Taurine Bromamine: Reactivity of an Endogenous and Exogenous Anti-Inflammatory and Antimicrobial Amino Acid Derivative
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Stability of Tau-NHBr
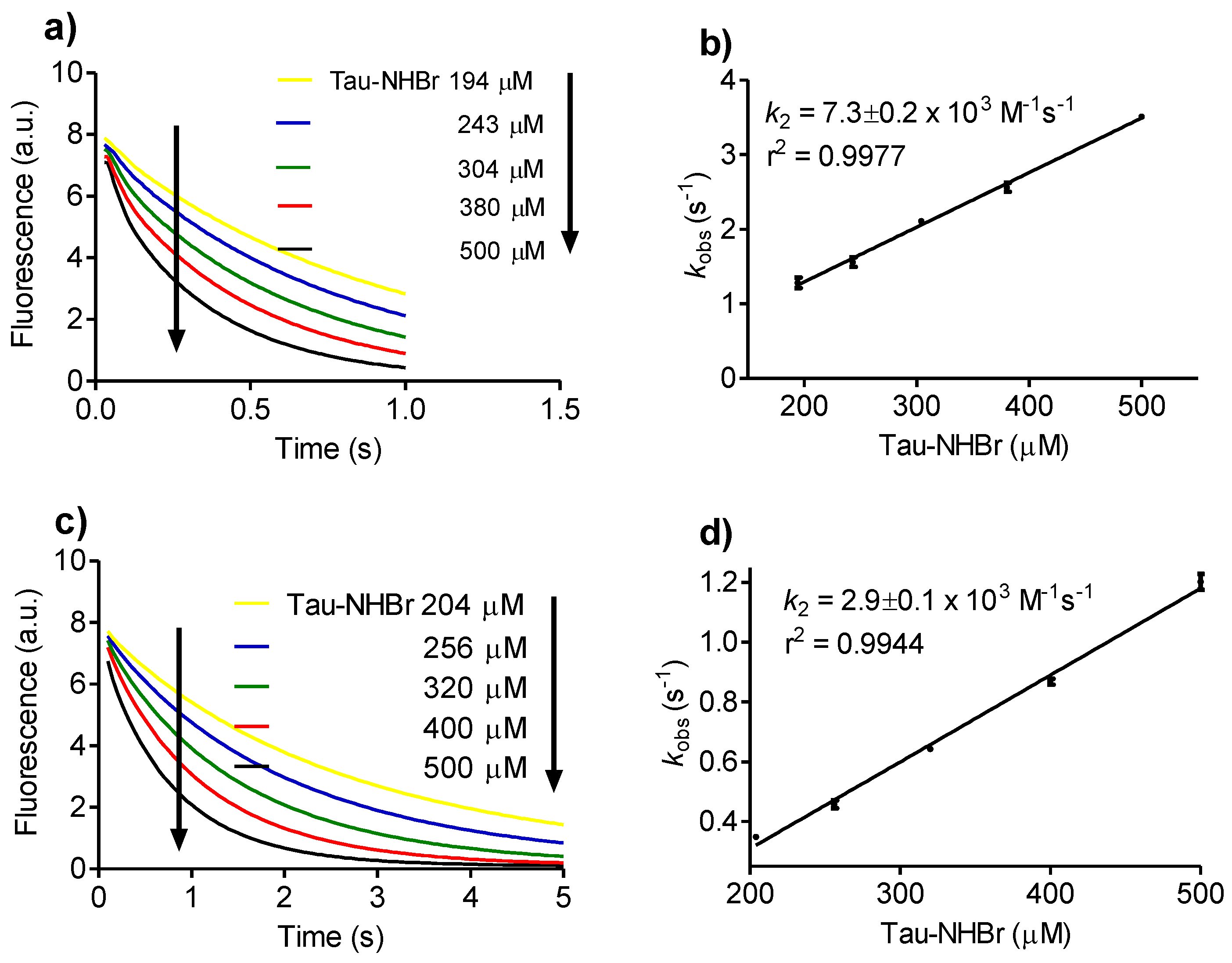
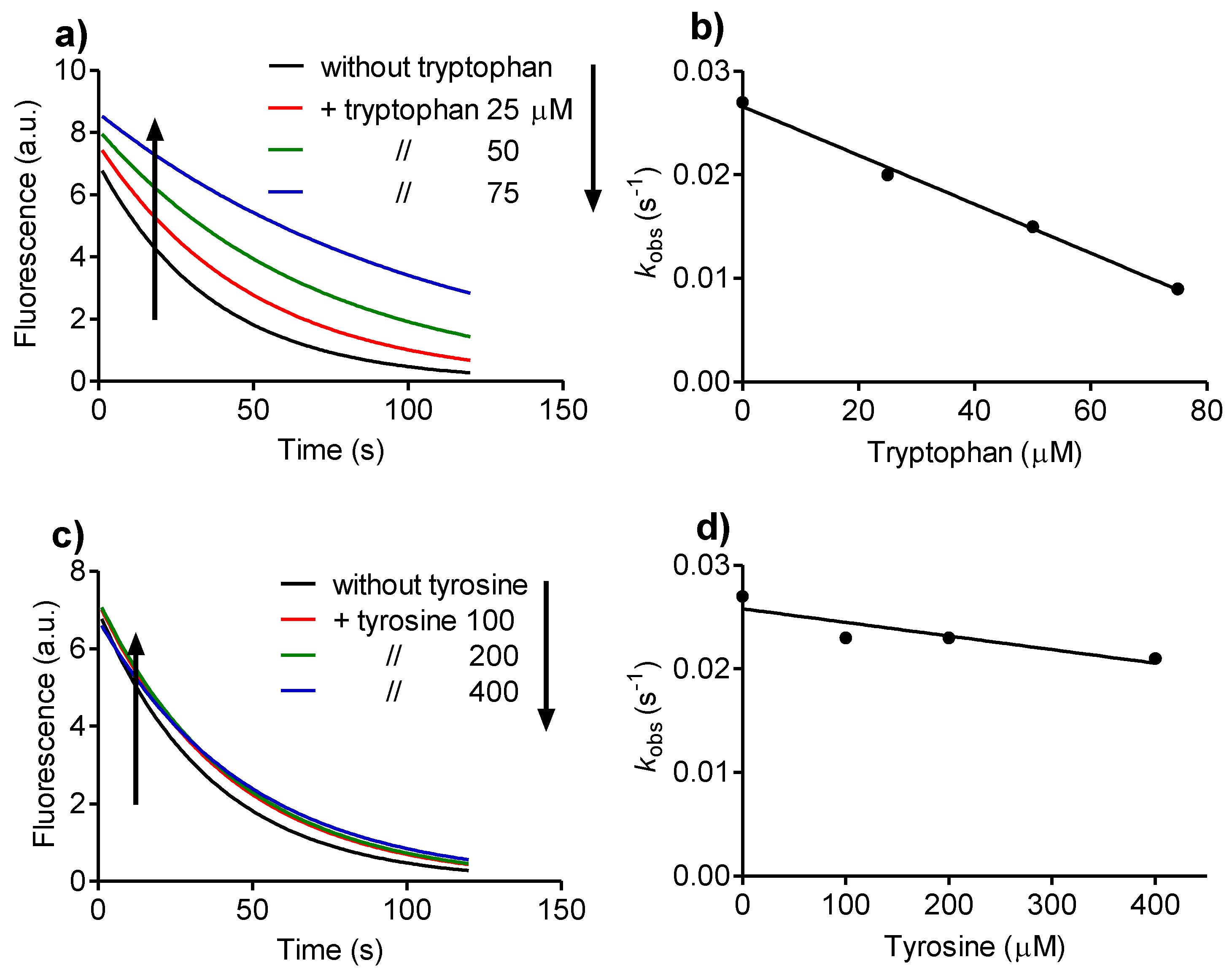
2.2. Reactivity with Tryptophan
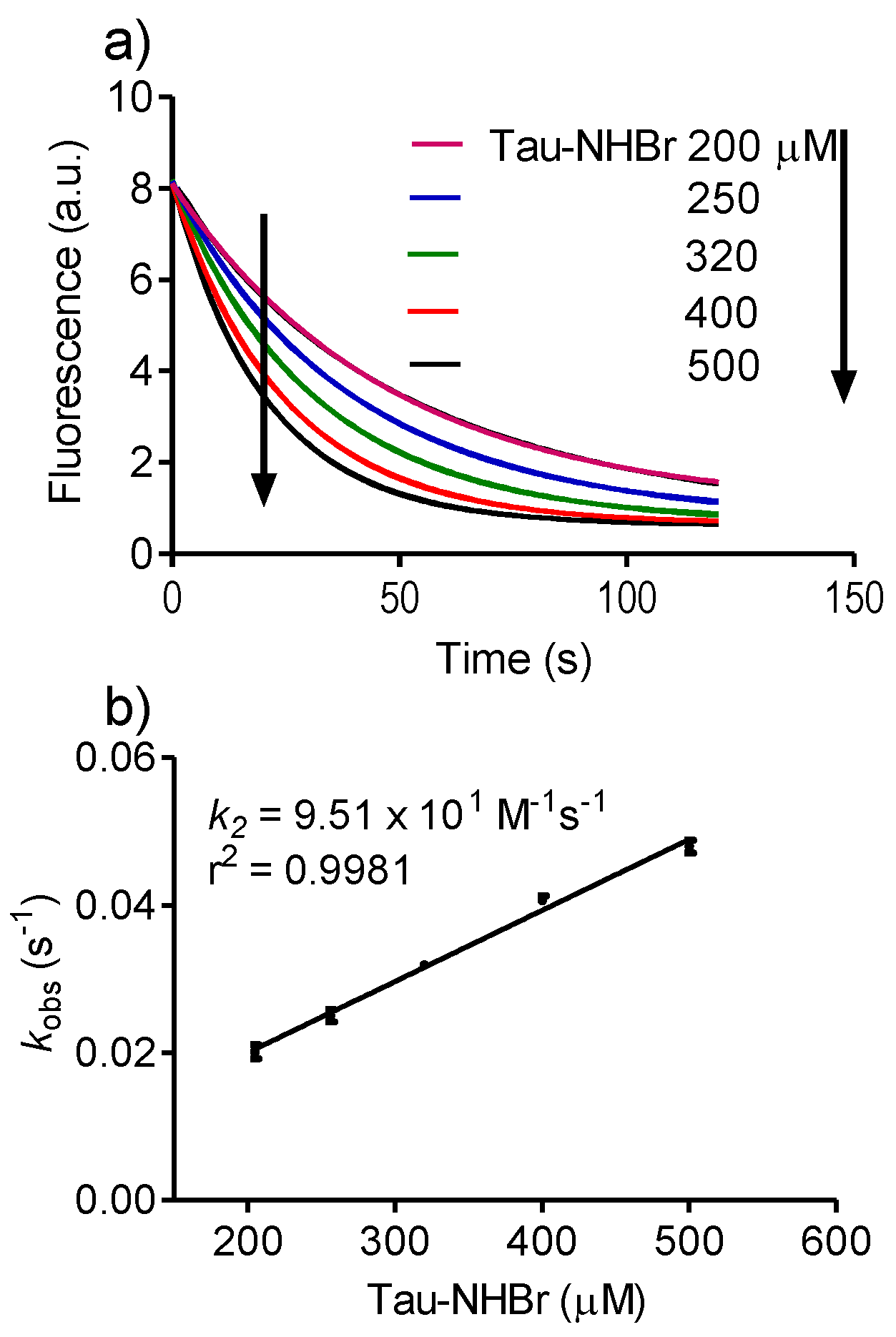
2.3. Reactivity with Dansylglycine
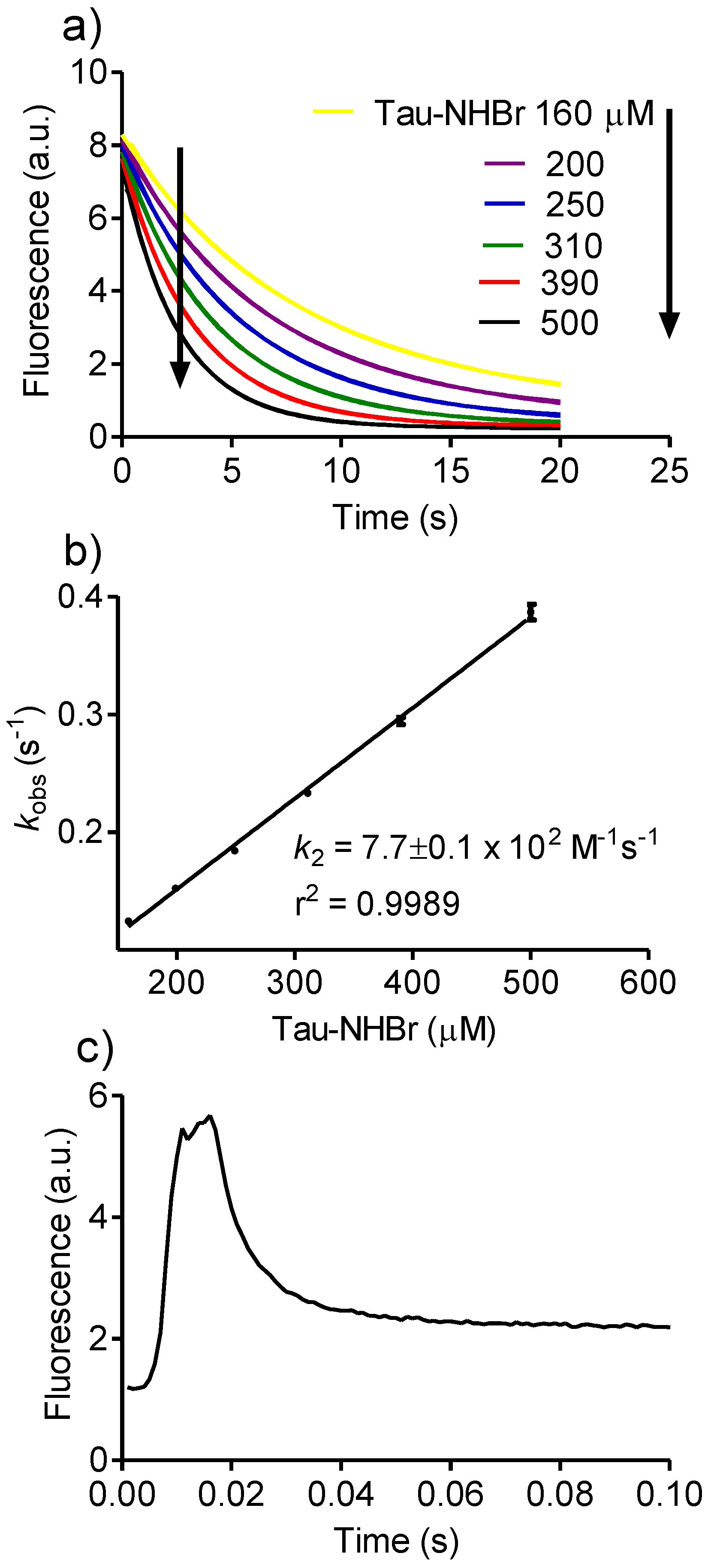
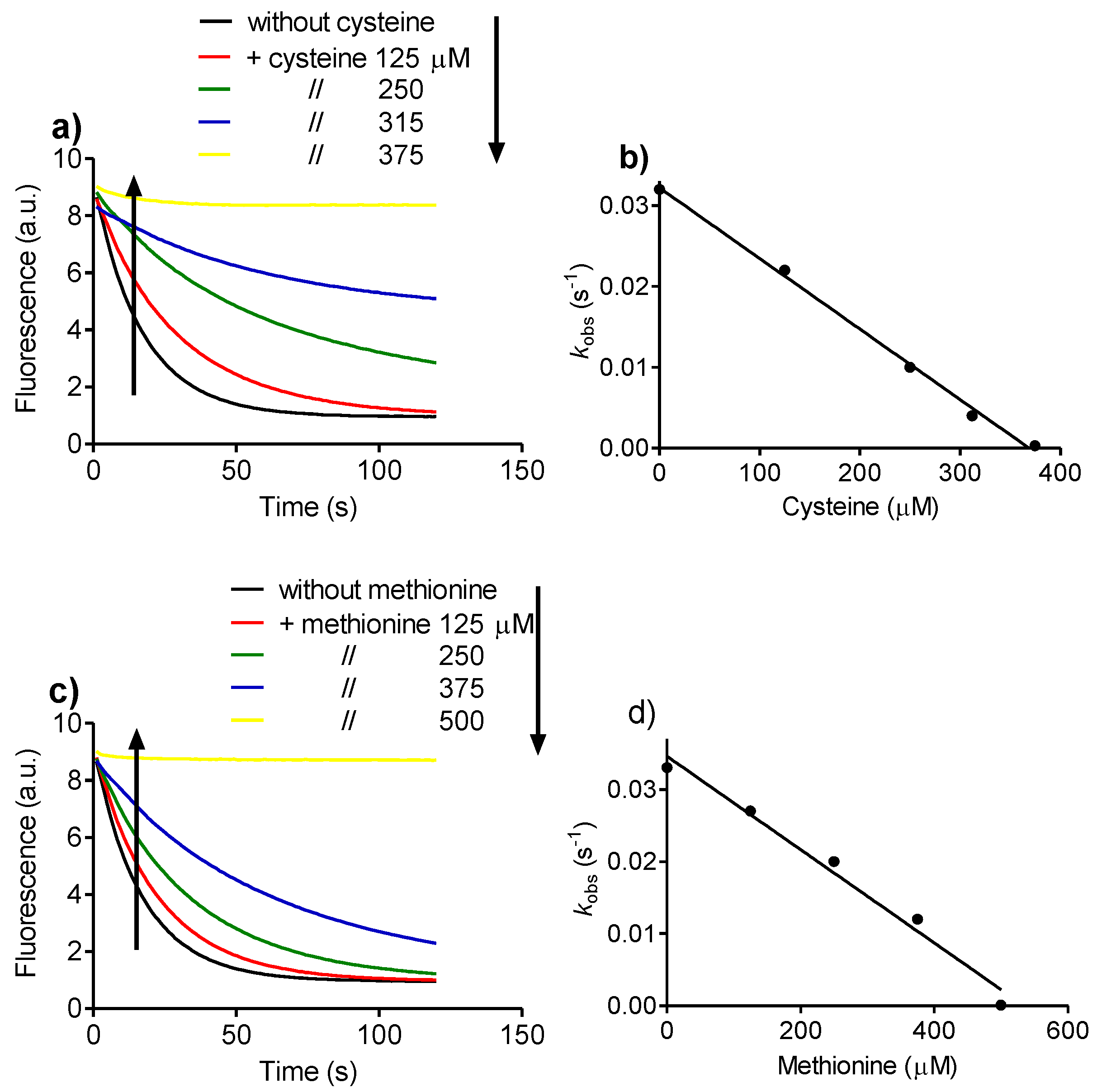
2.4. Relative Reactivity with Tryptophan, Tyrosine, Cysteine and Methionine
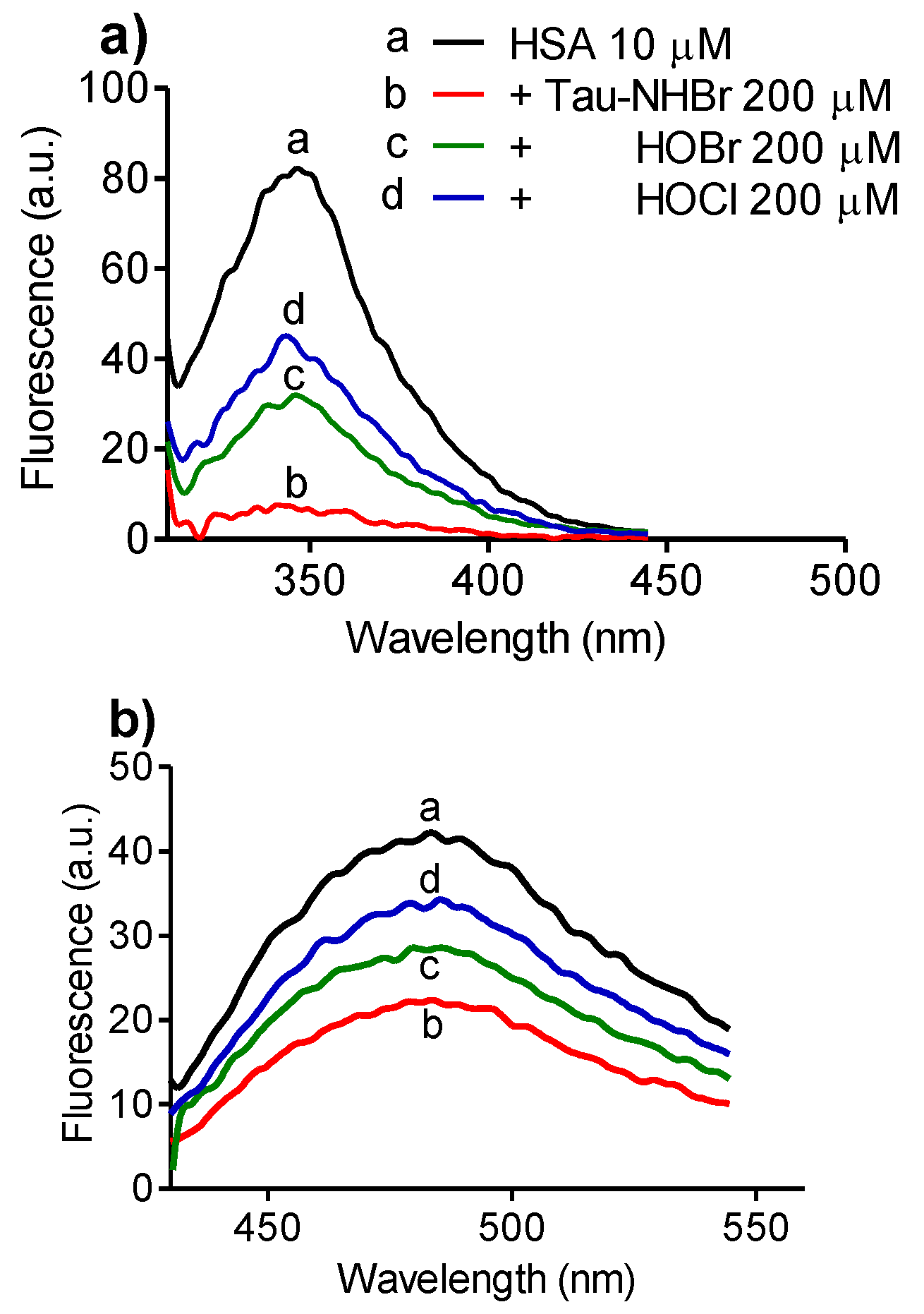
2.5. Selectivity upon Tryptophan Residues in Proteins
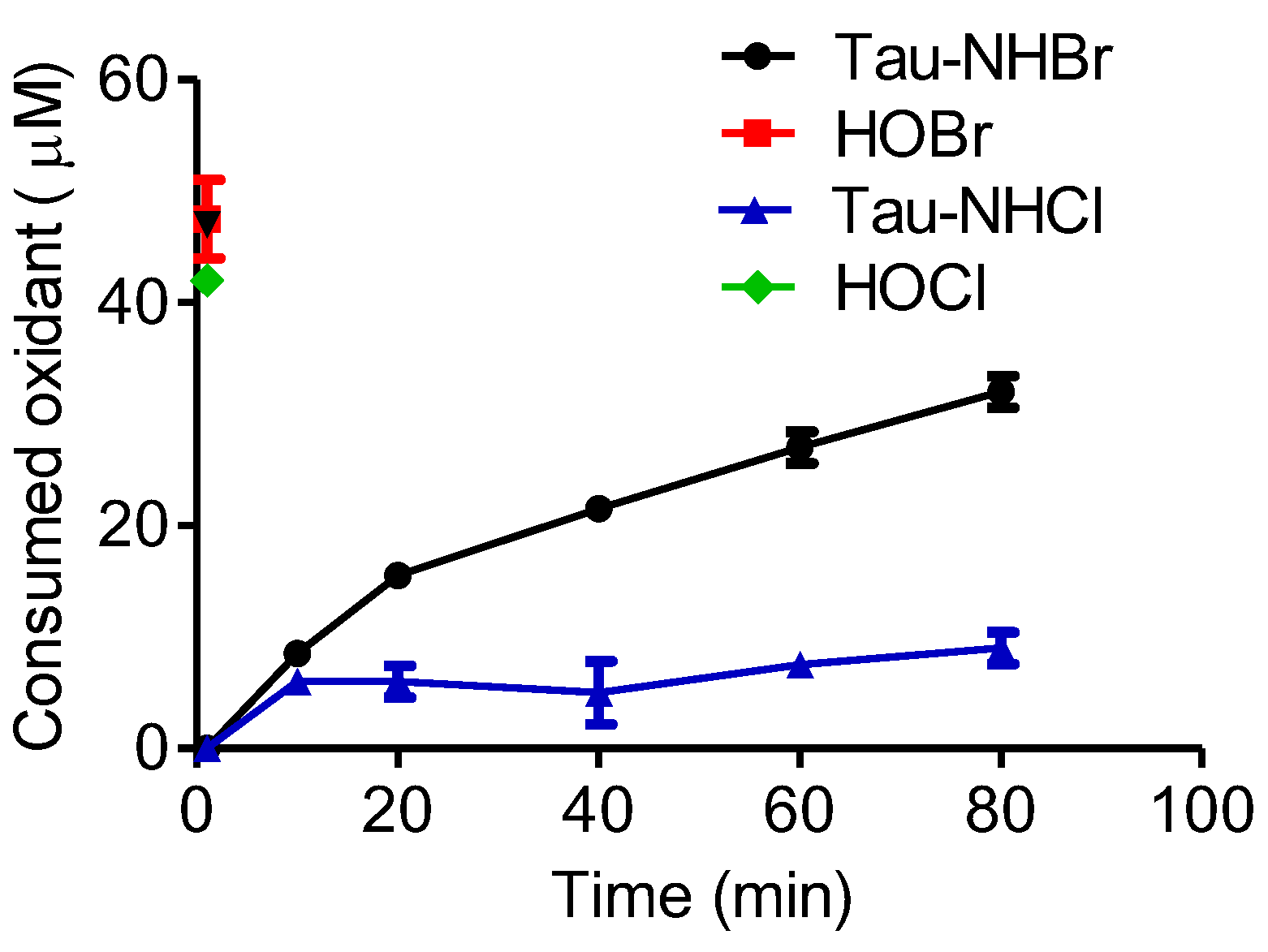
2.6. Comparison between Tau-NHCl and Tau-NHBr
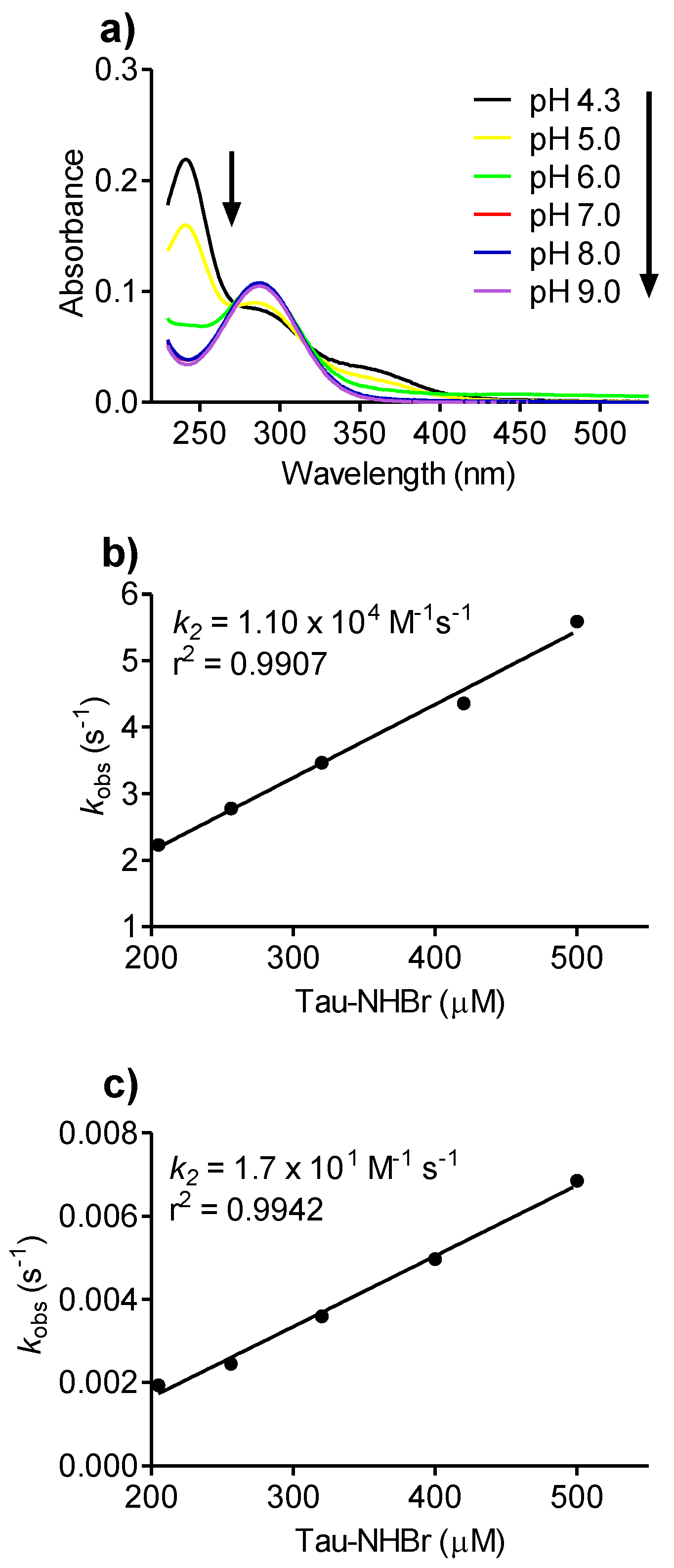
2.7. pH Effect on Tau-NHBr Reactivity
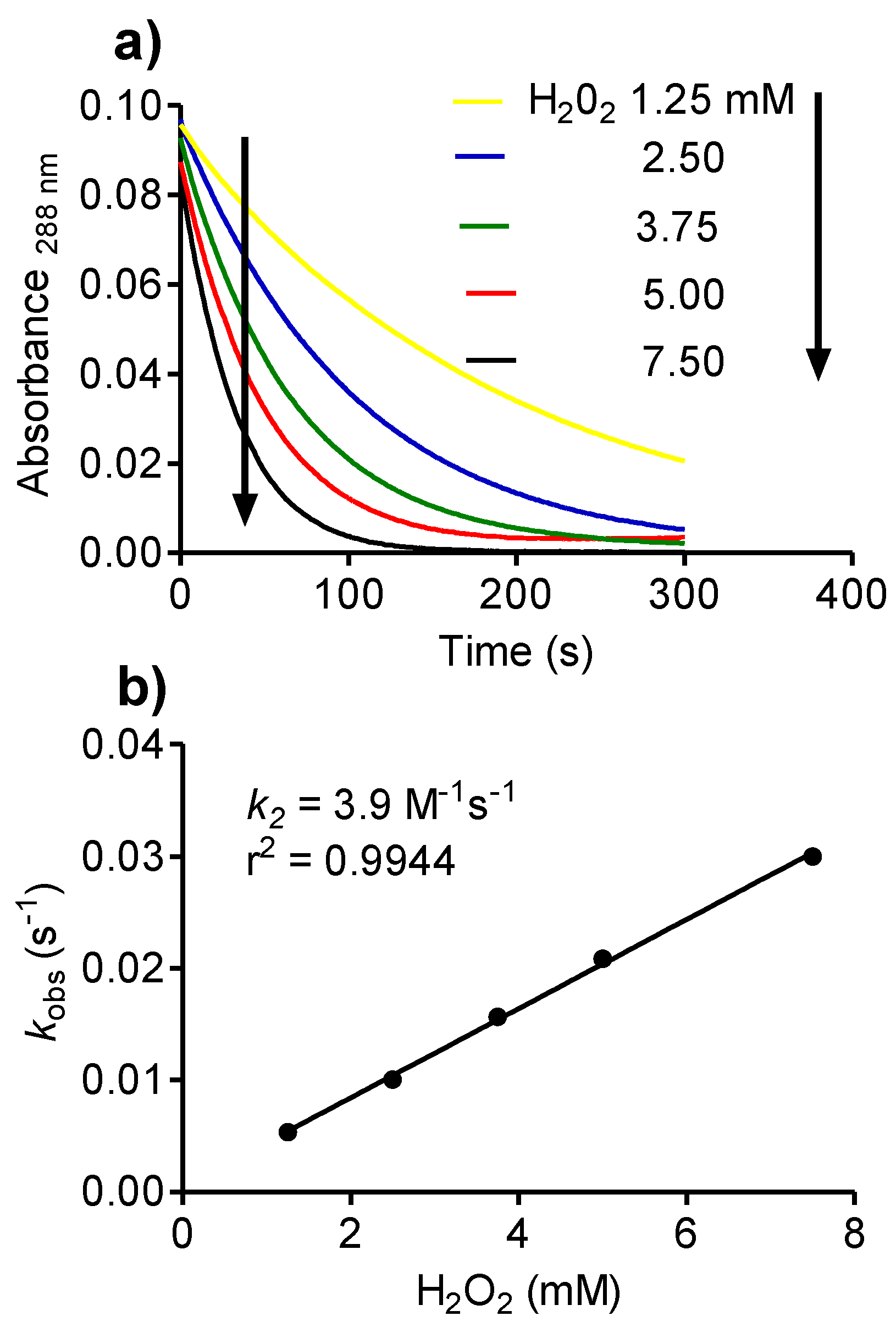
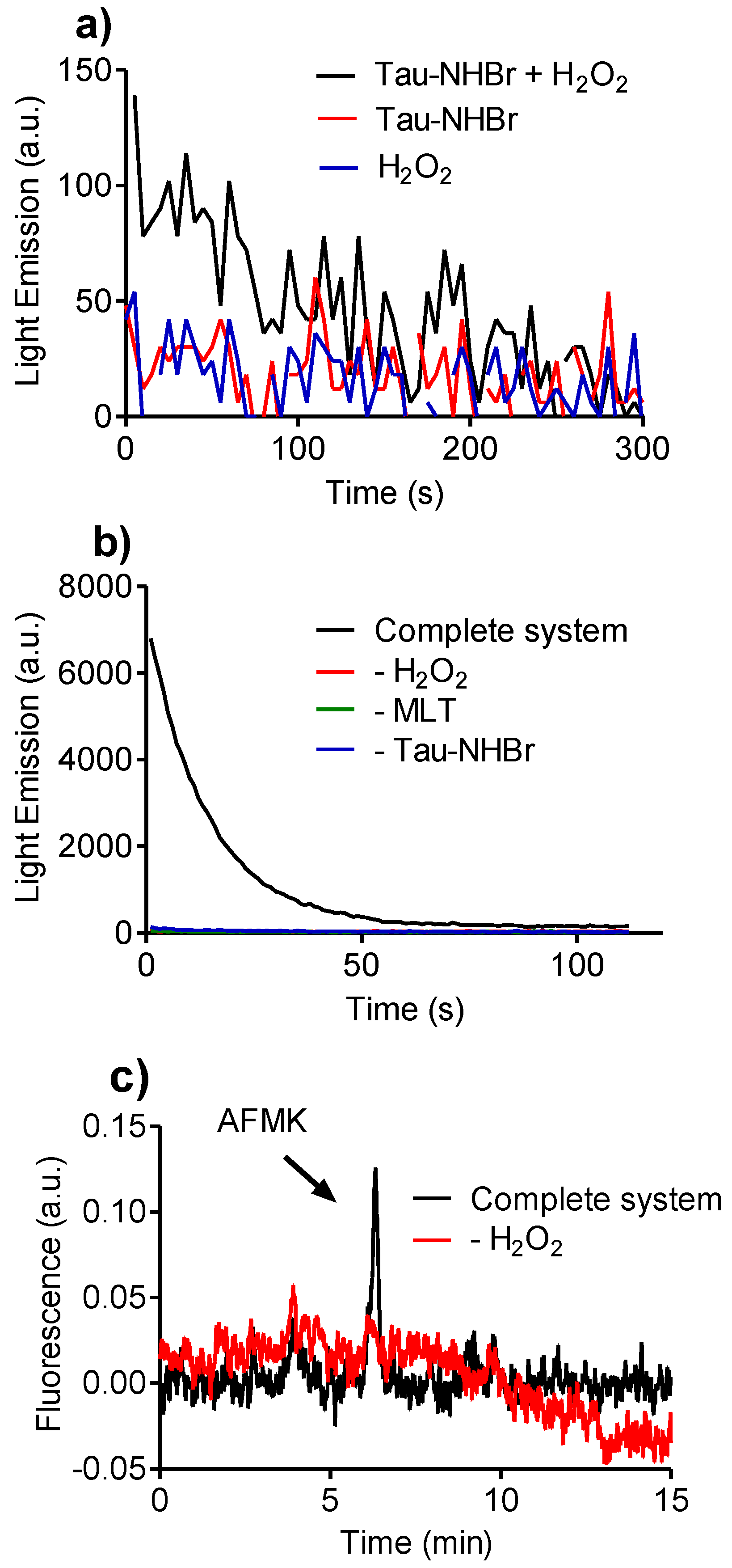
2.8. Reactivity of Tau-NHBr with Hydrogen Peroxide and Formation of Singlet Oxygen
2.9. Reactivity of Tau-NHBr with Linoleic Acid
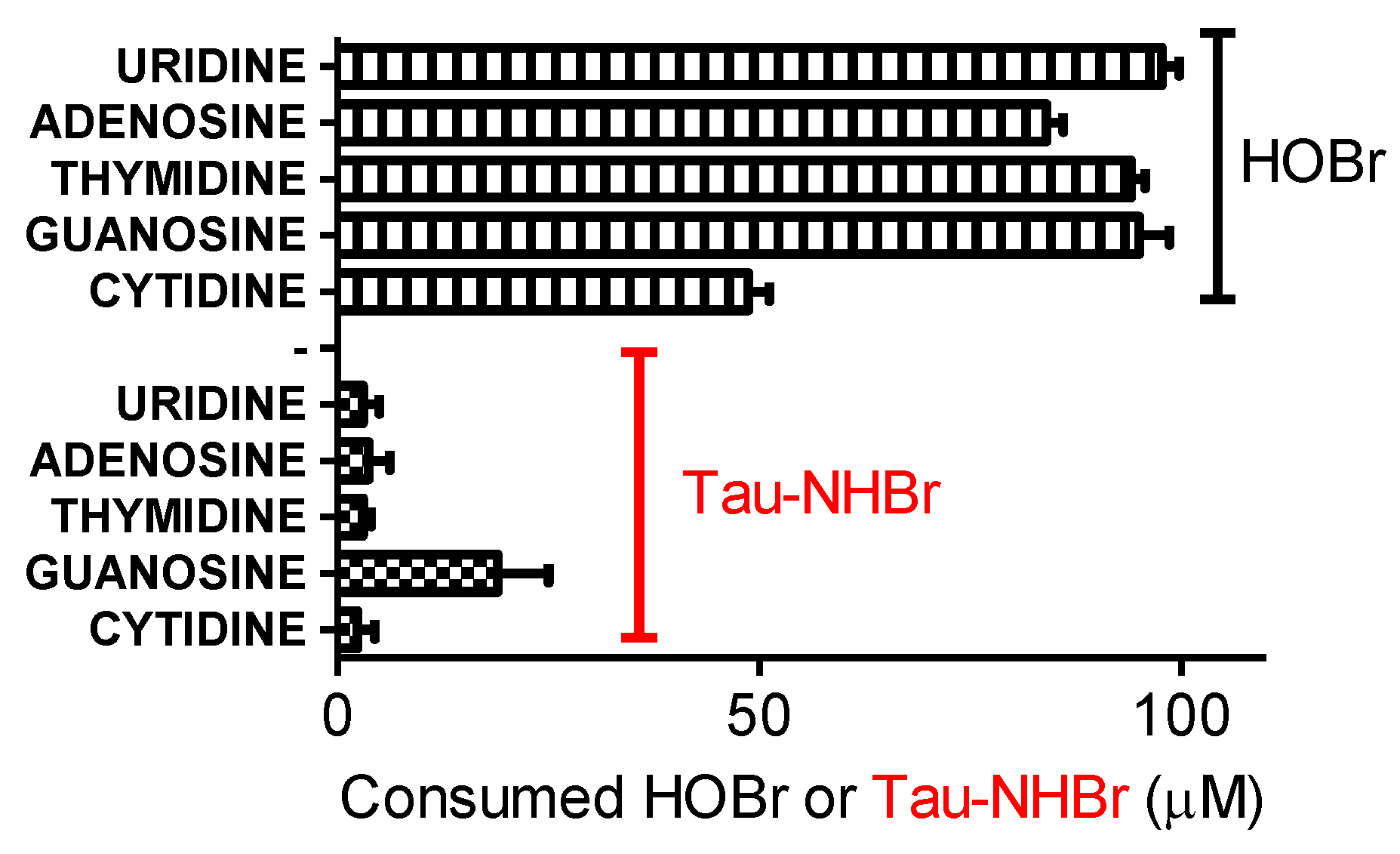
2.10. Reactivity of Tau-NHBr with Nucleosides
3. Experimental Section
3.1. Chemicals and Solutions
3.2. Determination of Rate Constants
- S is the fluorescence or absorbance as a function of time
- S0 is the initial fluorescence or absorbance
Then, kobs = k2 × [A],
3.3. Oxidation of Human Albumin and the Use of Dansylglycine as a Fluorescent Probe for Tryptophan Residues
3.4. Reactions with 3,3′,5,5′-tetramethylbenzidine (TMB), Curcumin, Hydrogen Peroxide and Linoleic Acid
