injury. J. Pathol. 2016, 239, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Rutanen, J.; Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Silvennoinen, P.; Kivela, A.; Hedman, A.; Hedman, M.; Heikura, T.; Orden, M.R.; et al. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation 2004, 109, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, J.; Hassinen, I.; Hedman, A.; Kivela, A.; Saraste, A.; Knuuti, J.; Husso, M.; Mussalo, H.; Hedman, M.; Rissanen, T.T.; et al. Adenoviral intramyocardial VEGF-D deltandeltac gene transfer increases myocardial perfusion reserve in refractory angina patients: A phase I/IIa study with 1-year follow-up. Eur. Heart J. 2017, 38, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Rutanen, J.; Turunen, A.M.; Teittinen, M.; Rissanen, T.T.; Heikura, T.; Koponen, J.K.; Gruchala, M.; Inkala, M.; Jauhiainen, S.; Hiltunen, M.O.; et al. Gene transfer using the mature form of VEGF-D reduces neointimal thickening through nitric oxide-dependent mechanism. Gene Ther. 2005, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Abi-Nader, K.N.; Shangaris, P.; Shaw, S.W.; Filippi, E.; Benjamin, E.; Boyd, M.; Peebles, D.M.; Martin, J.; Zachary, I.; et al. Local over-expression of VEGF-D∆N∆C in the uterine arteries of pregnant sheep results in long-term changes in uterine artery contractility and angiogenesis. PLoS ONE 2014, 9, e100021. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Heikura, T.; Puranen, A.; Kettunen, M.I.; Kholova, I.; Kauppinen, R.A.; Achen, M.G.; Stacker, S.A.; et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ. Res. 2003, 92, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Heikura, T.; Nieminen, T.; Roschier, M.M.; Karvinen, H.; Kaikkonen, M.U.; Mahonen, A.J.; Lesch, H.P.; Rissanen, T.T.; Laitinen, O.H.; Airenne, K.J.; et al. Baculovirus-mediated vascular endothelial growth factor-D(∆N∆C) gene transfer induces angiogenesis in rabbit skeletal muscle. J. Gene Med. 2012, 14, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.M.; Ueda, N.; Dryden, N.H.; Fleming, S.B.; Caesar, C.; Roufail, S.; Achen, M.G.; Stacker, S.A.; Mercer, A.A. Viral vascular endothelial growth factors vary extensively in amino acid sequence, receptor-binding specificities, and the ability to induce vascular permeability yet are uniformly active mitogens. J. Biol. Chem. 2003, 278, 38004–38014. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Le Couter, J.; Strauss, E.C.; Ferrara, N. Vascular endothelial growth factor A in intraocular vascular disease. Ophthalmology 2013, 120, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Ciulla, T.A. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin. Emerg. Drugs 2017, 22, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996, 15, 290–298. [Google Scholar] [PubMed]
- Joukov, V.; Kumar, V.; Sorsa, T.; Arighi, E.; Weich, H.; Saksela, O.; Alitalo, K. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J. Biol. Chem. 1998, 273, 6599–6602. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E. Opthea Presents Additional Positive Data from OPT-302 Phase 1/2a Wet AMD Trial at American Society of Retina Specialists 35th Annual Meeting. 2017. Available online: http://www.opthea.com/pub/OPT_OIS_ASRS%20CNV%20SHRM_Final.pdf (accessed on 15 August 2017).
- Yonemura, Y.; Endo, Y.; Tabata, K.; Kawamura, T.; Yun, H.Y.; Bandou, E.; Sasaki, T.; Miura, M. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int. J. Clin. Oncol. Jpn. Soc. Clin. Oncol. 2005, 10, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Thelen, A.; Scholz, A.; Benckert, C.; von Marschall, Z.; Schroder, M.; Wiedenmann, B.; Neuhaus, P.; Rosewicz, S.; Jonas, S. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int. J. Cancer 2008, 122, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Von Marschall, Z.; Scholz, A.; Stacker, S.A.; Achen, M.G.; Jackson, D.G.; Alves, F.; Schirner, M.; Haberey, M.; Thierauch, K.H.; Wiedenmann, B.; et al. Vascular endothelial growth factor-D induces lymphangiogenesis and lymphatic metastasis in models of ductal pancreatic cancer. Int. J. Oncol. 2005, 27, 669–679. [Google Scholar] [PubMed]
- Kopfstein, L.; Veikkola, T.; Djonov, V.G.; Baeriswyl, V.; Schomber, T.; Strittmatter, K.; Stacker, S.A.; Achen, M.G.; Alitalo, K.; Christofori, G. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am. J. Pathol. 2007, 170, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; Stacker, S.A. Vascular endothelial growth factor-D: Signalling mechanisms, biology and clinical relevance. Growth Factors 2012, 30, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomized controlled trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Holmes, M.D.; Chen, W.Y.; Li, L.; Hertzmark, E.; Spiegelman, D.; Hankinson, S.E. Aspirin intake and survival after breast cancer. J. Clin. Oncol. 2010, 28, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Fowkes, F.G.