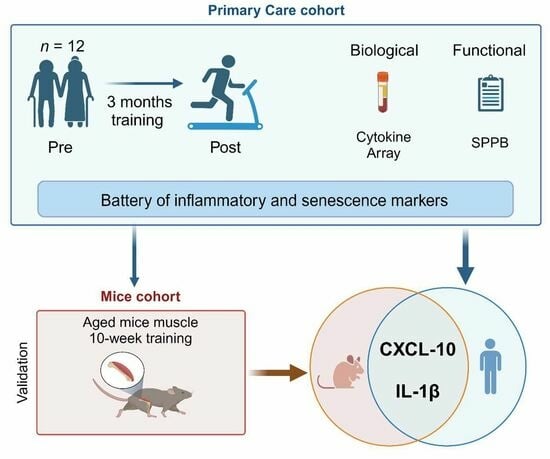
Physical Interventions Restore Physical Frailty and the Expression of CXCL-10 and IL-1β Inflammatory Biomarkers in Old Individuals and Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Care Setting-Enrolled Cohort
2.2. Animal Experimentation
2.3. RNA Extraction, cDNA Synthesis, and RT-qPCR
2.4. Human Circulating Inflammatory Markers
2.5. Statistical Analysis
3. Results
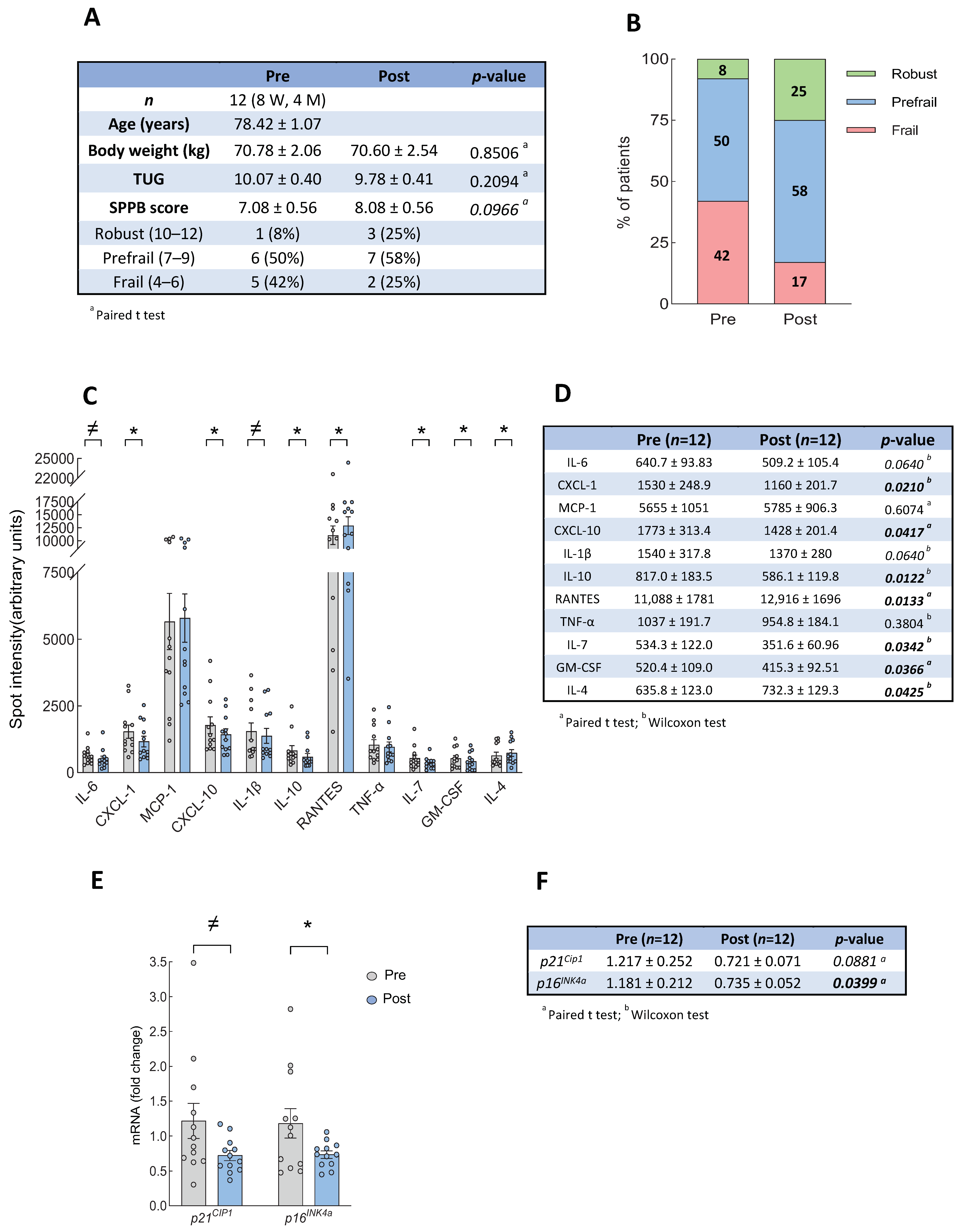
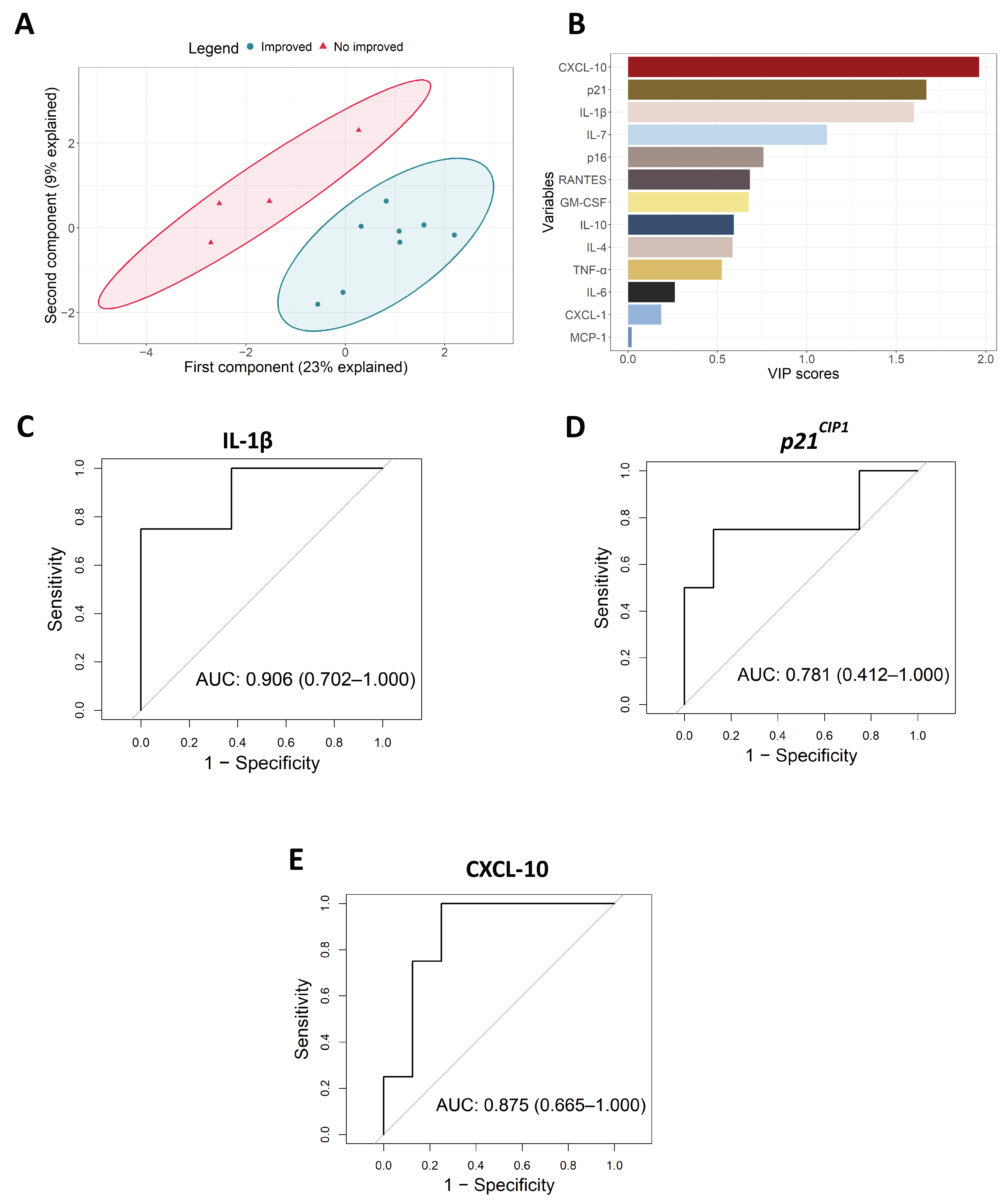
3.1. Intervention with Physical Exercise on Community Dwelling Older Persons
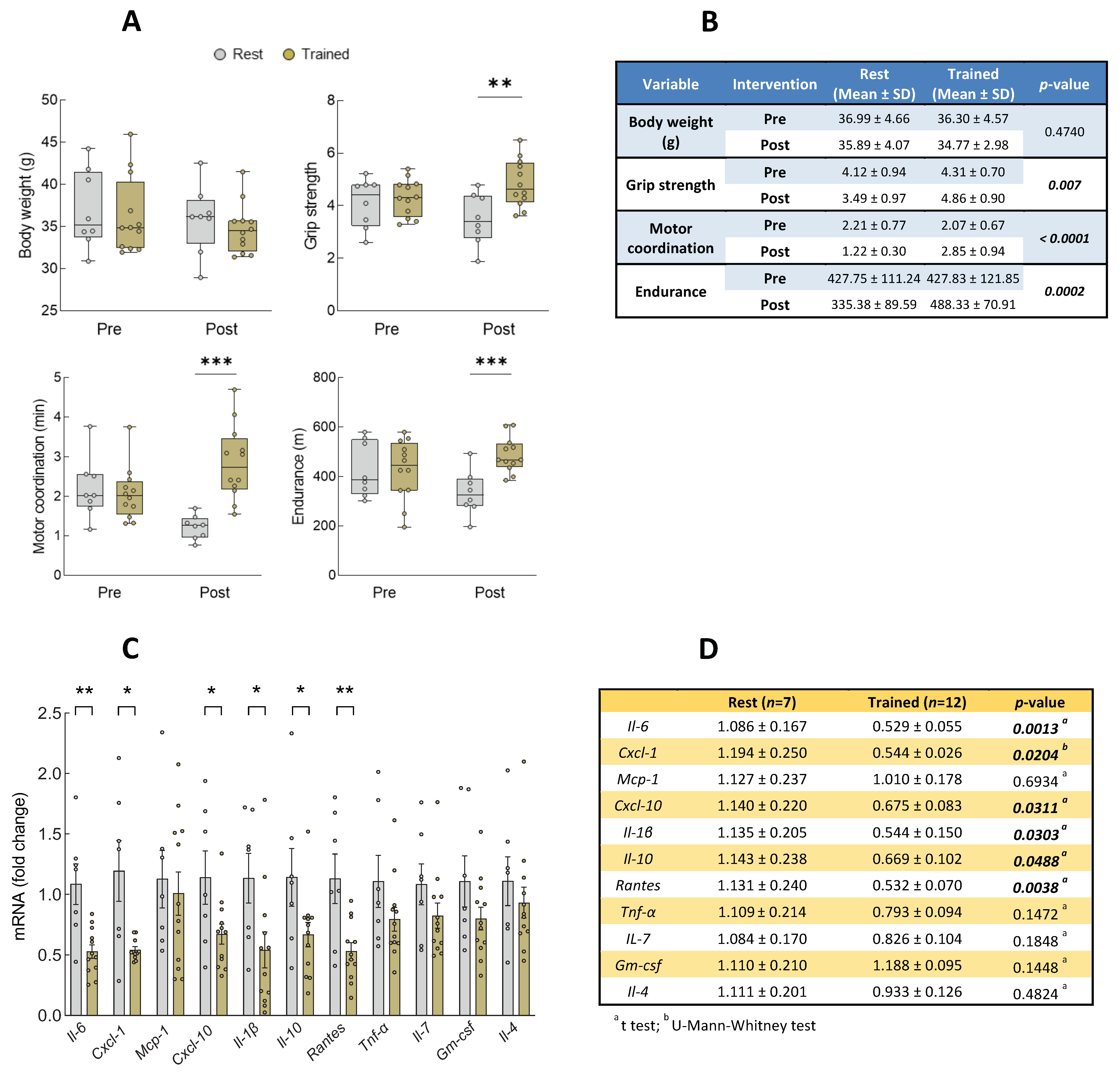
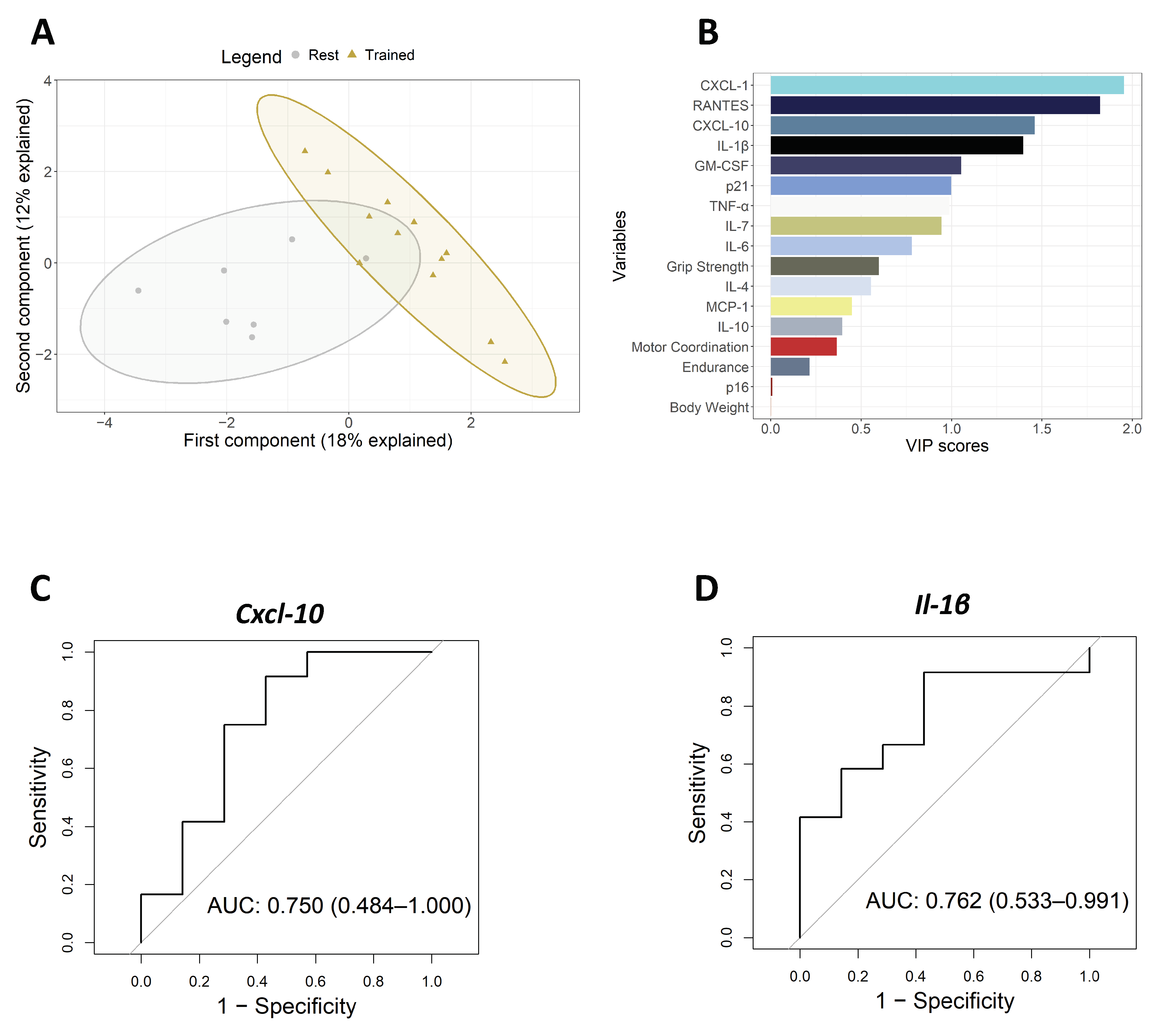
3.2. Intervention with Physical Exercise on Aged Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Satta, M.; Berna-Erro, A.; Carrasco-Garcia, E.; Alberro, A.; Saenz-Antonanzas, A.; Vergara, I.; Otaegui, D.; Matheu, A. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging 2020, 12, 9982–9999. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Prince, M.; Thiyagarajan, J.A.; De Carvalho, I.A.; Bernabei, R.; Chan, P.; Gutierrez-Robledo, L.M.; Michel, J.P.; Morley, J.E.; Ong, P.; et al. Frailty: An Emerging Public Health Priority. J. Am. Med. Dir. Assoc. 2016, 17, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Buta, B.; Xue, Q.L. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin. Geriatr. Med. 2018, 34, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Somech, J.; Joshi, A.; Mancini, R.; Chetrit, J.; Michel, C.; Sheppard, R.; Nguyen, V.; Walker, M.; Giannetti, N.; Sharma, A.; et al. Comparison of Questionnaire and Performance-Based Physical Frailty Scales to Predict Survival and Health-Related Quality of Life in Patients With Heart Failure. J. Am. Heart Assoc. 2023, 12, e026951. [Google Scholar] [CrossRef] [PubMed]