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, G.; Nauseef, W.M. Salt, chloride, bleach, and innate host defense. J. Leukoc. Biol. 2015, 98, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Nutman, T.B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004, 113, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Stoll, S.J.; Ahn, J.; Bittner, J.C.; Camargo, C.A., Jr. Prevalence of eosinophilia in hospitalized patients with asthma exacerbation. Respir. Med. 2015, 109, 1230–1232. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.; Brennan, S.; Senthilmohan, R.; Gangell, C.L.; Chapman, A.L.; Sly, P.D.; Kettle, A.J.; Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF); Balding, E.; Berry, L.J.; et al. Identifying peroxidases and their oxidants in the early pathology of cystic fibrosis. Free Radic. Biol. Med. 2010, 49, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Tang, C.; Sinha, A.; Mayer, P.S.; Davenport, G.D.; Brot, N.; Oda, M.N.; Zhao, X.Q.; Heinecke, J.W. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ. Res. 2014, 114, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.; Katz, L.; Gunsoy, N.; Keene, O.; Yancey, S. Blood eosinophil counts predict treatment response in patients with severe eosinophilic asthma. J. Allergy. Clin. Immunol. 2015, 136, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.E.; Chan, T.; van Dalen, C.J.; Senthilmohan, R.; Winn, M.; Venge, P.; Town, G.I.; Kettle, A.J. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic. Biol. Med. 2002, 33, 847–856. [Google Scholar] [CrossRef]
- Acharya, K.R.; Ackerman, S.J. Eosinophil granule proteins: Form and function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, Z.; Marcinkiewicz, J.; Katz, D.R.; Chain, B.M. Neutrophil myeloperoxidase: Soldier and statesman. Arch. Immunol. Ther. Exp. (Warsz) 2012, 60, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Furtmüller, P.G.; Regelsberger, G.; Obinger, C. Redox properties of the couple compound I/native enzyme of myeloperoxidase and eosinophil peroxidase. Eur. J. Biochem. 