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomized trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomized trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomized trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Peng, F.; Zhong, Y.; Liu, Y.; Zhang, Y.; Xie, Y.; Lu, Y.; Zhang, X.; Li, D. SPARC suppresses lymph node metastasis by regulating the expression of VEGFs in ovarian carcinoma. Int. J. Oncol. 2017, 51, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Schoppmann, S.F.; Birner, P.; Stockl, J.; Kalt, R.; Ullrich, R.; Caucig, C.; Kriehuber, E.; Nagy, K.; Alitalo, K.; Kerjaschki, D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002, 161, 947–956. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, R.A.; Achen, M.G. Lymphangiogenic growth factors as markers of tumor metastasis. APMIS 2004, 112, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; McColl, B.K.; Stacker, S.A. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell 2005, 7, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Charnock-Jones, D.S.; Licence, D.; Yanaihara, A.; Hastings, J.M.; Holland, C.M.; Emoto, M.; Umemoto, M.; Sakamoto, T.; Sato, S.; et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br. J. Cancer 2003, 88, 237–244. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Hewett, P.W.; Kosuge, D.; McCulloch, T.; Enholm, B.C.; Carmichael, J.; Murray, J.C. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002, 62, 1669–1675. [Google Scholar] [PubMed]
- Juttner, S.; Wissmann, C.; Jons, T.; Vieth, M.; Hertel, J.; Gretschel, S.; Schlag, P.M.; Kemmner, W.; Hocker, M. Vascular endothelial growth factor-D and its receptor VEGFR-3: Two novel independent prognostic markers in gastric adenocarcinoma. J. Clin. Oncol. 2006, 24, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Weickhardt, A.J.; Williams, D.S.; Lee, C.K.; Chionh, F.; Simes, J.; Murone, C.; Wilson, K.; Parry, M.M.; Asadi, K.; Scott, A.M.; et al. Vascular endothelial growth factor D expression is a potential biomarker of bevacizumab benefit in colorectal cancer. Br. J. Cancer 2015, 113, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.W.; Duraes, F.V.; Hirosue, S.; Raghavan, V.R.; Nembrini, C.; Thomas, S.N.; Issa, A.; Hugues, S.; Swartz, M.A. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012, 1, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, M.; Broggi, M.A.S.; Potin, L.; Bordry, N.; Jeanbart, L.; Lund, A.W.; Da Costa, E.; Hauert, S.; Rincon-Restrepo, M.; Tremblay, C.; et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.A.; Farnsworth, R.H.; Solomon, B.; Achen, M.G.; Stacker, S.A.; Fox, S.B. The role of the tumor vasculature in the host immune response: Implications for therapeutic strategies targeting the tumor microenvironment. Front. Immunol. 2016, 7, 621. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; Roufail, S.; Domagala, T.; Catimel, B.; Nice, E.C.; Geleick, D.M.; Murphy, R.; Scott, A.M.; Caesar, C.; Makinen, T.; et al. Monoclonal antibodies to vascular endothelial growth factor-D block interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem. 2000, 267, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Davydova, N.; Roufail, S.; Streltsov, V.A.; Stacker, S.A.; Achen, M.G. The VD1 neutralizing antibody to vascular endothelial growth factor-D: Binding epitope and relationship to receptor binding. J. Mol. Biol. 2011, 407, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Persaud, K.; Tille, J.C.; Liu, M.; Zhu, Z.; Jimenez, X.; Pereira, D.S.; Miao, H.Q.; Brennan, L.A.; Witte, L.; Pepper, M.S.; et al. Involvement of the VEGF receptor 3 in tubular morphogenesis demonstrated with a human anti-human VEGFR-3 monoclonal antibody that antagonizes receptor activation by VEGF-C. J. Cell Sci. 2004, 117, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Pytowski, B.; Goldman, J.; Persaud, K.; Wu, Y.; Witte, L.; Hicklin, D.J.; Skobe, M.; Boardman, K.C.; Swartz, M.A. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J. Natl. Cancer Inst. 2005, 97, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, P.; Waltari, M.; Holopainen, T.; Takahashi, T.; Pytowski, B.; Steiner, P.; Hicklin, D.; Persaud, K.; Tonra, J.R.; Witte, L.; et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007, 67, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kozaki, K.; Karpanen, T.; Koshikawa, K.; Yla-Herttuala, S.; Takahashi, T.; Alitalo, K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 2002, 94, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.; Egeblad, M.; Karkkainen, M.J.; Kubo, H.; Yla-Herttuala, S.; Jaattela, M.; Alitalo, K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001, 61, 1786–1790. [Google Scholar] [PubMed]
