Analysis of Guanine Oxidation Products in Double-Stranded DNA and Proposed Guanine Oxidation Pathways in Single-Stranded, Double-Stranded or Quadruplex DNA
Abstract
:1. Introduction
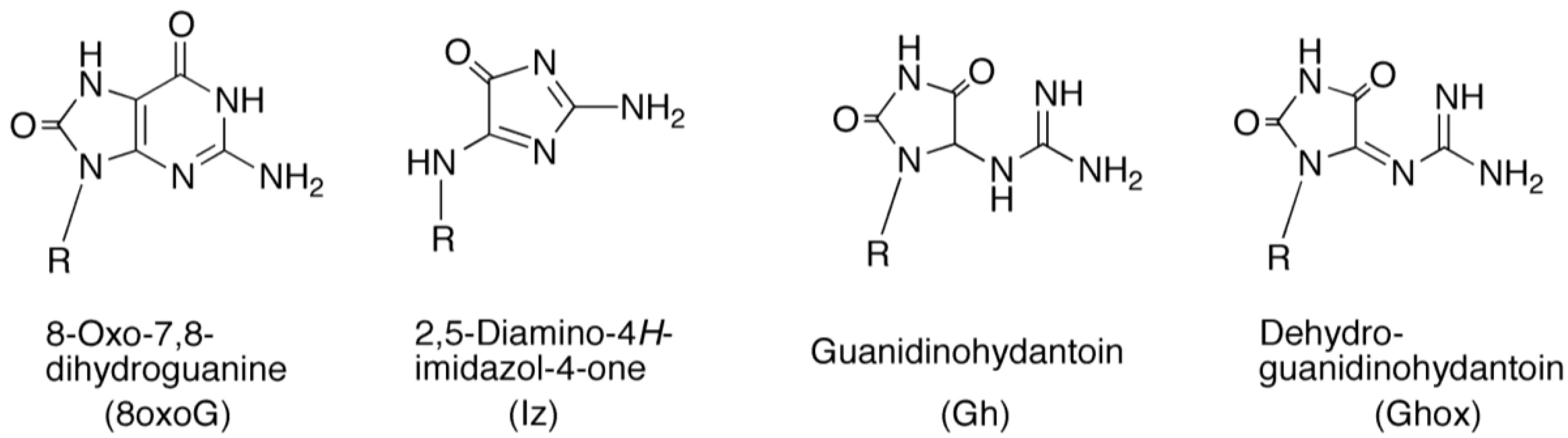
2. Results and Discussion
2.1. Formation of Double-Stranded DNA
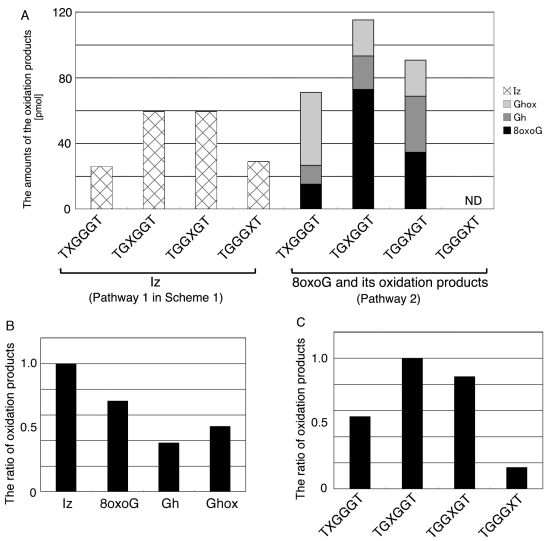
2.2. Identification of Guanine Oxidation Products in Double-Stranded DNA
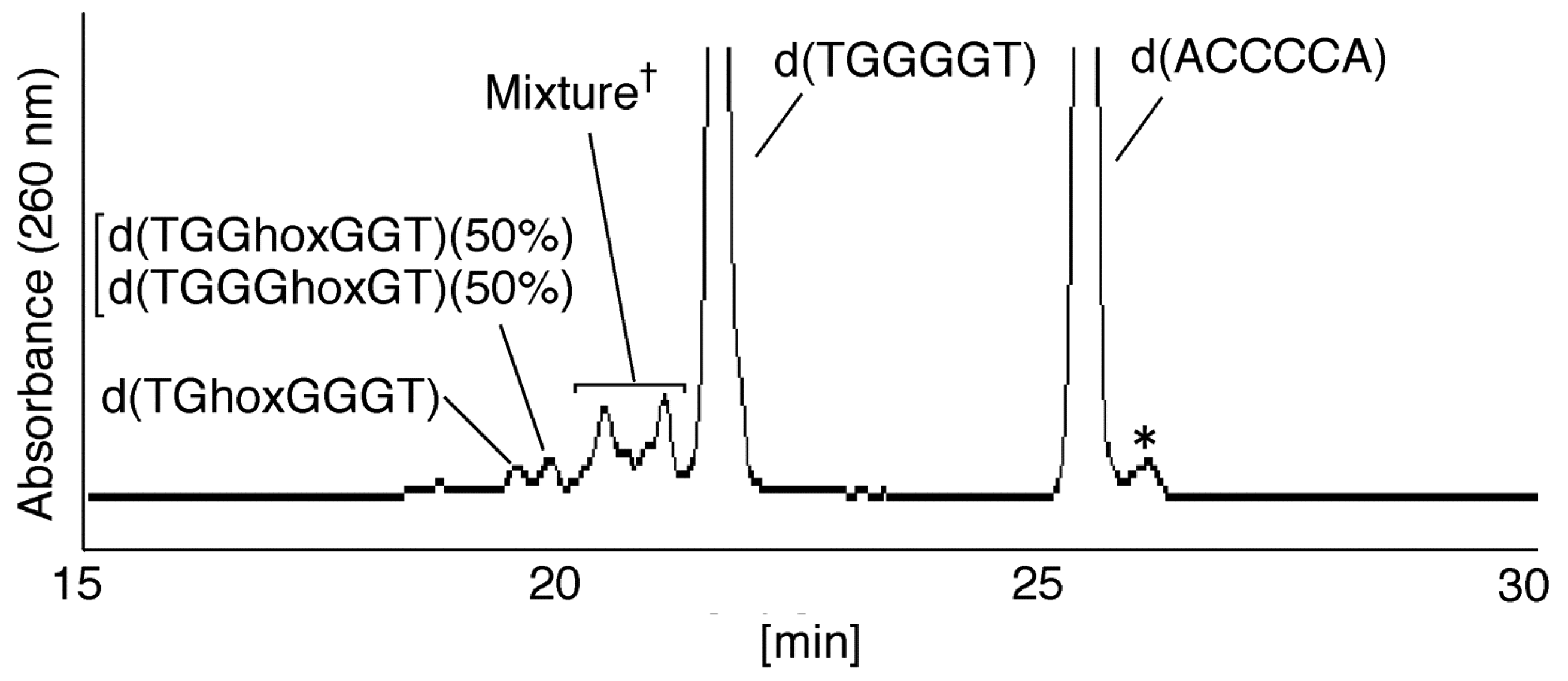
2.2.1. Isolation and Identification of Oligomers Containing Ghox
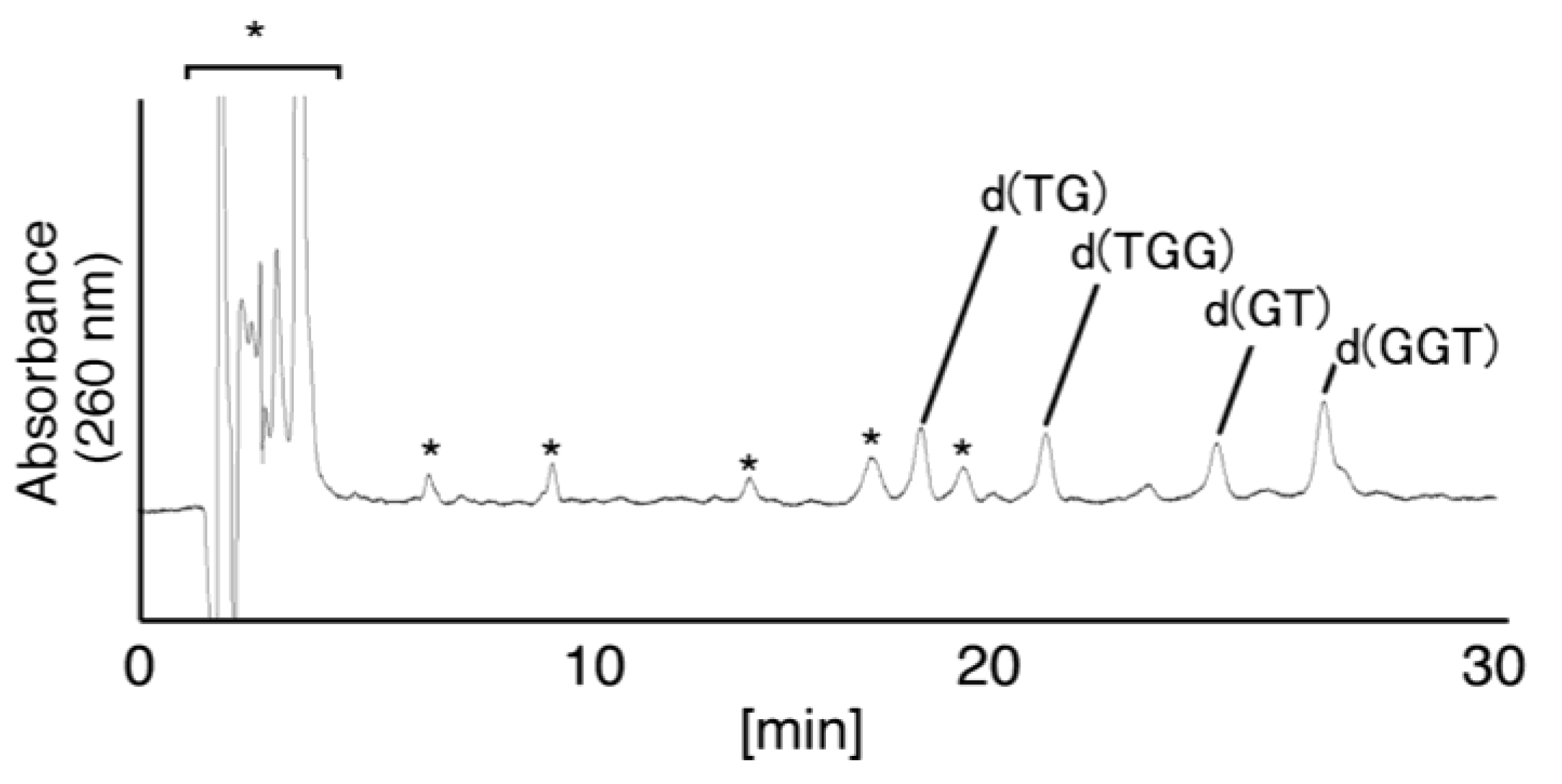
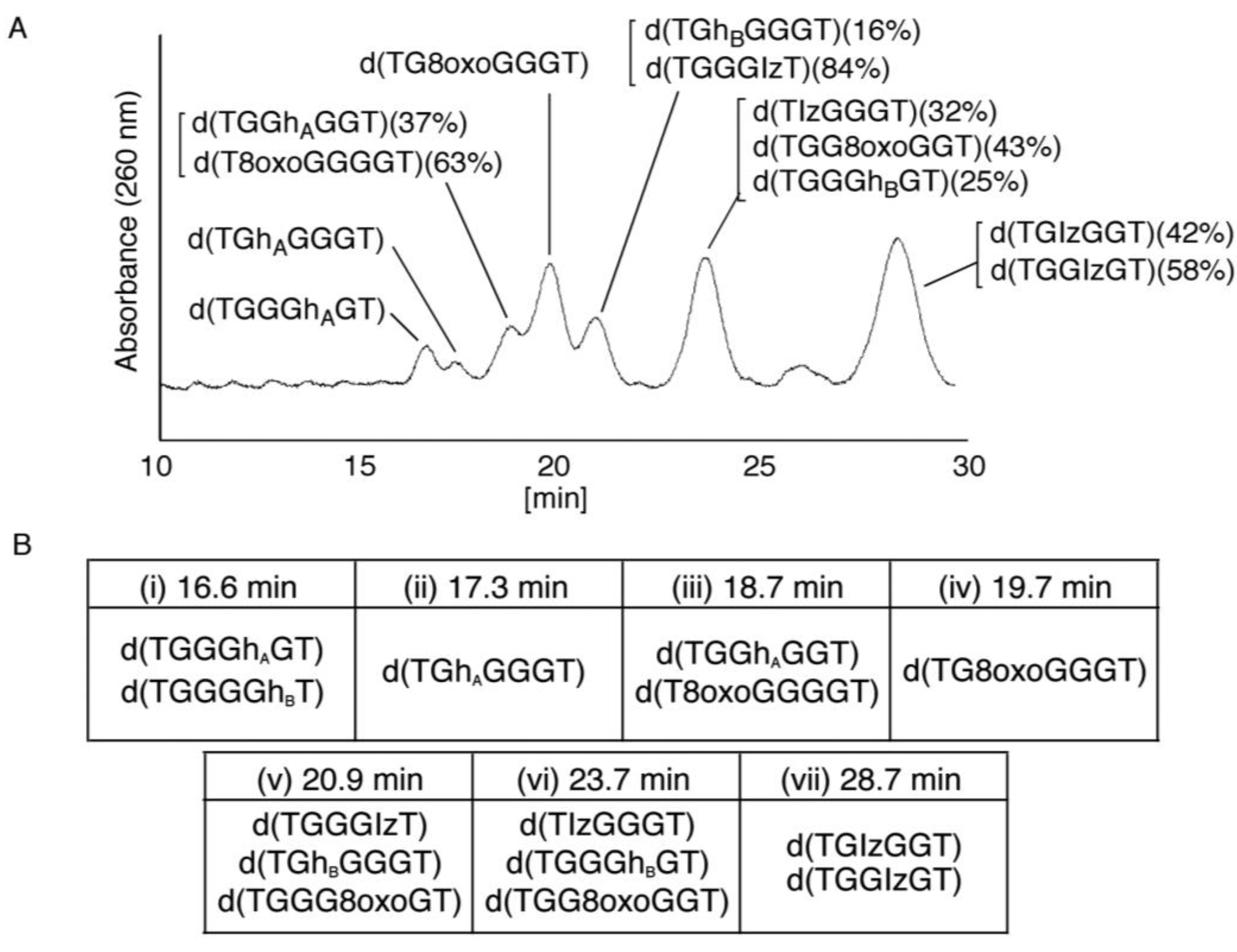
2.2.2. Isolation and Identification of Oligomers Containing Iz, Gh or 8oxoG
- (i)
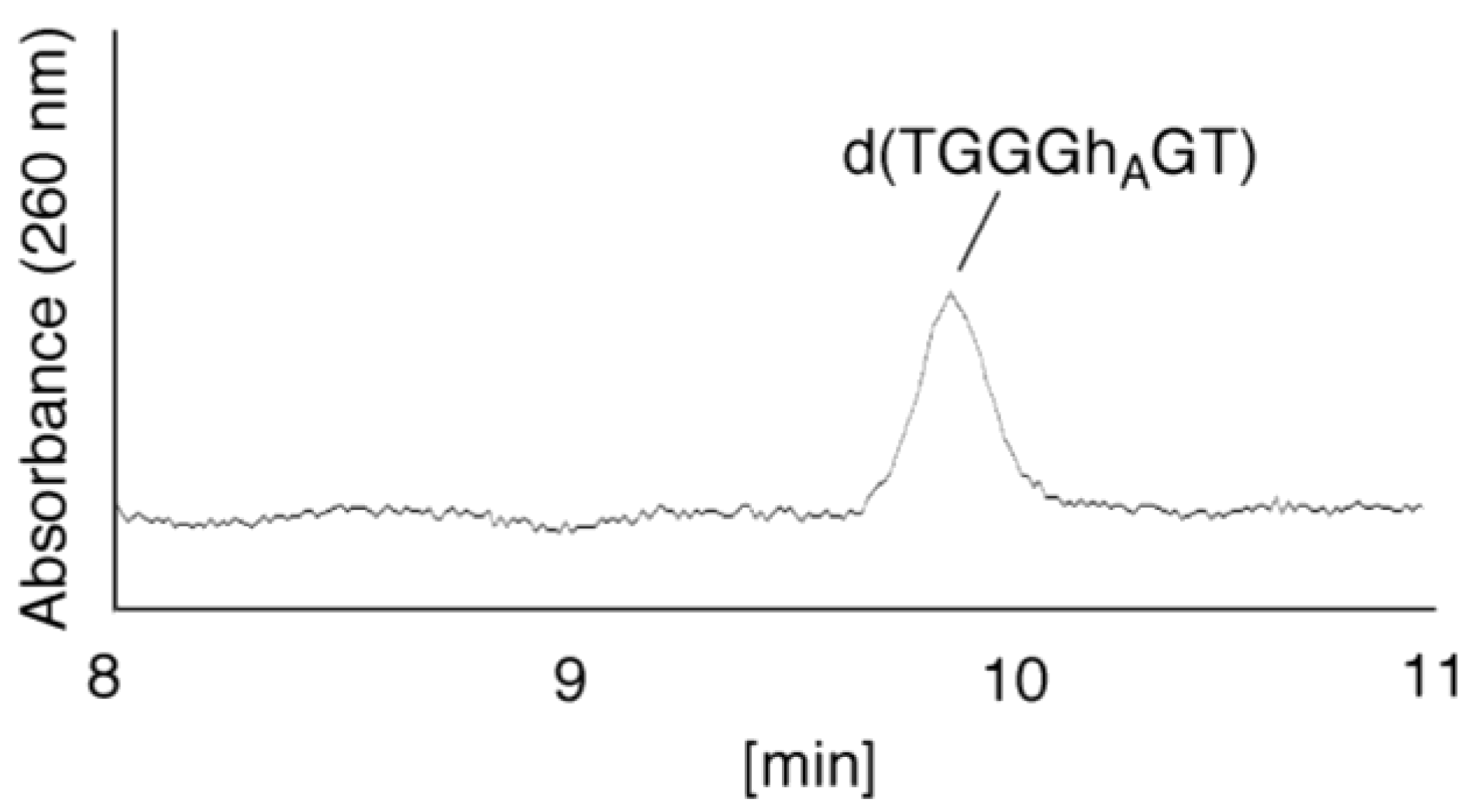
- Since the retention times of the standards d(TGGGhGT) and d(TGGGGhT) were identified as 16.6 min (Figure 7B), it was surmised that the peak at 16.6 min in Figure 7A contains d(TGGGhGT) and/or d(TGGGGhT). To distinguish d(TGGGhGT) from d(TGGGGhT), HPLC analysis using modified conditions (linear gradient of 4%–10% CH3CN/30 min in Figure 8) was performed, and the retention times of the standard samples d(TGGGGhT) and d(TGGGhGT) were identified as 9.3 and 9.7 min (data not shown). The 16.6 min peak in Figure 7A was isolated and reanalyzed using the HPLC conditions of Figure 8, which revealed that the 16.6 min peak in Figure 7A produced a single peak at 9.7 min. Thus, the peak at 16.6 min in Figure 7A contains only d(TGGGhGT).