2001, 268, 5142–5148. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Furtmüller, P.G.; Burner, U.; Regelsberger, G.; Obinger, C. Spectral and kinetic studies on the formation of eosinophil peroxidase compound I and its reaction with halides and thiocyanate. Biochemistry 2000, 39, 15578–15584. [Google Scholar] [CrossRef] [PubMed]
- Augusto, O.; Miyamoto, S. Oxygen radicals and related species. In Principles of free radical biomedicine; Pantopoulos, K., Schipper, H.M., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 19–42. [Google Scholar]
- Rayner, B.S.; Love, D.T.; Hawkins, C.L. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radic. Biol. Med. 2014, 71, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Morgon, N.H.; de Souza, A.R. Hypobromous acid, a powerful endogenous electrophile: Experimental and theoretical studies. J. Inorg. Biochem. 2015, 146, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sivey, J.D.; Arey, J.S.; Tentscher, P.R.; Roberts, A.L. Reactivity of BrCl, Br2, BrOCl, Br2O, and HOBr toward dimethenamid in solutions of bromide + aqueous free chlorine. Environ. Sci. Technol. 2013, 47, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, M.; Eckmann, C.M.; Roos, D. Killing of schistosomula by taurine chloramine and taurine bromamine. Am. J. Trop. Med. Hyg. 1987, 37, 106–110. [Google Scholar] [PubMed]