- He, Y.; Rajantie, I.; Pajusola, K.; Jeltsch, M.; Holopainen, T.; Yla-Herttuala, S.; Harding, T.; Jooss, K.; Takahashi, T.; Alitalo, K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005, 65, 4739–4746. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lalani, A.S.; Harding, T.C.; Gonzalez, M.; Wu, W.W.; Luan, B.; Tu, G.H.; Koprivnikar, K.; VanRoey, M.J.; He, Y.; et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005, 65, 6901–6909. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Knost, J.A.; Chiorean, E.G.; Kambhampati, S.R.; Yu, D.; Pytowski, B.; Qin, A.; Kauh, J.S.; O’Neil, B.H. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother. Pharmacol. 2016, 78, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.C.; Paavonen, K.; Davydova, N.; Roufail, S.; Sato, T.; Zhang, Y.F.; Karnezis, T.; Stacker, S.A.; Achen, M.G. Proteolytic processing of vascular endothelial growth factor-D is essential for its capacity to promote the growth and spread of cancer. FASEB J. 2011, 25, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.C.; Davydova, N.; Roufail, S.; Paquet-Fifield, S.; Paavonen, K.; Karnezis, T.; Zhang, Y.F.; Sato, T.; Rothacker, J.; Nice, E.C.; et al. The propeptides of VEGF-D determine heparin binding, receptor heterodimerization, and effects on tumor biology. J. Biol. Chem. 2013, 288, 8176–8186. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Siddiqui, S.S.; Brander, D.; Ullmann, S.; Zimmermann, K.; Antsiferova, M.; Werner, S.; Alitalo, K.; Detmar, M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 2011, 117, 4667–4678. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Tammela, T.; Ator, E.; Lyubynska, N.; Achen, M.G.; Hicklin, D.J.; Jeltsch, M.; Petrova, T.V.; Pytowski, B.; Stacker, S.A.; et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Investig. 2005, 115, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Hollmen, M.; Robciuc, M.R.; Alitalo, A.; Nurmi, H.; Morf, B.; Buschle, D.; Alkan, H.F.; Ochsenbein, A.M.; Alitalo, K.; et al. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol. Metab. 2015, 4, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.E.; Stacker, S.A.; Achen, M.G. Molecular control of lymphangiogenesis. Bioessays 2002, 24, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Debrah, L.B.; Albers, A.; Debrah, A.Y.; Brockschmidt, F.F.; Becker, T.; Herold, C.; Hofmann, A.; Osei-Mensah, J.; Mubarik, Y.; Frohlich, H.; et al. Single nucleotide polymorphisms in the angiogenic and lymphangiogenic pathways are associated with lymphedema caused by Wuchereria bancrofti. Hum. Genom. 2017, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Saaristo, A.; Holopainen, T.; Lyytikka, J.; Kotronen, A.; Pitkonen, M.; Abo-Ramadan, U.; Yla-Herttuala, S.; Petrova, T.V.; Alitalo, K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 2007, 13, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Lahteenvuo, M.; Honkonen, K.; Tervala, T.; Tammela, T.; Suominen, E.; Lahteenvuo, J.; Kholova, I.; Alitalo, K.; Yla-Herttuala, S.; Saaristo, A. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation 2011, 123, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Fifield, S.; Levy, S.M.; Sato, T.; Shayan, R.; Karnezis, T.; Davydova, N.; Nowell, C.J.; Roufail, S.; Ma, G.Z.; Zhang, Y.F.; et al. Vascular endothelial growth factor-D modulates caliber and function of initial lymphatics in the dermis. J. Investig. Dermatol. 2013, 133, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D.; Regele, H.M.; Moosberger, I.; Nagy-Bojarski, K.; Watschinger, B.; Soleiman, A.; Birner, P.; Krieger, S.; Hovorka, A.; Silberhumer, G.; et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 2004, 15, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D. Lymphatic neoangiogenesis in renal transplants: A driving force of chronic rejection? J. Nephrol. 2006, 19, 403–406. [Google Scholar] [PubMed]
- Mauceri, D.; Freitag, H.E.; Oliveira, A.M.; Bengtson, C.P.; Bading, H. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron 2011, 71, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Mauceri, D.; Hagenston, A.M.; Schramm, K.; Weiss, U.; Bading, H. Nuclear calcium buffering capacity shapes neuronal architecture. J. Biol. Chem. 2015, 290, 23039–23049. [Google Scholar] [CrossRef] [PubMed]
- Hemstedt, T.J.; Bengtson, C.P.; Ramirez, O.; Oliveira, A.M.M.; Bading, H. Reciprocal interaction of dendrite geometry and nuclear calcium-VEGFD signaling gates memory consolidation and extinction. J. Neurosci. 2017, 37, 6946–6955. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stacker, S.A.; Achen, M.G. Emerging Roles for VEGF-D in Human Disease. Biomolecules 2018, 8, 1. https://doi.org/10.3390/biom8010001
Stacker SA, Achen MG. Emerging Roles for VEGF-D in Human Disease. Biomolecules. 2018; 8(1):1. https://doi.org/10.3390/biom8010001
Chicago/Turabian StyleStacker, Steven A., and Marc G. Achen. 2018. "Emerging Roles for VEGF-D in Human Disease" Biomolecules 8, no. 1: 1. https://doi.org/10.3390/biom8010001