- (ii)
- (iii)
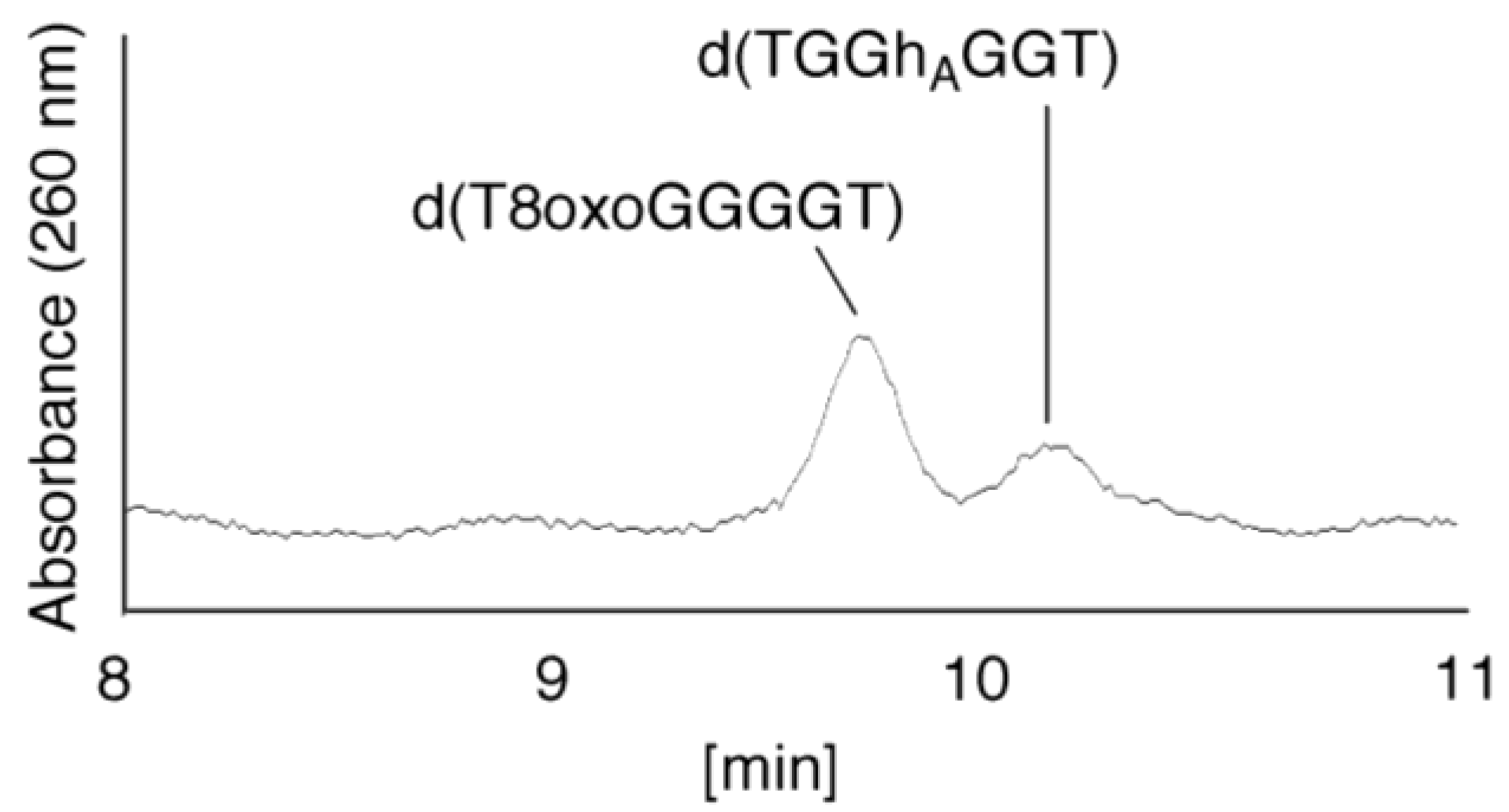
- Since the retention times of the standards d(T8oxoGGGGT) and d(TGGhGGT) (isocratic flow using 4% CH3CN/30 min) were both found to be 18.7 min (Figure 7B), the peak at 18.7 min in Figure 7A contains d(T8oxoGGGGT) and/or d(TGGhGGT). To distinguish d(T8oxoGGGGT) and d(TGGhGGT), modified HPLC conditions (linear gradient of 4%–10% CH3CN/30 min in Figure 9) were used to determine the retention times of the standards d(T8oxoGGGGT) and d(TGGhGGT), which were found to be 9.8 and 10.2 min, respectively. The peak at 18.7 min in Figure 7A was isolated and reanalyzed using HPLC (linear gradient of 4%–10% CH3CN/30 min) (Figure 9) and two peaks were detected at 9.8 and 10.2 min. The ratio of the peak areas at 9.8 and 10.2 min in Figure 9 was determined as 63:37. Thus, the peak at 18.7 min in Figure 7A contains d(T8oxoGGGGT) (63%) and d(TGGhGGT) (37%).
- (iv)
- (v)
- Since the retention times of the standards d(TGGGIzT), d(TGhGGGT), and d(TGGG8oxoGT) (isocratic flow using 4% CH3CN/30 min) were all 20.9 min (Figure 7B), the peak at 20.9 min in Figure 7A was presumed to contain d(TGGGIzT), d(TGhGGGT), and/or d(TGGG8oxoGT). Unfortunately, HPLC conditions that enabled separation of these three products were not found. Since HPLC with electrochemical detection (HPLC-ECD) can specifically detect oligomers containing 8oxoG, the peak at 20.9 min in Figure 7A was analyzed using this method. However, oligomers containing 8oxoG were not detected.