- Weiss, S.J.; Test, S.T.; Eckmann, C.M.; Roos, D.; Regiani, S. Brominating oxidants generated by human eosinophils. Science 1986, 234, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Simonart, T. Newer approaches to the treatment of acne vulgaris. Am. J. Clin. Dermatol. 2012, 13, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Biedroń, R.; Białecka, A.; Kasprowicz, A.; Mak, M.; Targosz, M. Susceptibility of Propionibacterium acnes and Staphylococcus epidermidis to killing by MPO-halide system products. Implication for taurine bromamine as a new candidate for topical therapy in treating acne vulgaris. Arch. Immunol. Ther. Exp. (Warsz) 2006, 54, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Strus, M.; Walczewska, M.; Machul, A.; Mikołajczyk, D. Influence of taurine haloamines (TauCl and TauBr) on the development of Pseudomonas aeruginosa biofilm: A preliminary study. Adv. Exp. Med. Biol. 2013, 775, 269–283. [Google Scholar] [PubMed]
- Strus, M.; Walczewska, M.; Machul, A.; Mikołajczyk, D.; Marcinkiewicz, J. Taurine Haloamines and Biofilm. Part I: Antimicrobial Activity of Taurine Bromamine and Chlorhexidine Against Biofilm Forming Pseudomonas aeruginosa. J. Adv. Exp. Med. Biol. 2015, 803, 121–132. [Google Scholar]
- Gottardi, W.; Klotz, S.; Nagl, M. Superior bactericidal activity of N-bromine compounds compared to their N-chlorine analogues can be reversed under protein load. J. Appl. Microbiol. 2014, 116, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Walczewska, M.; Olszanecki, R.; Bobek, M.; Biedroń, R.; Dulak, J.; Józkowicz, A.; Kontny, E.; Maślinski, W. Taurine haloamines and heme oxygenase-1 cooperate in the regulation of inflammation and attenuation of oxidative stress. Adv. Exp. Med. Biol. 2009, 643, 439–450. [Google Scholar] [PubMed]