- Vergara, I.; Mateo-Abad, M.; Saucedo-Figueredo, M.C.; Machon, M.; Montiel-Luque, A.; Vrotsou, K.; Nava Del Val, M.A.; Diez-Ruiz, A.; Guell, C.; Matheu, A.; et al. Description of frail older people profiles according to four screening tools applied in primary care settings: A cross sectional analysis. BMC Geriatr. 2019, 19, 342. [Google Scholar] [CrossRef]
- Heinze-Milne, S.D.; Banga, S.; Howlett, S.E. Frailty Assessment in Animal Models. Gerontology 2019, 65, 610–619. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Irina, G.; Refaela, C.; Adi, B.; Avia, D.; Liron, H.; Chen, A.; Gad, S. Low Blood ALT Activity and High FRAIL Questionnaire Scores Correlate with Increased Mortality and with Each Other. A Prospective Study in the Internal Medicine Department. J. Clin. Med. 2018, 7, 386. [Google Scholar] [CrossRef]
- Marcos-Perez, D.; Sanchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.P.; Fernandez-Tajes, J.; Pasaro, E.; Laffon, B.; Valdiglesias, V. Association of inflammatory mediators with frailty status in older adults: Results from a systematic review and meta-analysis. Geroscience 2020, 42, 1451–1473. [Google Scholar] [CrossRef] [PubMed]
- Heinze-Milne, S.D.; Banga, S.; Howlett, S.E. Frailty and cytokines in preclinical models: Comparisons with humans. Mech. Ageing Dev. 2022, 206, 111706. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Orkaby, A.R.; Ward, R.; Chen, J.; Shanbhag, A.; Sesso, H.D.; Gaziano, J.M.; Djousse, L.; Driver, J.A. Influence of Long-term Nonaspirin NSAID Use on Risk of Frailty in Men >/=60 Years: The Physicians’ Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1048–1054. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodriguez-Manas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Casas-Herrero, A.; Saez de Asteasu, M.L.; Anton-Rodrigo, I.; Sanchez-Sanchez, J.L.; Montero-Odasso, M.; Marin-Epelde, I.; Ramon-Espinoza, F.; Zambom-Ferraresi, F.; Petidier-Torregrosa, R.; Elexpuru-Estomba, J.; et al. Effects of Vivifrail multicomponent intervention on functional capacity: A multicentre, randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 884–893. [Google Scholar] [CrossRef]
- Gine-Garriga, M.; Roque-Figuls, M.; Coll-Planas, L.; Sitja-Rabert, M.; Salva, A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014, 95, 753–769.e3. [Google Scholar] [CrossRef]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef]
- Zheng, G.; Qiu, P.; Xia, R.; Lin, H.; Ye, B.; Tao, J.; Chen, L. Effect of Aerobic Exercise on Inflammatory Markers in Healthy Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Aging Neurosci. 2019, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Bruunsgaard, H.; Bjerregaard, E.; Schroll, M.; Pedersen, B.K. Muscle strength after resistance training is inversely correlated with baseline levels of soluble tumor necrosis factor receptors in the oldest old. J. Am. Geriatr. Soc. 2004, 52, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C.; et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. Geroscience 2022, 44, 2757–2770. [Google Scholar] [CrossRef] [PubMed]
- Englund, D.A.; Sakamoto, A.E.; Fritsche, C.M.; Heeren, A.A.; Zhang, X.; Kotajarvi, B.R.; Lecy, D.R.; Yousefzadeh, M.J.; Schafer, M.J.; White, T.A.; et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 2021, 20, e13415. [Google Scholar] [CrossRef]