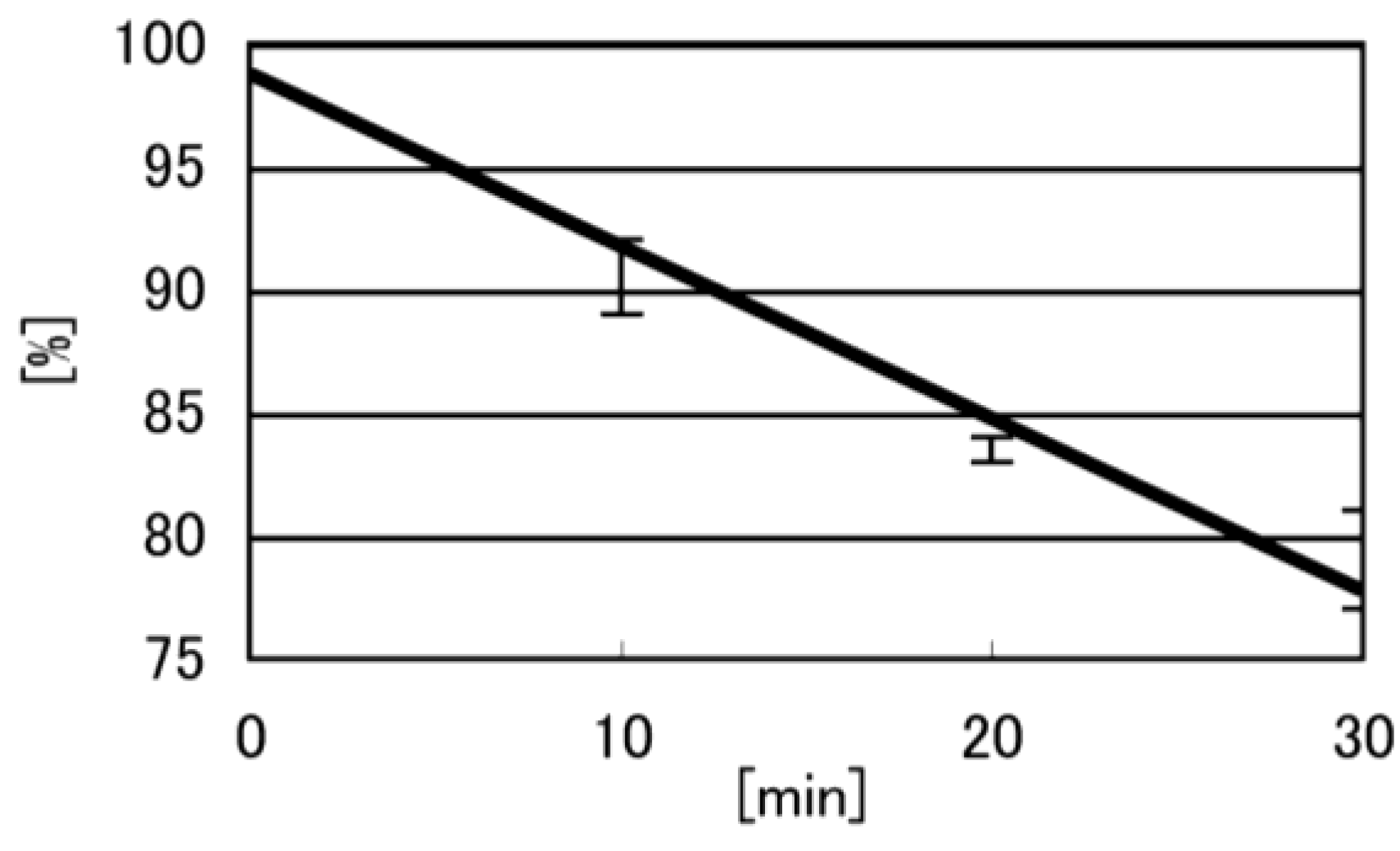
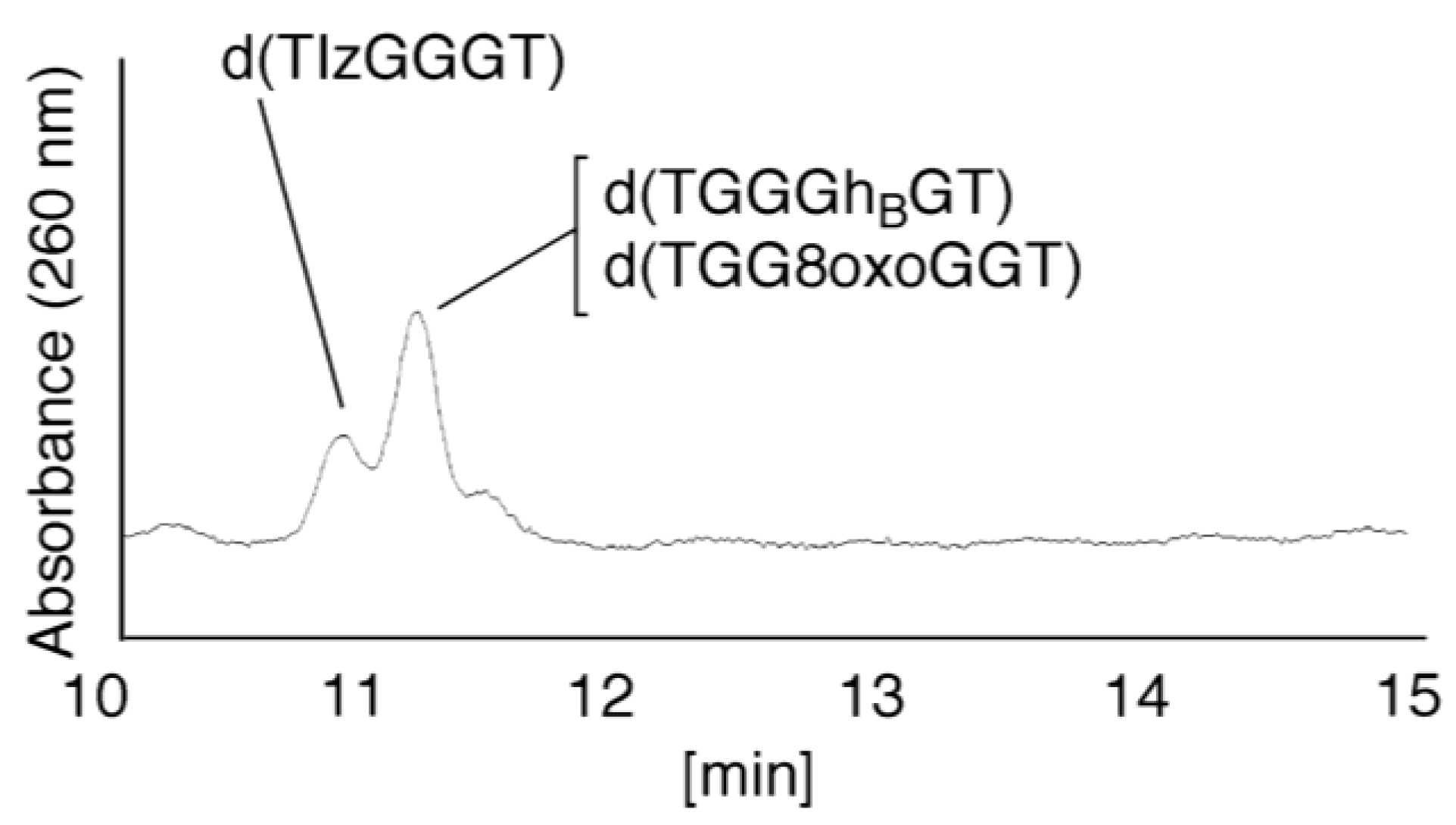
- Iz is degraded to 2,2,4-triamino-5(2H)-oxazolone (Oz) [27,28,29], while Gh and 8oxoG are the stable products [30,31]. A standard oligomer containing Iz was hardly detected in a solution heated to 80 °C for 60 min. Therefore, d(TGGGIzT) in the mixture was degraded and the amount of d(TGGGIzT) could be determined by measuring peak area loss. The peak at 20.9 min in Figure 7A was isolated and heated to 80 °C for 60 min. This resulted in the peak area decreasing to 16% of the original (Figure 10). Hence, the peak at 20.9 min in Figure 7A was determined to be d(TGhGGGT) (16%) and d(TGGGIzT) (84%).
