Revealing Accessibility of Cryptic Protein Binding Sites within the Functional Collagen Fibril
Abstract
:1. Introduction
2. The Complex Collagen Architecture
3. Collagen Interactions and the Fibril Surface
3.1. Identification of Binding Sequences
3.2. Fibril Surface Identity
4. Computational Studies of the Collagen Fibril
4.1. Building the Atomic Model of the Collagen Fibril
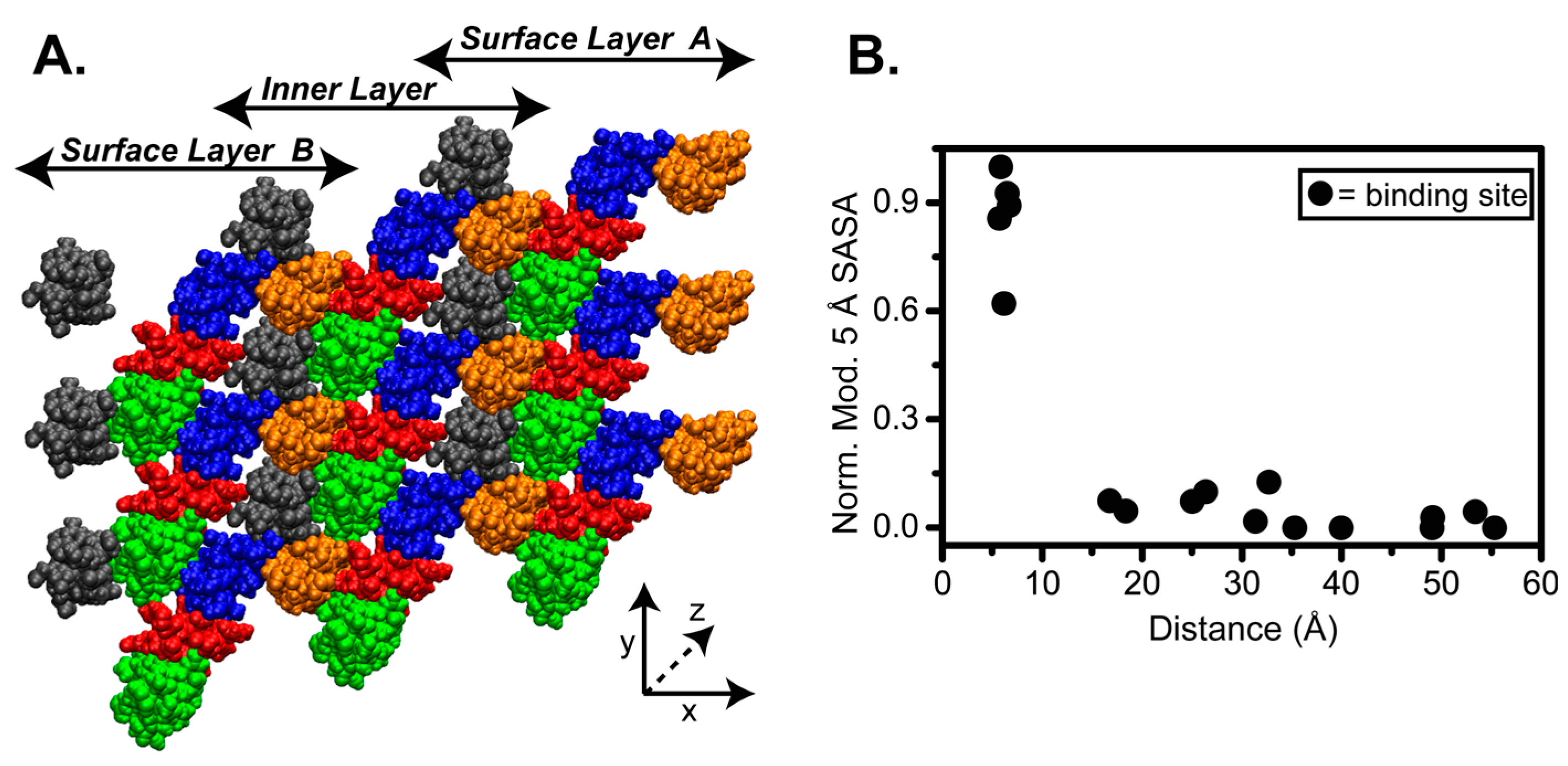
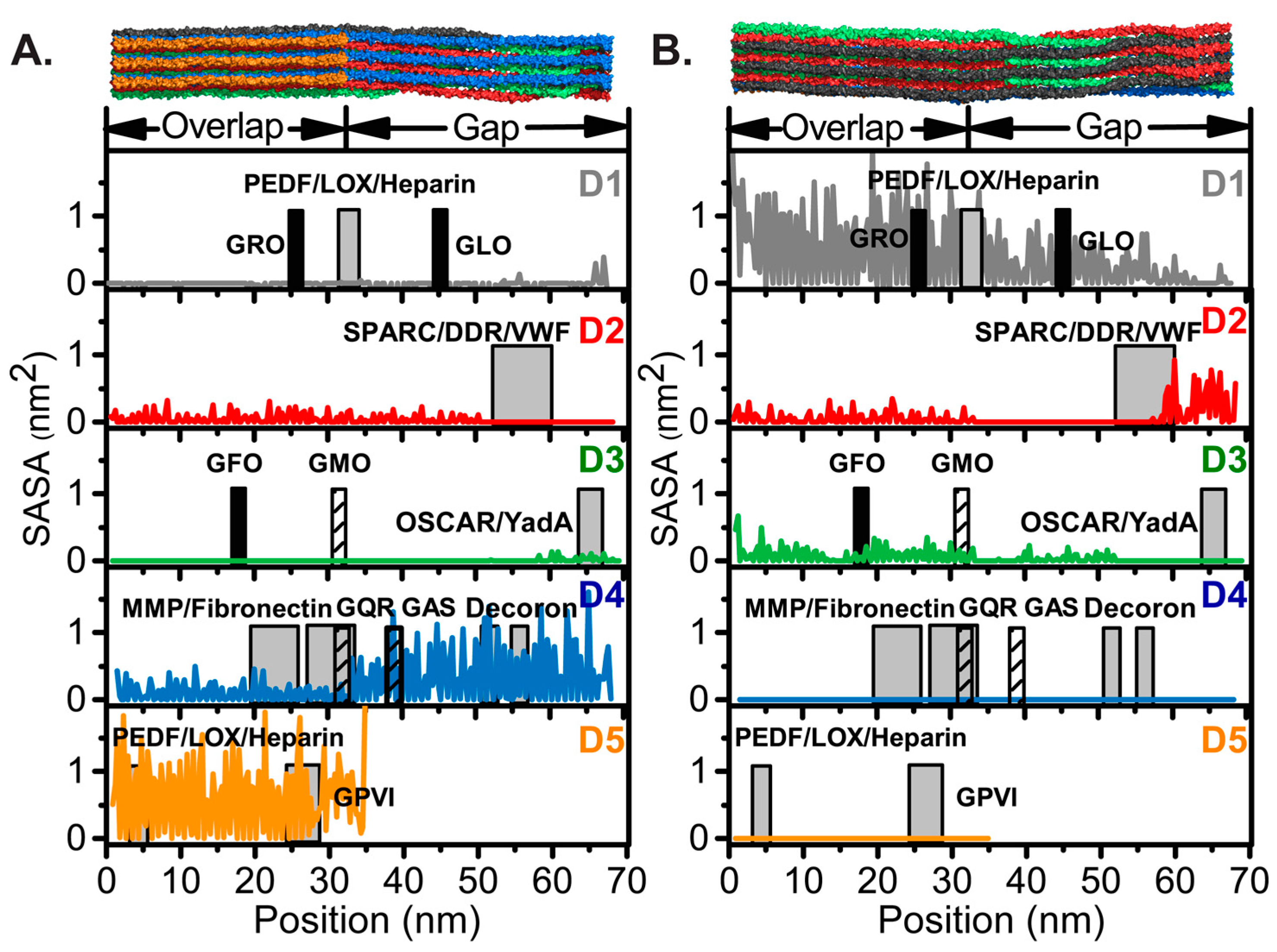
4.2. SASA of Collagen I Fibril Reveals Exposure of Partner Binding Sites from the Fibril Surface
5. Perspective
5.1. The Collagen Architecture as a “Smart Fibril”: Regulating Accessibility of Partner Binding Sites
5.2. Regulation of Protein Binding through Internal Dynamics of the Collagen Fibril
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Heino, J. The collagen family members as cell adhesion proteins. Bioessays 2007, 29, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.M.; Orgel, J.P.; Fertala, A.; McAuliffe, J.D.; Turner, K.R.; Di Lullo, G.A.; Chen, S.; Antipova, O.; Perumal, S.; Ala-Kokko, L.; et al. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J. Biol. Chem. 2008, 283, 21187–21197. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J. Structure of the integrin α2β1-binding collagen peptide. J. Mol. Biol. 2004, 335, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, H.M. Crystal and molecular structure of a collagen-like peptide at 1.9 Å resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Berisio, R.; Vitagliano, L.; Mazzarella, L.; Zagari, A. Crystal structure of a collagen-like polypeptide with repeating sequence Pro-Hyp-Gly at 1.4 Å resolution: Implications for collagen hydration. Biopolymers 2000, 56, 8–13. [Google Scholar] [CrossRef]
- Fallas, J.A.; Gauba, V.; Hartgerink, J.D. Solution structure of an ABC collagen heterotrimer reveals a single-register helix stabilized by electrostatic interactions. J. Biol. Chem. 2009, 284, 26851–26859. [Google Scholar] [CrossRef] [PubMed]
- Berisio, R.; Vitagliano, L.; Mazzarella, L.; Zagari, A. Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)(10)](3). Protein Sci. 2002, 11, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.Z.; Bella, J.; Brodsky, B.; Berman, H.M. The crystal and molecular structure of a collagen-like peptide with a biologically relevant sequence. J. Mol. Biol. 2001, 311, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.Z.; Bella, J.; Mayville, P.; Brodsky, B.; Berman, H.M. Sequence dependent conformational variations of collagen triple-helical structure. Nat. Struct. Biol. 1999, 6, 454–457. [Google Scholar] [PubMed]
- Kramer, R.Z.; Venugopal, M.G.; Bella, J.; Mayville, P.; Brodsky, B.; Berman, H.M. Staggered molecular packing in crystals of a collagen-like peptide with a single charged pair. J. Mol. Biol. 2000, 301, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.Z.; Vitagliano, L.; Bella, J.; Berisio, R.; Mazzarella, L.; Brodsky, B.; Zagari, A.; Berman, H.M. X-ray crystallographic determination of a collagen-like peptide with the repeating sequence (Pro-Pro-Gly). J. Mol. Biol. 1998, 280, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Madhan, B.; Li, Y.; Brodsky, B.; Baum, J. Osteogenesis imperfecta model peptides: Incorporation of residues replacing Gly within a triple helix achieved by renucleation and local flexibility. Biophys. J. 2011, 101, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cheng, H.; Silva, T.; Baum, J.; Brodsky, B.M. Osteogenesis imperfecta missense mutations in collagen: Structural consequences of a glycine to alanine replacement at a highly charged site. Biochemistry 2011, 50, 10771–10780. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Brodsky, B.; Baum, J. NMR conformational and dynamic consequences of a Gly to ser substitution in an osteogenesis imperfecta collagen model peptide. J. Biol. Chem. 2009, 284, 20660–20667. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Addabbo, R.M.; Lauer, J.L.; Fields, G.B.; Baum, J. Local conformation and dynamics of isoleucine in the collagenase cleavage site provide a recognition signal for matrix metalloproteinases. J. Biol. Chem. 2010, 285, 34181–34190. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Baum, J. Structural insights from 15N relaxation data for an anisotropic collagen peptide. J. Am. Chem. Soc. 2009, 131, 18194–18195. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, G.; Li, Y.; Mohs, A.; Strafaci, C.; Popiel, M.; Baum, J.; Brodsky, B. Common interruptions in the repeating tripeptide sequence of non-fibrillar collagens: Sequence analysis and structural studies on triple-helix peptide models. J. Mol. Biol. 2008, 376, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Xiao, J.; Thiagarajan, G.; Baum, J.; Brodsky, B. NMR monitoring of chain-specific stability in heterotrimeric collagen peptides. J. Am. Chem. Soc. 2008, 130, 13520–13521. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Brodsky, B.; Baum, J. NMR shows hydrophobic interactions replace glycine packing in the triple helix at a natural break in the (Gly-X-Y)n repeat. J. Biol. Chem. 2007, 282, 22699–22706. [Google Scholar] [CrossRef] [PubMed]
- Mohs, A.; Popiel, M.; Li, Y.; Baum, J.; Brodsky, B. Conformational features of a natural break in the type IV collagen Gly-X-Y repeat. J. Biol. Chem. 2006, 281, 17197–17202. [Google Scholar] [CrossRef] [PubMed]
- Hyde, T.J.; Bryan, M.A.; Brodsky, B.; Baum, J. Sequence dependence of renucleation after a Gly mutation in model collagen peptides. J. Biol. Chem. 2006, 281, 36937–36943. [Google Scholar] [CrossRef] [PubMed]
- Buevich, A.V.; Silva, T.; Brodsky, B.; Baum, J. Transformation of the mechanism of triple-helix peptide folding in the absence of a C-terminal nucleation domain and its implications for mutations in collagen disorders. J. Biol. Chem. 2004, 279, 46890–46895. [Google Scholar] [CrossRef] [PubMed]
- Bhate, M.; Wang, X.; Baum, J.; Brodsky, B. Folding and conformational consequences of glycine to alanine replacements at different positions in a collagen model peptide. Biochemistry 2002, 41, 6539–6547. [Google Scholar] [CrossRef] [PubMed]
- Buevich, A.V.; Baum, J. Residue-specific real-time NMR diffusion experiments define the association states of proteins during folding. J. Am. Chem. Soc. 2002, 124, 7156–7162. [Google Scholar] [CrossRef] [PubMed]
- Buevich, A.; Baum, J. Nuclear magnetic resonance characterization of peptide models of collagen-folding diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Buevich, A.V.; Shinde, U.P.; Inouye, M.; Baum, J. Backbone dynamics of the natively unfolded pro-peptide of subtilisin by heteronuclear NMR relaxation studies. J. Biomol. NMR 2001, 20, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, M.H.; Brodsky, B.; Baum, J. Backbone dynamics of (pro-hyp-gly)10 and a designed collagen-like triple-helical peptide by 15N-NMR relaxation and hydrogen-exchange measurements. Biochemistry 1993, 32, 13299–13309. [Google Scholar] [CrossRef] [PubMed]