- Zhang, X.; Englund, D.A.; Aversa, Z.; Jachim, S.K.; White, T.A.; LeBrasseur, N.K. Exercise Counters the Age-Related Accumulation of Senescent Cells. Exerc. Sport. Sci. Rev. 2022, 50, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bisset, E.S.; Heinze-Milne, S.; Grandy, S.A.; Howlett, S.E. Aerobic Exercise Attenuates Frailty in Aging Male and Female C57Bl/6 Mice and Effects Systemic Cytokines Differentially by Sex. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 41–46. [Google Scholar] [CrossRef]
- Kane, A.E.; Bisset, E.S.; Heinze-Milne, S.; Keller, K.M.; Grandy, S.A.; Howlett, S.E. Maladaptive Changes Associated With Cardiac Aging Are Sex-Specific and Graded by Frailty and Inflammation in C57BL/6 Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 233–243. [Google Scholar] [CrossRef]
- Iparraguirre, L.; Alberro, A.; Iniguez, S.G.; Munoz-Culla, M.; Vergara, I.; Matheu, A.; Otaegui, D. Blood RNA-Seq profiling reveals a set of circular RNAs differentially expressed in frail individuals. Immun. Ageing 2023, 20, 33. [Google Scholar] [CrossRef]
- Wall, J.C.; Bell, C.; Campbell, S.; Davis, J. The Timed Get-up-and-Go test revisited: Measurement of the component tasks. J. Rehabil. Res. Dev. 2000, 37, 109–113. [Google Scholar]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Bautmans, I.; Salimans, L.; Njemini, R.; Beyer, I.; Lieten, S.; Liberman, K. The effects of exercise interventions on the inflammatory profile of older adults: A systematic review of the recent literature. Exp. Gerontol. 2021, 146, 111236. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Justice, J.N.; Gioscia-Ryan, R.A.; Johnson, L.C.; Battson, M.L.; de Picciotto, N.E.; Beck, H.J.; Jiang, H.; Sindler, A.L.; Bryan, N.S.; Enoka, R.M.; et al. Sodium nitrite supplementation improves motor function and skeletal muscle inflammatory profile in old male mice. J. Appl. Physiol. (1985) 2015, 118, 163–169. [Google Scholar] [CrossRef]
- da Silva, P.F.L.; Ogrodnik, M.; Kucheryavenko, O.; Glibert, J.; Miwa, S.; Cameron, K.; Ishaq, A.; Saretzki, G.; Nagaraja-Grellscheid, S.; Nelson, G.; et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 2019, 18, e12848. [Google Scholar] [CrossRef]
- Zhang, X.; Habiballa, L.; Aversa, Z.; Ng, Y.E.; Sakamoto, A.E.; Englund, D.A.; Pearsall, V.M.; White, T.A.; Robinson, M.M.; Rivas, D.A.; et al. Characterization of cellular senescence in aging skeletal muscle. Nat. Aging 2022, 2, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Rall, L.C.; Roubenoff, R.; Cannon, J.G.; Abad, L.W.; Dinarello, C.A.; Meydani, S.N. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med. Sci. Sports Exerc. 1996, 28, 1356–1365. [Google Scholar] [CrossRef]
- Bisset, E.S.; Howlett, S.E. The biology of frailty in humans and animals: Understanding frailty and promoting translation. Aging Med. 2019, 2, 27–34. [Google Scholar] [CrossRef]
- Samson, L.D.; Buisman, A.M.; Ferreira, J.A.; Picavet, H.S.J.; Verschuren, W.M.M.; Boots, A.M.; Engelfriet, P. Inflammatory marker trajectories associated with frailty and ageing in a 20-year longitudinal study. Clin. Transl. Immunol. 2022, 11, e1374. [Google Scholar] [CrossRef]
- Bouzakri, K.; Plomgaard, P.; Berney, T.; Donath, M.Y.; Pedersen, B.K.; Halban, P.A. Bimodal effect on pancreatic beta-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes 2011, 60, 1111–1121. [Google Scholar] [CrossRef]