- (vi)
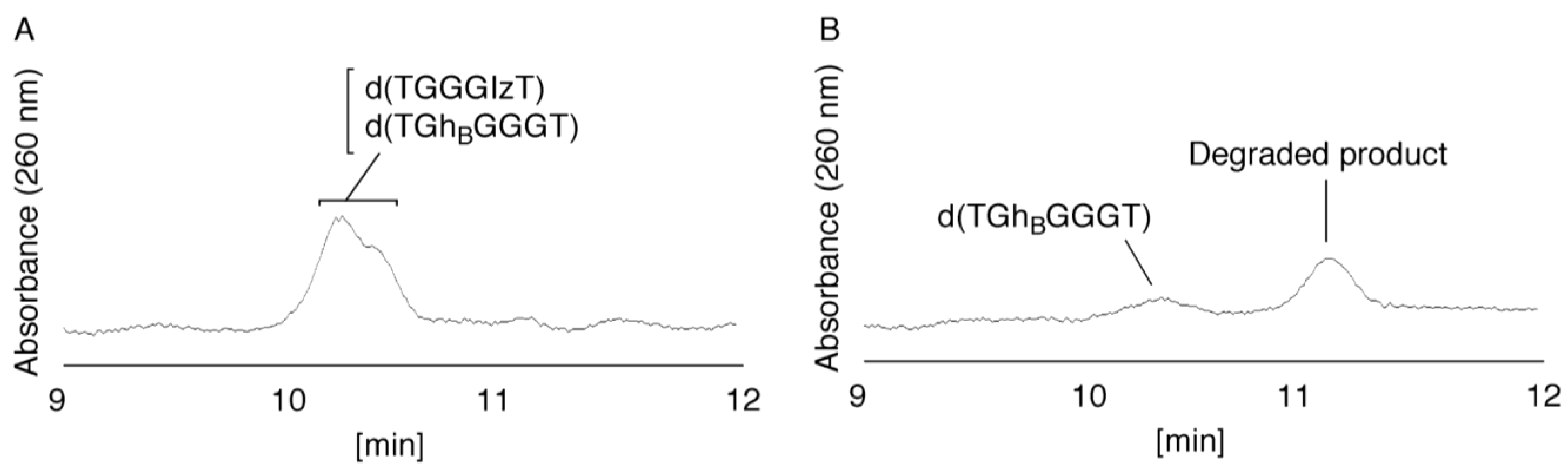
- Since the retention times of the standards d(TIzGGGT), d(TGGGhGT), and d(TGG8oxoGGT) (isocratic flow using 4% CH3CN/30 min) were identified as 23.7 min (Figure 7B), it was surmised that the peak at 23.7 min in Figure 7A contains d(TIzGGGT), d(TGGGhGT), and/or d(TGG8oxoGGT). Therefore, HPLC analysis using modified HPLC conditions (linear gradient of 4%–10% of CH3CN/30 min in Figure 11) was performed to separate the three products. The retention times of d(TIzGGGT), d(TGGGhGT), and d(TGG8oxoGGT) were identified as 10.9, 11.2, and 11.2 min under these conditions. The peak at 23.7 min in Figure 7A was isolated and reanalyzed using HPLC with a linear gradient of 4%–10% CH3CN/30 min (Figure 11). This resulted in peaks being detected at 10.9 and 11.2 min and the ratio of these peak areas were determined to be 32:68, respectively. Therefore, d(TIzGGGT) accounts for 32% of the peak area at 23.7 min in Figure 7A.
- Unfortunately, d(TGGGhGT) and d(TGG8oxoGGT) could not be separated using HPLC. The peak at 23.7 min in Figure 7A was then analyzed using HPLC-ECD. This analysis revealed that the oligomer containing 8oxoG accounts for 43% of the peak area at 23.7 min in Figure 7A. Hence, it was determined that the peak at 23.7 min in Figure 7A contained d(TIzGGGT) (32%), d(TGGGhGT) (25%), and d(TGG8oxoGGT) (43%).
- (vii)
- Since the retention times of the standards d(TGIzGGT) and d(TGGIzGT) (isocratic flow using 4% CH3CN/30 min) were both 28.7 min (Figure 7B), the peak at 28.7 min in Figure 7A contains d(TGIzGGT) and/or d(TGGIzGT). Therefore, the location of Iz in the oligomer at 28.7 min in Figure 7A was identified by piperidine treatment, as previously reported (Figure 12) [25,32]. As a result, the peak at 28.7 min in Figure 7A was identified as containing d(TGIzGGT) (42%) and d(TGGIzGT) (58%).
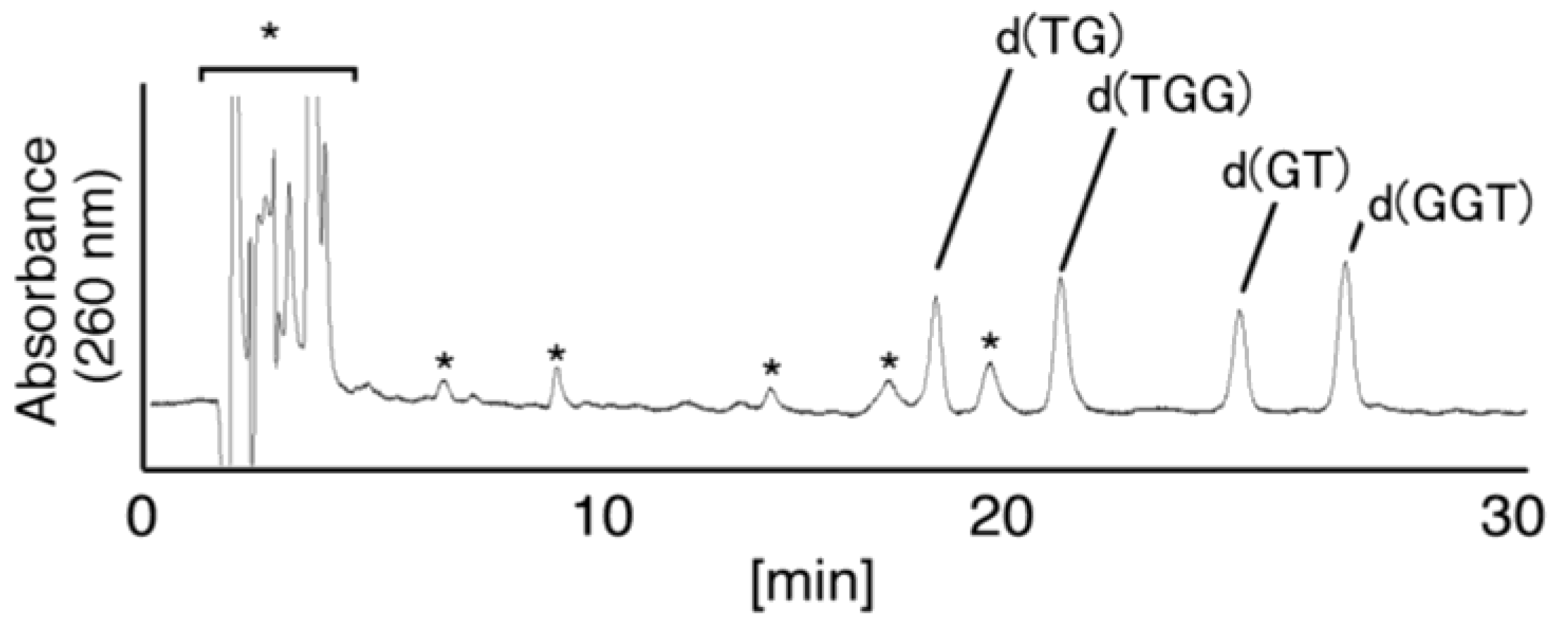

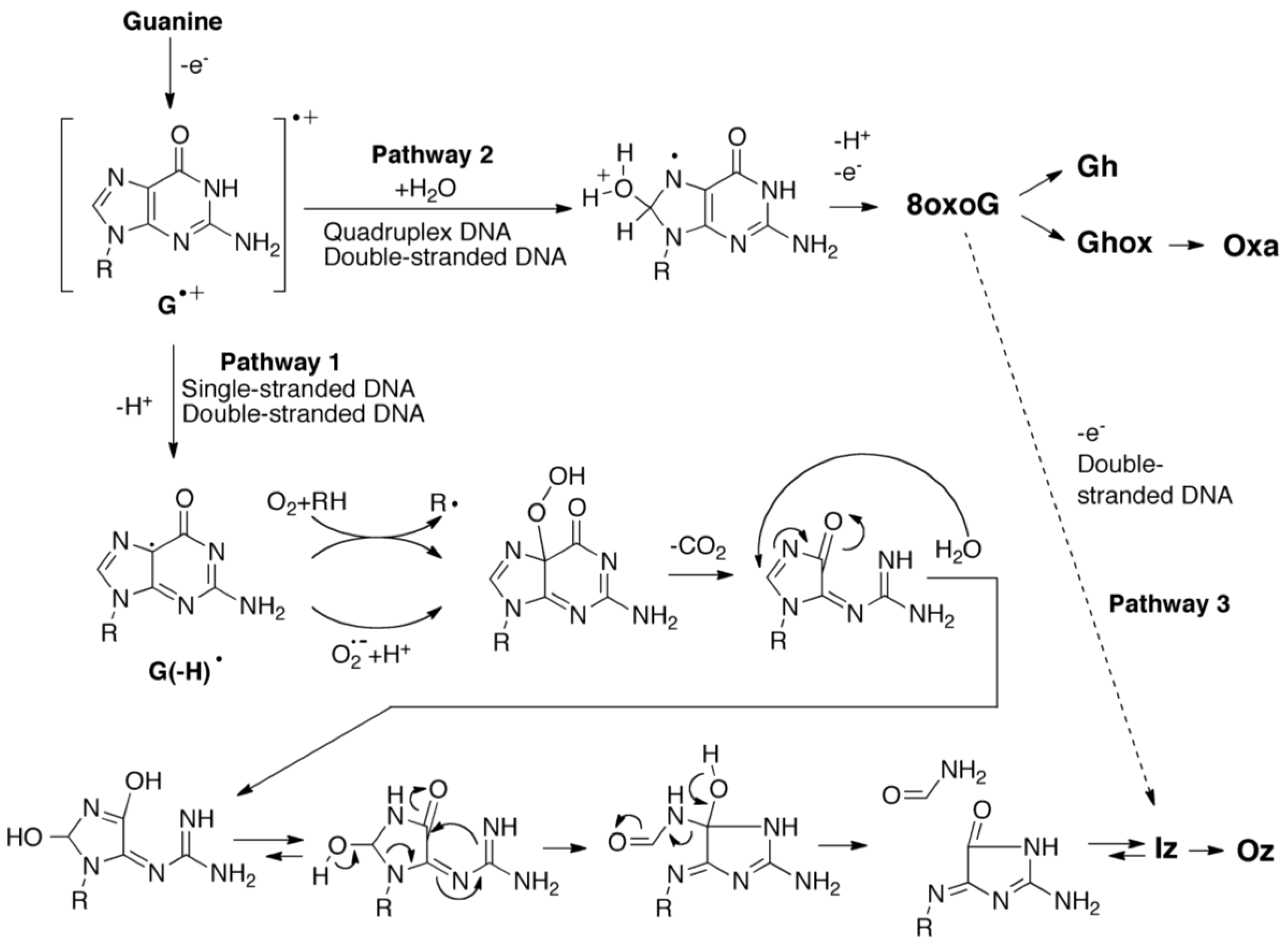
2.3. Proposed Guanine Oxidation Pathways
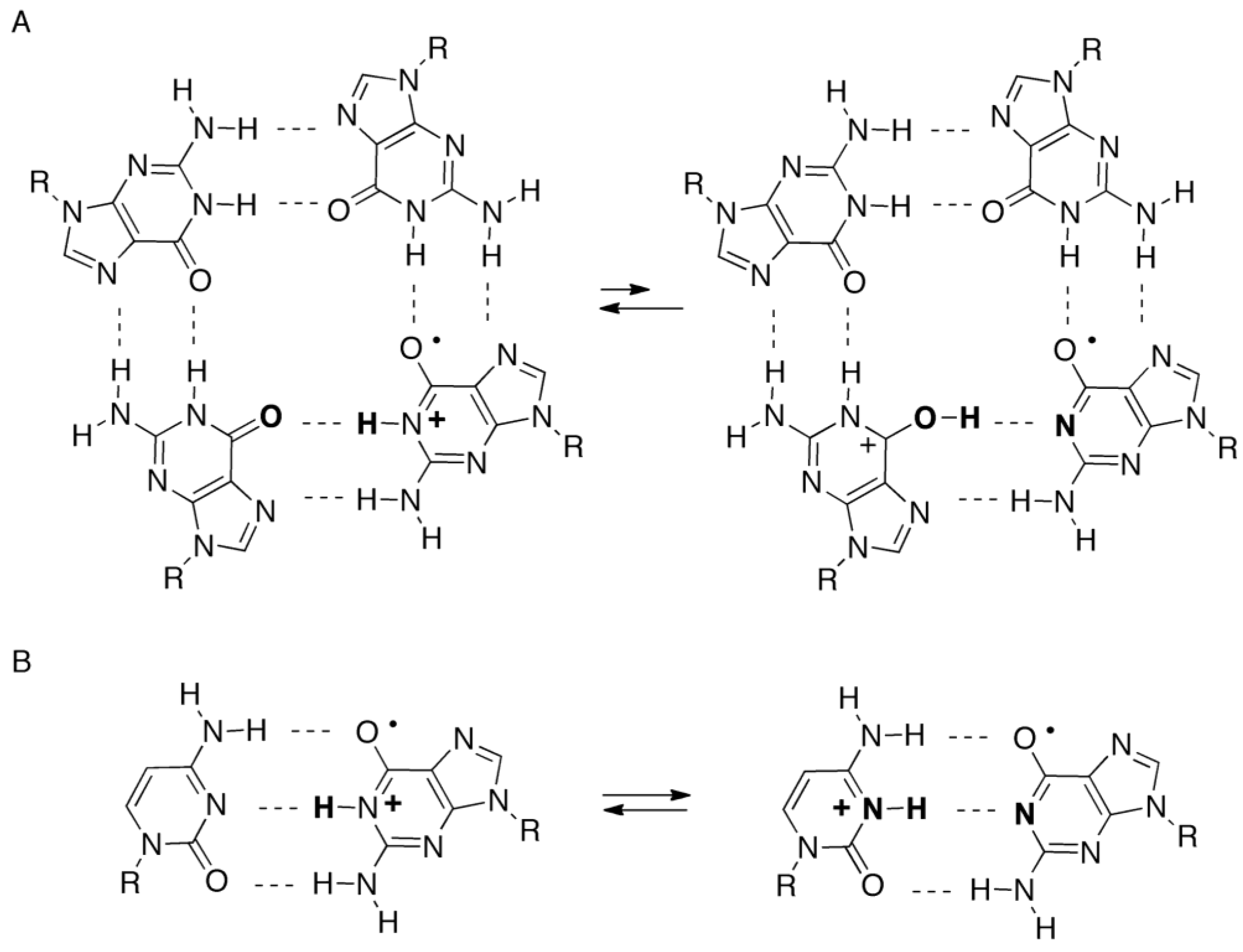
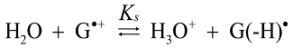
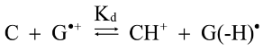
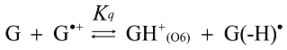
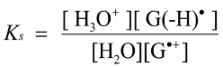