- Tokunaga, S.; Kanayama, A.; Miyamoto, Y. Modification of IkappaBalpha by taurine bromamine inhibits tumor necrosis factor alpha-induced NF-kappaB activation. Inflamm. Res. 2007, 56, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cha, Y.N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2014, 46, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Peskin, A.V.; Winterbourn, C.C. Taurine chloramine is more selective than hypochlorous acid at targeting critical cysteines and inactivating creatine kinase and glyceraldehyde-3-phosphate dehydrogenase. Free Radic. Biol. Med. 2006, 40, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sun Jang, J.; Piao, S.; Cha, Y.N.; Kim, C. Taurine Chloramine Activates Nrf2, Increases HO-1 Expression and Protects Cells from Death Caused by Hydrogen Peroxide. J. Clin. Biochem. Nutr. 2009, 45, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Ji, H.I.; Chung, H.; Kim, C.; Lee, S.H.; Lee, Y.A.; Yang, H.I.; Yoo, M.C.; Hong, S.J. Taurine chloramine modulates the expression of adipokines through inhibition of the STAT-3 signaling pathway in differentiated human adipocytes. Amino Acids 2013, 45, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kang, I.S. Taurine Chloramine, a Taurine Metabolite from Activated Neutrophils, Inhibits Osteoclastogenesis by Suppressing NFATc1 Expression. Adv. Exp. Med. Biol. 2015, 803, 99–107. [Google Scholar] [PubMed]
- Gottardi, W.; Nagl, M. Chemical properties of N-chlorotaurine sodium, a key compound in the human defence system. Arch. Pharm. (Weinheim) 2002, 335, 411–421. [Google Scholar] [CrossRef]
- Gottardi, W.; Debabov, D.; Nagl, M. N-chloramines, a promising class of well-tolerated topical anti-infectives. Antimicrob. Agents Chemother. 2013, 57, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Bozeman, P.M.; Jefferson, M.M.; King, C.C. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase eosinophil peroxidase. Formation of bromamines. J. Biol. Chem. 1995, 270, 2906–2913. [Google Scholar] [PubMed]