- Antonelli, A.; Rotondi, M.; Fallahi, P.; Ferrari, S.M.; Paolicchi, A.; Romagnani, P.; Serio, M.; Ferrannini, E. Increase of CXC chemokine CXCL10 and CC chemokine CCL2 serum levels in normal ageing. Cytokine 2006, 34, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, H.L.; Almeida, C.S.; Abramo, C.; Grunewald, S.T.F.; Levy, R.A.; Teixeira, H.C. CCL2, CXCL8, CXCL9 and CXCL10 serum levels increase with age but are not altered by treatment with hydroxychloroquine in patients with osteoarthritis of the knees. Int. J. Rheum. Dis. 2017, 20, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Shurin, G.V.; Yurkovetsky, Z.R.; Chatta, G.S.; Tourkova, I.L.; Shurin, M.R.; Lokshin, A.E. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine 2007, 39, 123–129. [Google Scholar] [CrossRef]
- Qu, T.; Yang, H.; Walston, J.D.; Fedarko, N.S.; Leng, S.X. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine 2009, 46, 319–324. [Google Scholar] [CrossRef]
- Meyer, J.D.; Hayney, M.S.; Coe, C.L.; Ninos, C.L.; Barrett, B.P. Differential Reduction of IP-10 and C-Reactive Protein via Aerobic Exercise or Mindfulness-Based Stress-Reduction Training in a Large Randomized Controlled Trial. J. Sport. Exerc. Psychol. 2019, 41, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, Y.; Sato, H.; Tsujimura, K.; Kawaguchi, H.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M.; Nedachi, T. Skeletal muscle cell contraction reduces a novel myokine, chemokine (C-X-C motif) ligand 10 (CXCL10): Potential roles in exercise-regulated angiogenesis. Biosci. Biotechnol. Biochem. 2018, 82, 97–105. [Google Scholar] [CrossRef]