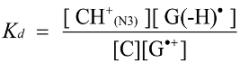
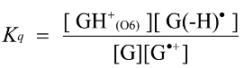
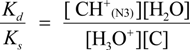
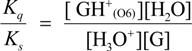
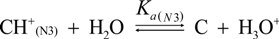
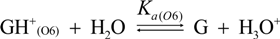
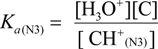
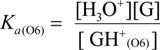
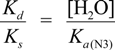
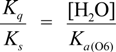
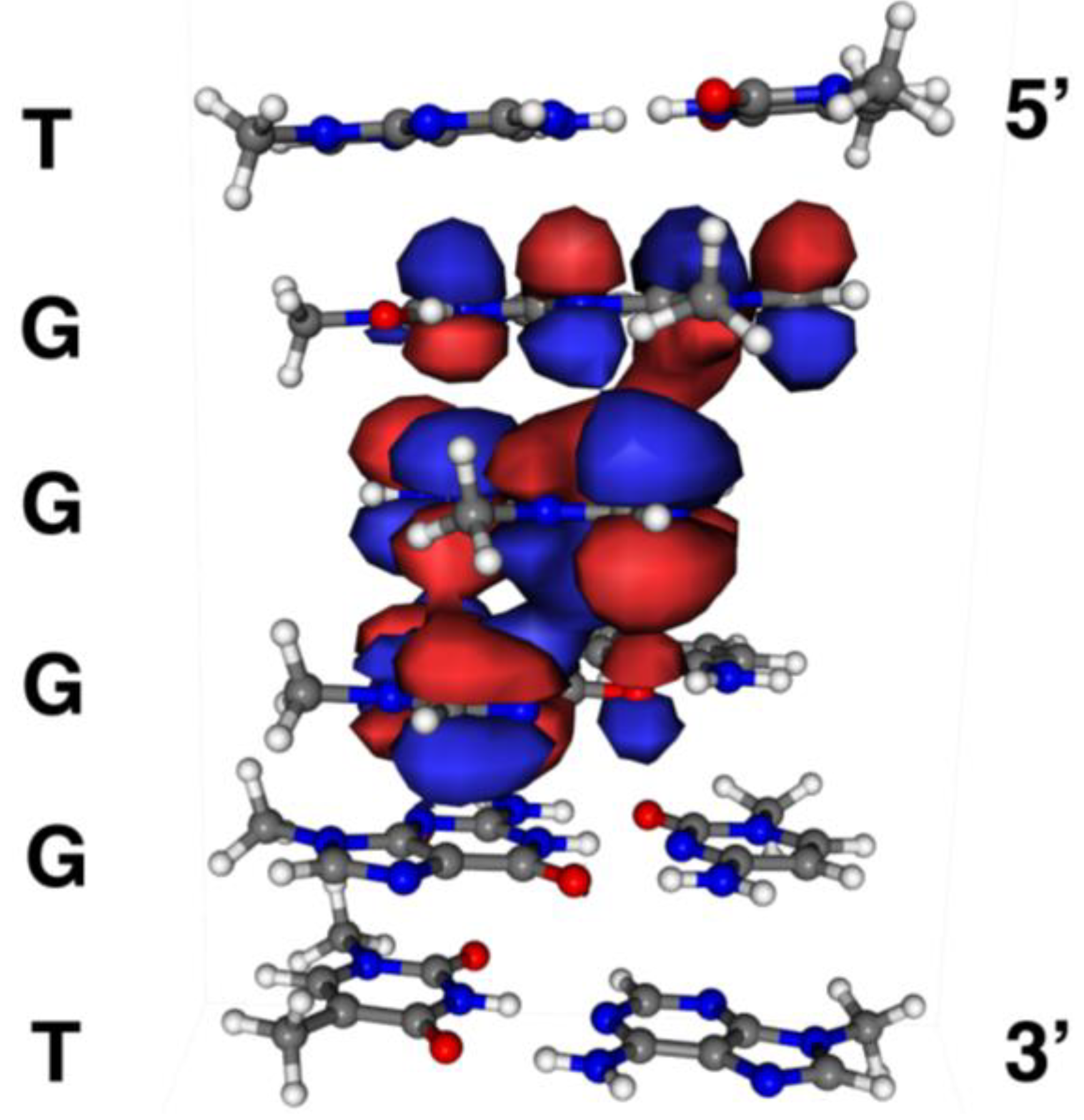
2.4. Formation of Iz by One-Electron Oxidation of Double-Stranded DNA
2.5. Formation of 8oxoG in Quadruplex DNA and Its Effects
2.6. Localization of Guanine Oxidation Products
3. Experimental Section
3.1. Materials
3.2. Analysis of Oxidation Reactions
3.3. Calculation of the Highest Occupied Molecular Orbital (HOMO)
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Kreutzer, D.A.; Essigmann, J.M. Mutagenicity and repair of oxidative DNA damage: Insights from studies using defined lesions. Mutat. Res. 1998, 400, 99–115. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.-L. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat. Res. 2003, 531, 5–23. [Google Scholar] [CrossRef]
- Barnes, D.E.; Lindahl, T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu. Rev. Genet. 2004, 38, 445–476. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Sakumi, K.; Sakamoto, K.; Tsuchimoto, D.; Tsuzuki, T.; Nakatsu, Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol. Chem. 2006, 387, 373–379. [Google Scholar]
- Wilson, D.M., III; Bohr, V.A. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair 2007, 6, 544–559. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Feig, D.I.; Loeb, L.A. Oxygen radical induced mutagenesis is DNA polymerase specific. J. Mol. Biol. 1994, 235, 33–41. [Google Scholar]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar]
- Steenken, S.; Jovanovic, S.V. How easily oxidizable is DNA? One-Electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Phillips, K.; Dauter, Z.; Murchie, A.I.H.; Lilley, D.M.J.; Luisi, B. The crystal structure of a parallel-stranded guanine tetraplex at 0.95 Å resolution. J. Mol. Biol. 1997, 273, 171–182. [Google Scholar]