- Petrônio, M.S.; Ximenes, V.F. Inhibition of lysozyme by taurine dibromamine. Protein. Pept. Lett. 2013, 20, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.; Estevão, M.S.; Ferreira, L.M.; Fernandes, E.; Marques, M.M. A new insight on the hypochlorous acid scavenging mechanism of tryptamine and tryptophan derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 6475–6478. [Google Scholar] [CrossRef] [PubMed]
- Gulçin, I. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J. Enzyme Inhib. Med. Chem. 2008, 23, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Jantschko, W.; Furtmüller, P.G.; Allegra, M.; Livrea, M.A.; Jakopitsch, C.; Regelsberger, G.; Obinger, C. Redox intermediates of plant and mammalian peroxidases: A comparative transient-kinetic study of their reactivity toward indole derivatives. Arch. Biochem. Biophys. 2002, 398, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Graciani, F.S.; Ximenes, V.F. Investigation of human albumin-induced circular dichroism in dansylglycine. PLoS ONE 2013, 8, e76849. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; da Fonseca, L.M.; de Almeida, A.C. Taurine bromamine: A potent oxidant of tryptophan residues in albumin. Arch. Biochem. Biophys. 2011, 507, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Petrônio, M.S.; Ximenes, V.F. Light emission from tryptophan oxidation by hypobromous acid. Luminescence 2013, 28, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Horie, T.; Mizuma, T.; Awazu, S. Fluorescence energy transfer study of the relationship between the lone tryptophan residue and drug binding sites in human serum albumin. J. Pharm. Sci. 1987, 76, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer US: New York, NY, USA, 2006; pp. 45–135. [Google Scholar]