- Long, C.G.; Braswell, E.; Zhu, D.; Apigo, J.; Baum, J.; Brodsky, B. Characterization of collagen-like peptides containing interruptions in the repeating Gly-X-Y sequence. Biochemistry 1993, 32, 11688–11695. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Arnott, S.; Takayanagi, M.; Kakudo, M. Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly)10. J. Mol. Biol. 1981, 152, 427–443. [Google Scholar] [CrossRef]
- Nagarajan, V.; Kamitori, S.; Okuyama, K. Structure analysis of a collagen-model peptide with a (Pro-Hyp-Gly) sequence repeat. J. Biochem. 1999, 125, 310–318. [Google Scholar] [CrossRef] [PubMed]
- O'Leary, L.E.; Fallas, J.A.; Hartgerink, J.D. Positive and negative design leads to compositional control in AAB collagen heterotrimers. J. Am. Chem. Soc. 2011, 133, 5432–5443. [Google Scholar] [CrossRef] [PubMed]
- Gauba, V.; Hartgerink, J.D. Synthetic collagen heterotrimers: Structural mimics of wild-type and mutant collagen type I. J. Am. Chem. Soc. 2008, 130, 7509–7515. [Google Scholar] [CrossRef] [PubMed]
- Gauba, V.; Hartgerink, J.D. Surprisingly high stability of collagen ABC heterotrimer: Evaluation of side chain charge pairs. J. Am. Chem. Soc. 2007, 129, 15034–15041. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N.; Kartha, G. Structure of collagen. Nature 1955, 176, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N. Structure of Collagen at the Molecular Level. Treatise on Collagen; Ramachandran, G.N., Ed.; Academic Press, Inc.: New York, NY, USA, 1967; Volume 1, Chapter 3. [Google Scholar]
- Rich, A.; Crick, F.H. The molecular structure of collagen. J. Mol. Biol. 1961, 3, 483. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F.H. The structure of collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Colige, A.; Vandenberghe, I.; Thiry, M.; Lambert, C.A.; Van Beeumen, J.; Li, S.W.; Prockop, D.J.; Lapiere, C.M.; Nusgens, B.V. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J. Biol. Chem. 2002, 277, 5756–5766. [Google Scholar] [CrossRef] [PubMed]
- Vadon-Le Goff, S.; Hulmes, D.J.; Moali, C. BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biol. 2015, 44–46, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Vadon-Le Goff, S.; Kronenberg, D.; Bourhis, J.M.; Bijakowski, C.; Raynal, N.; Ruggiero, F.; Farndale, R.W.; Stocker, W.; Hulmes, D.J.; Moali, C. Procollagen C-proteinase enhancer stimulates procollagen processing by binding to the C-propeptide region only. J. Biol. Chem. 2011, 286, 38932–38938. [Google Scholar] [CrossRef] [PubMed]
- Wess, T.J.; Orgel, J.P. Changes in collagen structure: Drying, dehydrothermal treatment and relation to long term deterioration. Thermochim. Acta 2000, 365, 119–128. [Google Scholar] [CrossRef]
- Petruska, J.A.; Hodge, A.J. A subunit model for the tropocollagen macromolecule. Proc. Natl. Acad. Sci. USA 1964, 51, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.C. Biosynthesis of collagen crosslinks: Increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc. Natl. Acad. Sci. USA 1974, 71, 4826–4830. [Google Scholar] [CrossRef] [PubMed]