- Haider, S.; Parkinson, G.N.; Neidle, S. Crystal structure of the potassium form of an Oxytricha nova G-quadruplex. J. Mol. Biol. 2002, 320, 189–200. [Google Scholar] [CrossRef]
- Parkinson, G.N.; Lee, M.P.H.; Neidle, S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature 2002, 417, 876–880. [Google Scholar] [CrossRef]
- Xu, Y.; Sugiyama, H. Highly efficient photochemical 2’-deoxyribonolactone formation at the diagonal loop of a 5-iodouracil-containing antiparallel G-quartet. J. Am. Chem. Soc. 2004, 126, 6274–6279. [Google Scholar] [CrossRef]
- Simonsson, T.; Pecinka, P.; Kubista, M. DNA tetraplex formation in the control region of c-myc. Nucleic Acids Res. 1998, 26, 1167–1172. [Google Scholar] [CrossRef]
- Phan, A.T.; Modi, Y.S.; Patel, D.J. Propeller-type parallel-stranded G-quadruplexes in the human c-myc promoter. J. Am. Chem. Soc. 2004, 126, 8710–8716. [Google Scholar] [CrossRef]
- Aboul-Ela, F.; Murchie, A.I.H.; Lilley, D.M.J. NMR study of parallel-stranded tetraplex formation by the hexadeoxynucleotide d(TG4T). Nature 1992, 360, 280–282. [Google Scholar] [CrossRef]
- Morikawa, M.; Kino, K.; Oyoshi, T.; Suzuki, M.; Kobayashi, T.; Miyazawa, H. Product analysis of photooxidation in isolated quadruplex DNA; 8-oxo-7,8-dihydroguanine and its oxidation product at 3’-G are formed instead of 2,5-diamino-4H-imidazol-4-one. RSC Adv. 2013, 3, 25694–25697. [Google Scholar]
- Xu, Y.; Tashiro, R.; Sugiyama, H. Photochemical determination of different DNA structures. Nat. Protoc. 2007, 2, 78–87. [Google Scholar]
- Xu, Y. Chemistry in human telomere biology: Structure, function and targeting of telomere DNA/RNA. Chem. Soc. Rev. 2011, 40, 2719–2740. [Google Scholar] [CrossRef]
- Fleming, A.M.; Orendt, A.M.; He, Y.; Zhu, J.; Dukor, R.K.; Burrows, C.J. Reconciliation of chemical, enzymatic, spectroscopic and computational data to assign the absolute configuration of the DNA base lesion spiroiminodihydantoin. J. Am. Chem. Soc. 2013, 135, 18191–18204. [Google Scholar]
- Kypr, J.; Kejnovská, I.; Renciuk, D.; Vorlícková, M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713–1725. [Google Scholar] [CrossRef]
- OligoAnalyzer 3.1. Available online: http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/ (accssed on 17 January 2014).