- Kettle, A.J.; Albrett, A.M.; Chapman, A.L.; Dickerhof, N.; Forbes, L.V.; Khalilova, I.; Turner, R. Measuring chlorine bleach in biology and medicine. Biochim. Biophys. Acta 2014, 1840, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Padovan, C.Z.; Carvalho, D.A.; Fernandes, J.R. Oxidation of melatonin by taurine chloramine. J. Pineal Res. 2010, 49, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, R.; Hoogland, H.; Wever, R.; Weiss, S.J. Singlet oxygen production by human eosinophils. J. Biol. Chem. 1988, 263, 9692–9696. [Google Scholar] [PubMed]
- Kładna, A.; Aboul-Enein, H.Y.; Kruk, I. Enhancing effect of melatonin on chemiluminescence accompanying decomposition of hydrogen peroxide in the presence of copper. Free Radic. Biol. Med. 2003, 34, 1544–1554. [Google Scholar] [CrossRef]
- De Almeida, E.A.; Martinez, G.R.; Klitzke, C.F.; de Medeiros, M.H.; Di Mascio, P. Oxidation of melatonin by singlet molecular oxygen (O2(1deltag)) produces N1-acetyl-N2-formyl-5-methoxykynurenine. J. Pineal Res. 2003, 35, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Spalteholz, H.; Panasenko, O.M.; Arnhold, J. Formation of reactive halide species by myeloperoxidase and eosinophil peroxidase. Arch. Biochem. Biophys. 2006, 445, 225–324. [Google Scholar] [CrossRef] [PubMed]