- Mardare, C.; Kruger, K.; Liebisch, G.; Seimetz, M.; Couturier, A.; Ringseis, R.; Wilhelm, J.; Weissmann, N.; Eder, K.; Mooren, F.C. Endurance and Resistance Training Affect High Fat Diet-Induced Increase of Ceramides, Inflammasome Expression, and Systemic Inflammation in Mice. J. Diabetes Res. 2016, 2016, 4536470. [Google Scholar] [CrossRef]
- Trott, D.W.; Lesniewski, L.A.; Donato, A.J. Selected life-extending interventions reduce arterial CXCL10 and macrophage colony-stimulating factor in aged mouse arteries. Cytokine 2017, 96, 102–106. [Google Scholar] [CrossRef]
- Bakhashab, S.; Ahmed, F.W.; Schulten, H.J.; Bashir, A.; Karim, S.; Al-Malki, A.L.; Gari, M.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Alqahtani, M.H.; et al. Metformin improves the angiogenic potential of human CD34+ cells co-incident with downregulating CXCL10 and TIMP1 gene expression and increasing VEGFA under hyperglycemia and hypoxia within a therapeutic window for myocardial infarction. Cardiovasc. Diabetol. 2016, 15, 27. [Google Scholar] [CrossRef]
- Palomera-Avalos, V.; Grinan-Ferre, C.; Izquierdo, V.; Camins, A.; Sanfeliu, C.; Canudas, A.M.; Pallas, M. Resveratrol modulates response against acute inflammatory stimuli in aged mouse brain. Exp. Gerontol. 2018, 102, 3–11. [Google Scholar] [CrossRef]
- Pansarasa, O.; Mimmi, M.C.; Davin, A.; Giannini, M.; Guaita, A.; Cereda, C. Inflammation and cell-to-cell communication, two related aspects in frailty. Immun. Ageing 2022, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Yu, Q.; Xue, Q.L.; Yao, W.; Brayton, C.; Yang, H.; Fedarko, N.; Walston, J. Inflammation and mortality in a frail mouse model. Age 2012, 34, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, X. Effects of regular exercise on inflammasome activation-related inflammatory cytokine levels in older adults: A systematic review and meta-analysis. J. Sports Sci. 2021, 39, 2338–2352. [Google Scholar] [CrossRef]