- Hulmes, D.J.S.; Miller, A. Quasi-hexagonal molecular packing in collagen fibrils. Nature 1979, 282, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.D.; MacRae, T.P.; Miller, A.; Suzuki, E. Molecular conformation and packing in collagen fibrils. J. Mol. Biol. 1983, 167, 497–521. [Google Scholar] [CrossRef]
- Fraser, R.D.; MacRae, T.P.; Miller, A. Molecular packing in type I collagen fibrils. J. Mol. Biol. 1987, 193, 115–125. [Google Scholar] [CrossRef]
- Wess, T.J.; Hammersley, A.; Wess, L.; Miller, A. Type I collagen packing, conformation of the triclinic unit cell. J. Mol. Biol. 1995, 248, 487–493. [Google Scholar] [CrossRef]
- Wess, T.J.; Hammersley, A.P.; Wess, L.; Miller, A. Molecular packing of type I collagen in tendon. J. Mol. Biol. 1998, 275, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.; Miller, A.; Irving, T.C.; Fischetti, R.F.; Hammersley, A.P.; Wess, T.J. The in situ supermolecular structure of type I collagen. Structure 2001, 9, 1061–1069. [Google Scholar] [CrossRef]
- Orgel, J.P.; Irving, T.C.; Miller, A.; Wess, T.J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.; Antipova, O.; Sagi, I.; Bitler, A.; Qiu, D.; Wang, R.; Xu, Y.; San Antonio, J.D. Collagen fibril surface displays a constellation of sites capable of promoting fibril assembly, stability, and hemostasis. Connect. Tissue Res. 2011, 52, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Siljander, P.R.; Onley, D.J.; Sundaresan, P.; Knight, C.G.; Barnes, M.J. Collagen–platelet interactions: Recognition and signalling. Biochem. Soc. Symp. 2003, 70, 81–94. [Google Scholar] [CrossRef]
- An, B.; Lin, Y.-S.; Brodsky, B. Collagen interactions: Drug design and delivery. Adv. Drug Deliv. Rev. 2015, 97, 69. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, G.A.; Sweeney, S.M.; Korkko, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Herr, A.B.; Farndale, R.W. Structural insights into the interactions between platelet receptors and fibrillar collagen. J. Biol. Chem. 2009, 284, 19781–19785. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.M.; Lu, Y.; Canty, E.G.; Kadler, K.E. Active negative control of collagen fibrillogenesis in vivo. Intracellular cleavage of the type I procollagen propeptides in tendon fibroblasts without intracellular fibrils. J. Biol. Chem. 2008, 283, 12129–12135. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.G.; Morton, L.F.; Onley, D.J.; Peachey, A.R.; Messent, A.J.; Smethurst, P.A.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. Identification in collagen type I of an integrin α2β1-binding site containing an essential ger sequence. J. Biol. Chem. 1998, 273, 33287–33294. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The collagen-binding A-domains of integrins α1β1 and α2β1 recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Lisman, T.; Bihan, D.; Hamaia, S.; Smerling, C.S.; Pugh, N.; Konitsiotis, A.; Leitinger, B.; de Groot, P.G.; Jarvis, G.E.; et al. Cell-collagen interactions: The use of peptide toolkits to investigate collagen–receptor interactions. Biochem. Soc. Trans. 2008, 36, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Nishikawa, Y.; Asada, S.; Yamazaki, C.M.; Takahara, Y.; Homma, D.L.; Otaka, A.; Ohtani, K.; Wakamiya, N.; Nagata, K.; et al. Specific recognition of the collagen triple helix by chaperone HSP47. II. The HSP47-binding structural motif in collagens and related proteins. J. Biol. Chem. 2006, 281, 11177–11185. [Google Scholar] [CrossRef] [PubMed]