- Pitt, A.R.; Spickett, C.M. Mass spectrometric analysis of HOCl− and free-radical-induced damage to lipids and proteins. Biochem. Soc. Trans. 2008, 36, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Stanley, N.R.; Pattison, D.I.; Hawkins, C.L. Ability of hypochlorous acid and N-chloramines to chlorinate DNA and its constituents. Chem. Res. Toxicol. 2010, 23, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Sassa, A.; Kamoshita, N.; Matsuda, T.; Ishii, Y.; Kuraoka, I.; Nohmi, T.; Ohta, T.; Honma, M.; Yasui, M. Miscoding properties of 8-chloro-2′-deoxyguanosine, a hypochlorous acid-induced DNA adduct, catalysed by human DNA polymerases. Mutagenesis 2013, 28, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sassa, A.; Ohta, T.; Nohmi, T.; Honma, M.; Yasui, M. Mutational specificities of brominated DNA adducts catalyzed by human DNA polymerases. J. Mol. Biol. 2011, 406, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Winterbourn, C.C. Assays for the Chlorination Activity of Myeloperoxidase. Methods Enzymol. 1994, 233, 502–512. [Google Scholar] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Carvalho Bertozo, L.; Morgon, N.H.; De Souza, A.R.; Ximenes, V.F. Taurine Bromamine: Reactivity of an Endogenous and Exogenous Anti-Inflammatory and Antimicrobial Amino Acid Derivative. Biomolecules 2016, 6, 23. https://doi.org/10.3390/biom6020023
De Carvalho Bertozo L, Morgon NH, De Souza AR, Ximenes VF. Taurine Bromamine: Reactivity of an Endogenous and Exogenous Anti-Inflammatory and Antimicrobial Amino Acid Derivative. Biomolecules. 2016; 6(2):23. https://doi.org/10.3390/biom6020023
Chicago/Turabian StyleDe Carvalho Bertozo, Luiza, Nelson Henrique Morgon, Aguinaldo Robinson De Souza, and Valdecir Farias Ximenes. 2016. "Taurine Bromamine: Reactivity of an Endogenous and Exogenous Anti-Inflammatory and Antimicrobial Amino Acid Derivative" Biomolecules 6, no. 2: 23. https://doi.org/10.3390/biom6020023