- So, W.Y.; Song, M.; Park, Y.H.; Cho, B.L.; Lim, J.Y.; Kim, S.H.; Song, W. Body composition, fitness level, anabolic hormones, and inflammatory cytokines in the elderly: A randomized controlled trial. Aging Clin. Exp. Res. 2013, 25, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Lanza, I.R. Age-associated inflammation and implications for skeletal muscle responses to exercise. Exp. Gerontol. 2023, 177, 112177. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Pérez, D.; Cruces-Salguero, S.; García-Domínguez, E.; Araúzo-Bravo, M.J.; Gómez-Cabrera, M.C.; Viña, J.; Vergara, I.; Matheu, A. Physical Interventions Restore Physical Frailty and the Expression of CXCL-10 and IL-1β Inflammatory Biomarkers in Old Individuals and Mice. Biomolecules 2024, 14, 166. https://doi.org/10.3390/biom14020166
Marcos-Pérez D, Cruces-Salguero S, García-Domínguez E, Araúzo-Bravo MJ, Gómez-Cabrera MC, Viña J, Vergara I, Matheu A. Physical Interventions Restore Physical Frailty and the Expression of CXCL-10 and IL-1β Inflammatory Biomarkers in Old Individuals and Mice. Biomolecules. 2024; 14(2):166. https://doi.org/10.3390/biom14020166
Chicago/Turabian StyleMarcos-Pérez, Diego, Sara Cruces-Salguero, Esther García-Domínguez, Marcos J. Araúzo-Bravo, Mari Carmen Gómez-Cabrera, José Viña, Itziar Vergara, and Ander Matheu. 2024. "Physical Interventions Restore Physical Frailty and the Expression of CXCL-10 and IL-1β Inflammatory Biomarkers in Old Individuals and Mice" Biomolecules 14, no. 2: 166. https://doi.org/10.3390/biom14020166