- Konitsiotis, A.D.; Raynal, N.; Bihan, D.; Hohenester, E.; Farndale, R.W.; Leitinger, B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J. Biol. Chem. 2008, 283, 6861–6868. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Raynal, N.; Stathopoulos, S.; Myllyharju, J.; Farndale, R.W.; Leitinger, B. Collagen binding specificity of the discoidin domain receptors: Binding sites on collagens II and III and molecular determinants for collagen iv recognition by DDR1. Matrix Biol. 2011, 30, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, A.; Okano-Kosugi, H.; Yamazaki, C.M.; Koide, T. Pigment epithelium-derived factor (PEDF) shares binding sites in collagen with heparin/heparan sulfate proteoglycans. J. Biol. Chem. 2011, 286, 26364–26374. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.; Russell, B.H.; Rivera, J.J.; Liang, X.; Xu, X.; Afshar-Kharghan, V.; Hook, M. An engineered α1 integrin-binding collagenous sequence. J. Biol. Chem. 2010, 285, 31046–31054. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Visse, R.; Inouye, M.; Nagase, H.; Brodsky, B. Defining requirements for collagenase cleavage in collagen type III using a bacterial collagen system. J. Biol. Chem. 2012, 287, 22988–22997. [Google Scholar] [CrossRef] [PubMed]
- An, B.; DesRochers, T.M.; Qin, G.; Xia, X.; Thiagarajan, G.; Brodsky, B.; Kaplan, D.L. The influence of specific binding of collagen-silk chimeras to silk biomaterials on hMSC behavior. Biomaterials 2013, 34, 402–412. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Abbonante, V.; Yigit, S.; Balduini, A.; Kaplan, D.L.; Brodsky, B. Definition of the native and denatured type II collagen binding site for fibronectin using a recombinant collagen system. J. Biol. Chem. 2014, 289, 4941–4951. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Stoichevska, V.; Schacht, K.; Werkmeister, J.A.; Ramshaw, J.A. Engineering multiple biological functional motifs into a blank collagen-like protein template from streptococcus pyogenes. J. Biomed. Mater. Res. Part A 2014, 102, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Hamaia, S.W.; Pugh, N.; Raynal, N.; Nemoz, B.; Stone, R.; Gullberg, D.; Bihan, D.; Farndale, R.W. Mapping of potent and specific binding motifs, GLOGEN and GVOGEA, for integrin α1β1 using collagen toolkits II and III. J. Biol. Chem. 2012, 287, 26019–26028. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural basis of collagen recognition by integrin α2β1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Raynal, N.; Hamaia, S.W.; Siljander, P.R.; Maddox, B.; Peachey, A.R.; Fernandez, R.; Foley, L.J.; Slatter, D.A.; Jarvis, G.E.; Farndale, R.W. Use of synthetic peptides to locate novel integrin α2β1-binding motifs in human collagen III. J. Biol. Chem. 2006, 281, 3821–3831. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, F.; Hamaia, S.W.; Bihan, D.; Hohenester, E.; Farndale, R.W. An activating mutation reveals a second binding mode of the integrin α2 i domain to the GFOGER motif in collagens. PLoS ONE 2013, 8, e69833. [Google Scholar] [CrossRef] [PubMed]
- Hamaia, S.; Farndale, R.W. Integrin recognition motifs in the human collagens. Adv. Exp. Med. Biol. 2014, 819, 127–142. [Google Scholar] [PubMed]