- Tashiro, R.; Ohtsuki, A.; Sugiyama, H. The distance between donor and acceptor affects the proportion of C1’ and C2’ oxidation products of DNA in a BrU-containing excess electron transfer system. J. Am. Chem. Soc. 2010, 132, 14361–14363. [Google Scholar] [CrossRef]
- Mourgues, S.; Trzcionka, J.; Vasseur, J.J.; Pratviel, G.; Meunier, B. Incorporation of oxidized guanine nucleoside 5’-triphosphates in DNA with DNA polymerases and preparation of single-lesion carrying DNA. Biochemistry 2008, 47, 4788–4799. [Google Scholar]
- Kino, K.; Saito, I.; Sugiyama, H. Product analysis of GG-specific photooxidation of DNA via electron transfer: 2-aminoimidazolone as a major guanine oxidation product. J. Am. Chem. Soc. 1998, 120, 7373–7374. [Google Scholar]
- Gremaud, J.N.; Martin, B.D.; Sugden, K.D. Influence of substrate complexity on the diastereoselective formation of spiroiminodihydantoin and guanidinohydantoin from chromate oxidation. Chem. Res. Toxicol. 2010, 23, 379–385. [Google Scholar] [CrossRef]
- Cadet, J.; Berger, M.; Buchko, G.W.; Joshi, P.C.; Raoul, S.; Ravanat, J.-L. 2,2-Diamino-4-[(3,5-di-O-acetyl-2-deoxy-β-d-erythro-pentofuranosyl)amino]-5-(2H)-oxazolone: A novel and predominant radical oxidation product of 3’,5’-Di-O-acetyl-2’-deoxyguanosine. J. Am. Chem. Soc. 1994, 116, 7403–7404. [Google Scholar] [CrossRef]
- Kino, K.; Sugasawa, K.; Mizuno, T.; Bando, T.; Sugiyama, H.; Akita, M.; Miyazawa, H.; Hanaoka, F. Eukaryotic DNA polymerases α, β and ε incorporate guanine opposite 2,2,4-triamino-5(2H)-oxazolone. ChemBioChem 2009, 10, 2613–2616. [Google Scholar]
- Kino, K.; Morikawa, M.; Kobayashi, T.; Kobayashi, T.; Komori, R.; Sei, Y.; Miyazawa, H. The oxidation of 8-oxo-7,8-dihydroguanine by iodine. Bioorg. Med. Chem. Lett. 2010, 20, 3818–3820. [Google Scholar]
- Luo, W.; Muller, J.G.; Rachlin, E.M.; Burrows, C.J. Characterization of spiroiminodihydantoin as a product of one-electron oxidation of 8-oxo-7,8-dihydroguanosine. Org. Lett. 2000, 2, 613–616. [Google Scholar] [CrossRef]
- Hu, C.-W.; Chao, M.-R.; Sie, C.-H. Urinary analysis of 8-oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanosine by isotope-dilution LC-MS/MS with automated solid-phase extraction: Study of 8-oxo-7,8-dihydroguanine stability. Free Radic. Biol. Med. 2010, 48, 89–97. [Google Scholar] [CrossRef]
- Tretyakova, N.Y.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-induced secondary oxidative lesions at guanine nucleobases: Chemical stability and recognition by the Fpg DNA repair enzyme. Chem. Res. Toxicol. 2000, 13, 658–664. [Google Scholar] [CrossRef]
- García, N.A.; Criado, S.N.; Massad, W.A. Riboflavin as a Visible-Light-Sensitiser in the Aerobic Photodegradation of Ophthalmic and Sympathomimetic Drugs. In Flavins: Photochemistry and Photobilogy; Silva, E., Edwards, A.M., Eds.; RSC Publishing: Cambridge, UK, 2006; pp. 61–82. [Google Scholar]
- Chacon, J.N.; McLearie, J.; Sinclair, R.S. Singlet oxygen yields and radical contributions in the dye-sensitised photo-oxidation in methanol of esters of polyunsaturated fatty acids (oleic, linoleic, linolenic and arachidonic). Photochem. Photobiol. 1988, 47, 647–656. [Google Scholar]
- Kino, K.; Kobayashi, T.; Arima, E.; Komori, R.; Kobayashi, T.; Miyazawa, H. Photoirradiation products of flavin derivatives, and the effects of photooxidation on guanine. Bioorg. Med. Chem. Lett. 2009, 19, 2070–2074. [Google Scholar]
- Cadet, J.; Douki, T.; Ravanat, J.L. One-electron oxidation of DNA and inflammation processes. Nat. Chem. Biol. 2006, 2, 348–349. [Google Scholar]
- Kobayashi, K.; Tagawa, S. Direct observation of guanine radical cation deprotonation in duplex DNA using pulse radiolysis. J. Am. Chem. Soc. 2003, 125, 10213–10218. [Google Scholar]
- Misiaszek, R.; Crean, C.; Joffe, A.; Geacintov, N.E.; Shafirovich, V. Oxidative DNA damage associated with combination of guanine and superoxide radicals and repair mechanisms via radical trapping. J. Biol. Chem. 2004, 279, 32106–32115. [Google Scholar]
- Osakada, Y.; Kawai, K.; Tachikawa, T.; Fujitsuka, M.; Tainaka, K.; Tero-Kubota, S.; Majima, T. Generation of singlet oxygen during photosensitized one-electron oxidation of DNA. Chem. Eur. J. 2012, 18, 1060–1063. [Google Scholar]
- Vialas, C.; Pratviel, G.; Claparols, C.; Meunier, B. Efficient oxidation of 2’-deoxyguanosine by Mn-TMPyP/KHSO5 to imidazolone dIz without formation of 8-oxo-dG. J. Am. Chem. Soc. 1998, 120, 11548–11553. [Google Scholar]
- Steenken, S. Purine bases, nucleosides, and nucleotides: Aqueous solution redox chemistry and transformation reactions of their radical cations and e− and OH adducts. Chem. Rev. 1989, 89, 503–520. [Google Scholar] [CrossRef]
- Verdolino, V.; Cammi, R.; Munk, B.H.; Schlegel, H.B. Calculation of pKa values of nucleobases and the guanine oxidation products guanidinohydantoin and spiroiminodihydantoin using density functional theory and a polarizable continuum model. J. Phys. Chem. B 2008, 112, 16860–16873. [Google Scholar] [CrossRef]
- Kino, K.; Sugiyama, H. Possible cause of G·C → C·G transversion mutation by guanine oxidation product, imidazolone. Chem. Biol. 2001, 8, 369–378. [Google Scholar] [CrossRef]
- Suzuki, M.; Kino, K.; Morikawa, M.; Kobayashi, T.; Komori, R.; Miyazawa, H. Calculation of the stabilization energies of oxidatively damaged guanine base pairs with guanine. Molecules 2012, 17, 6705–6715. [Google Scholar] [CrossRef]
- Kino, K.; Takao, M.; Miyazawa, H.; Hanaoka, F. A DNA oligomer containing 2,2,4-triamino-5(2H)-oxazolone is incised by human NEIL1 and NTH1. Mutat Res. 2012, 734, 73–77. [Google Scholar] [CrossRef]
- Luo, W.; Muller, J.G.; Rachlin, E.M.; Burrows, C.J. Characterization of hydantoin products from one-electron oxidation of 8-oxo-7,8-dihydroguanosine in a nucleoside model. Chem. Res. Toxicol. 2001, 14, 927–938. [Google Scholar] [CrossRef]
- Duarte, V.; Gasparutto, D.; Jaquinod, M.; Ravanat, J.-L.; Cadet, J. Repair and mutagenic potential of oxaluric acid, a major product of singlet oxygen-mediated oxidation of 8-oxo-7,8-dihydroguanine. Chem. Res. Toxicol. 2001, 14, 46–53. [Google Scholar] [CrossRef]
- Ito, K.; Inoue, S.; Yamamoto, K.; Kawanishi, S. 8-Hydroxydeoxyguanosine formation at the 5’ site of 5’-GG-3’ sequences in double-stranded DNA by UV radiation with riboflavin. J. Biol. Chem. 1993, 268, 13221–13227. [Google Scholar]
- Sugiyama, H.; Saito, I. Theoretical studies of GG-specific photocleavage of DNA via electron transfer: Significant lowering of ionization potential and 5'-localization of HOMO of stacked GG bases in B-form DNA. J. Am. Chem. Soc. 1996, 118, 7063–7068. [Google Scholar] [CrossRef]
- Ito, K.; Kawanishi, S. Photoinduced hydroxylation of deoxyguanosine in DNA by pterins: Sequence specificity and mechanism. Biochemistry 1997, 36, 1774–1781. [Google Scholar] [CrossRef]
- Maestro, Version 9.0.211, Schrödinger, LLC: New York, NY, USA, 2009.
- Frisch, M.J.; et al. Gaussian 03, Revision C.02; Gaussian Inc.: Wallingford, CT, USA, 1995. [Google Scholar]
- Varetto, U. Molekel 5.4.0.8, Swiss National Supercomputing Centre: Lugano, Switzerland, 2009.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morikawa, M.; Kino, K.; Oyoshi, T.; Suzuki, M.; Kobayashi, T.; Miyazawa, H. Analysis of Guanine Oxidation Products in Double-Stranded DNA and Proposed Guanine Oxidation Pathways in Single-Stranded, Double-Stranded or Quadruplex DNA. Biomolecules 2014, 4, 140-159. https://doi.org/10.3390/biom4010140
Morikawa M, Kino K, Oyoshi T, Suzuki M, Kobayashi T, Miyazawa H. Analysis of Guanine Oxidation Products in Double-Stranded DNA and Proposed Guanine Oxidation Pathways in Single-Stranded, Double-Stranded or Quadruplex DNA. Biomolecules. 2014; 4(1):140-159. https://doi.org/10.3390/biom4010140
Chicago/Turabian StyleMorikawa, Masayuki, Katsuhito Kino, Takanori Oyoshi, Masayo Suzuki, Takanobu Kobayashi, and Hiroshi Miyazawa. 2014. "Analysis of Guanine Oxidation Products in Double-Stranded DNA and Proposed Guanine Oxidation Pathways in Single-Stranded, Double-Stranded or Quadruplex DNA" Biomolecules 4, no. 1: 140-159. https://doi.org/10.3390/biom4010140
APA StyleMorikawa, M., Kino, K., Oyoshi, T., Suzuki, M., Kobayashi, T., & Miyazawa, H. (2014). Analysis of Guanine Oxidation Products in Double-Stranded DNA and Proposed Guanine Oxidation Pathways in Single-Stranded, Double-Stranded or Quadruplex DNA. Biomolecules, 4(1), 140-159. https://doi.org/10.3390/biom4010140