- Carafoli, F.; Bihan, D.; Stathopoulos, S.; Konitsiotis, A.D.; Kvansakul, M.; Farndale, R.W.; Leitinger, B.; Hohenester, E. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure 2009, 17, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T.; Raynal, N.; Groeneveld, D.; Maddox, B.; Peachey, A.R.; Huizinga, E.G.; de Groot, P.G.; Farndale, R.W. A single high-affinity binding site for von willebrand factor in collagen III, identified using synthetic triple-helical peptides. Blood 2006, 108, 3753–3756. [Google Scholar] [CrossRef] [PubMed]
- Brondijk, T.H.; Bihan, D.; Farndale, R.W.; Huizinga, E.G. Implications for collagen I chain registry from the structure of the collagen von willebrand factor A3 domain complex. Proc. Natl. Acad. Sci. USA 2012, 109, 5253–5258. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, G.E.; Raynal, N.; Langford, J.P.; Onley, D.J.; Andrews, A.; Smethurst, P.A.; Farndale, R.W. Identification of a major GpVI-binding locus in human type III collagen. Blood 2008, 111, 4986–4996. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Moroi, M.; Soejima, K.; Nakagaki, T.; Miura, Y.; Berndt, M.C.; Gardiner, E.E.; Howes, J.M.; Pugh, N.; Bihan, D.; et al. Constitutive dimerization of glycoprotein VI (GPVI) in resting platelets is essential for binding to collagen and activation in flowing blood. J. Biol. Chem. 2012, 287, 30000–30013. [Google Scholar] [CrossRef] [PubMed]
- Lebbink, R.J.; Raynal, N.; de Ruiter, T.; Bihan, D.G.; Farndale, R.W.; Meyaard, L. Identification of multiple potent binding sites for human leukocyte associated Ig-like receptor lair on collagens II and III. Matrix Biol. 2009, 28, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Brondijk, T.H.; de Ruiter, T.; Ballering, J.; Wienk, H.; Lebbink, R.J.; van Ingen, H.; Boelens, R.; Farndale, R.W.; Meyaard, L.; Huizinga, E.G. Crystal structure and collagen-binding site of immune inhibitory receptor lair-1: Unexpected implications for collagen binding by platelet receptor GPVI. Blood 2010, 115, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Raynal, N.; Andersen, T.L.; Slatter, D.A.; Bihan, D.; Pugh, N.; Cella, M.; Kim, T.; Rho, J.; Negishi-Koga, T.; et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Investig. 2011, 121, 3505–3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Hinerman, J.M.; Blaszczyk, M.; Miller, J.L.; Conrady, D.G.; Barrow, A.D.; Chirgadze, D.Y.; Bihan, D.; Farndale, R.W.; Herr, A.B. Structural basis for collagen recognition by the immune receptor oscar. Blood 2016, 127, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Giudici, C.; Raynal, N.; Wiedemann, H.; Cabral, W.A.; Marini, J.C.; Timpl, R.; Bachinger, H.P.; Farndale, R.W.; Sasaki, T.; Tenni, R. Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J. Biol. Chem. 2008, 283, 19551–19560. [Google Scholar] [CrossRef] [PubMed]
- Leo, J.C.; Elovaara, H.; Bihan, D.; Pugh, N.; Kilpinen, S.K.; Raynal, N.; Skurnik, M.; Farndale, R.W.; Goldman, A. First analysis of a bacterial collagen-binding protein with collagen toolkits: Promiscuous binding of yada to collagens may explain how yada interferes with host processes. Infect. Immun. 2010, 78, 3226–3236. [Google Scholar] [CrossRef] [PubMed]
- Manka, S.W.; Carafoli, F.; Visse, R.; Bihan, D.; Raynal, N.; Farndale, R.W.; Murphy, G.; Enghild, J.J.; Hohenester, E.; Nagase, H. Structural insights into triple-helical collagen cleavage by matrix metalloproteinase 1. Proc. Natl. Acad. Sci. USA 2012, 109, 12461–12466. [Google Scholar] [CrossRef] [PubMed]
- Howes, J.M.; Bihan, D.; Slatter, D.A.; Hamaia, S.W.; Packman, L.C.; Knauper, V.; Visse, R.; Farndale, R.W. The recognition of collagen and triple-helical toolkit peptides by MMP-13: Sequence specificity for binding and cleavage. J. Biol. Chem. 2014, 289, 24091–24101. [Google Scholar] [CrossRef] [PubMed]
- Kalamajski, S.; Bihan, D.; Bonna, A.; Rubin, K.; Farndale, R.W. Fibromodulin interacts with collagen cross-linking sites and activates lysyl oxidase. J. Biol. Chem. 2016, 291, 7951–7960. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.D.; San Antonio, J.D.; Persikov, A.V.; Dagher, H.; Dalgleish, R.; Jensen, S.T.; Jeunemaitre, X.; Savige, J. The collagen III fibril has a “flexi-rod” structure of flexible sequences interspersed with rigid bioactive domains including two with hemostatic roles. PLoS ONE 2017, 12, e0175582. [Google Scholar] [CrossRef] [PubMed]
- San Antonio, J.D.; Lander, A.D.; Karnovsky, M.J.; Slayter, H.S. Mapping the heparin-binding sites on type I collagen monomers and fibrils. J. Cell Biol. 1994, 125, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fertala, A.; Ratner, B.D.; Sage, E.H.; Jiang, S. Identifying the sparc binding sites on collagen I and procollagen I by atomic force microscopy. Anal. Chem. 2005, 77, 6765–6771. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.; San Antonio, J.D.; Antipova, O. Molecular and structural mapping of collagen fibril interactions. Connect. Tissue Res. 2011, 52, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Orgel, J.; Gullberg, D. Molecular composition and function of integrin-based collagen glues-introducing colinbris. Biochim. Biophys. Acta 2014, 1840, 2533–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspanti, M.; Alessandrini, A.; Gobbi, P.; Ruggeri, A. Collagen fibril surface: TMAFM, FEG-SEM and freeze-etching observations. Microsc. Res. Tech. 1996, 35, 87–93. [Google Scholar] [CrossRef]
- Hulmes, D.J.; Jesior, J.C.; Miller, A.; Berthet-Colominas, C.; Wolff, C. Electron microscopy shows periodic structure in collagen fibril cross sections. Proc. Natl. Acad. Sci USA 1981, 78, 3567–3571. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Antipova, O.; Orgel, J.P. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc. Natl. Acad. Sci. USA 2008, 105, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Streeter, I.; de Leeuw, N.H. Atomistic modeling of collagen proteins in their fibrillar environment. J. Phys. Chem. B 2010, 114, 13263–13270. [Google Scholar] [CrossRef] [PubMed]
- Streeter, I.; de Leeuw, N.H. A molecular dynamics study of the interprotein interactions in collagen fibrils. Soft Matter 2011, 7, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Botlani, M.; Hammond, J.R.; Scott, H.L.; Orgel, J.P.; Schieber, J.D. Effect of intrinsic and extrinsic factors on the simulated d-band length of type I collagen. Proteins 2015, 83, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M. Analytical molecular surface calculation. J. Appl. Crystallogr. 1983, 16, 548–558. [Google Scholar] [CrossRef]
- Connolly, M.L. Solvent-accessible surfaces of proteins and nucleic acids. Science 1983, 221, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.M.; Guy, C.A.; Fields, G.B.; San Antonio, J.D. Defining the domains of type I collagen involved in heparin-binding and endothelial tube formation. Proc. Natl. Acad. Sci. USA 1998, 95, 7275–7280. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, M.L.; Becerra, S.P.; Choong, P.F.; Dass, C.R. The applied biochemistry of pedf and implications for tissue homeostasis. Growth Factors 2010, 28, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.; Eid, A.; Antipova, O.; Bella, J.; Scott, J.E. Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS ONE 2009, 4, e7028. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Jamieson, G.A.; Okuma, M.; Kambayashi, J.; Tandon, N.N. Platelet adhesion to native type I collagen fibrils. Role of GPVI in divalent cation-dependent and -independent adhesion and thromboxane A2 generation. J. Biol. Chem. 1998, 273, 4338–4344. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar] [PubMed]
- Keene, D.R.; San Antonio, J.D.; Mayne, R.; McQuillan, D.J.; Sarris, G.; Santoro, S.A.; Iozzo, R.V. Decorin binds near the C terminus of type I collagen. J. Biol. Chem. 2000, 275, 21801–21804. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Dinakarpandian, D.; Yoshida, N.; Lauer-Fields, J.L.; Fields, G.B.; Visse, R.; Nagase, H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004, 23, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.G.; Stultz, C.M. Insight into the degradation of type-I collagen fibrils by MMP-8. J. Mol. Biol. 2013, 425, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Moroi, M.; Jung, S.M. Platelet receptors for collagen. Thromb. Haemost. 1997, 78, 439–444. [Google Scholar] [PubMed]
- Jokinen, J.; Dadu, E.; Nykvist, P.; Kapyla, J.; White, D.J.; Ivaska, J.; Vehvilainen, P.; Reunanen, H.; Larjava, H.; Hakkinen, L.; et al. Integrin-mediated cell adhesion to type I collagen fibrils. J. Biol. Chem. 2004, 279, 31956–31963. [Google Scholar] [CrossRef] [PubMed]
- Siljander, P.R.; Hamaia, S.; Peachey, A.R.; Slatter, D.A.; Smethurst, P.A.; Ouwehand, W.H.; Knight, C.G.; Farndale, R.W. Integrin activation state determines selectivity for novel recognition sites in fibrillar collagens. J. Biol. Chem. 2004, 279, 47763–47772. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Slatter, D.A.; Siljander, P.R.; Jarvis, G.E. Platelet receptor recognition and cross-talk in collagen-induced activation of platelets. J. Thromb. Haemost. 2007, 5 (Suppl 1), 220–229. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W. Collagen-induced platelet activation. Blood Cells Mol. Dis. 2006, 36, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Woltersdorf, C.; Bonk, M.; Leitinger, B.; Huhtala, M.; Kapyla, J.; Heino, J.; Gil Girol, C.; Niland, S.; Eble, J.A.; Bruckner, P.; et al. The binding capacity of α1β1-, α2β1- and α10β1-integrins depends on non-collagenous surface macromolecules rather than the collagens in cartilage fibrils. Matrix Biol. 2017, 63, 91–105. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoop, C.L.; Zhu, J.; Nunes, A.M.; Case, D.A.; Baum, J. Revealing Accessibility of Cryptic Protein Binding Sites within the Functional Collagen Fibril. Biomolecules 2017, 7, 76. https://doi.org/10.3390/biom7040076
Hoop CL, Zhu J, Nunes AM, Case DA, Baum J. Revealing Accessibility of Cryptic Protein Binding Sites within the Functional Collagen Fibril. Biomolecules. 2017; 7(4):76. https://doi.org/10.3390/biom7040076
Chicago/Turabian StyleHoop, Cody L., Jie Zhu, Ana Monica Nunes, David A. Case, and Jean Baum. 2017. "Revealing Accessibility of Cryptic Protein Binding Sites within the Functional Collagen Fibril" Biomolecules 7, no. 4: 76. https://doi.org/10.3390/biom7040076





