A Combination of Heavy Metals and Intracellular Pathway Modulators Induces Alzheimer Disease-like Pathologies in Organotypic Brain Slices
Abstract
:1. Introduction
1.1. Alzheimer’s Disease
1.2. Study of AD-Like Pathologies in Organotypic Brain Slices
1.3. Culture of Postnatal Brain Slices
1.4. Culture of Adult Brain Slices
1.5. Use of Exogenous Human Spreading on Mouse Brain Slices
2. Materials and Methods
2.1. Animals
2.2. Organotypic Brain Slice Cultures
2.3. Proteins/Peptides
2.4. Collagen Hydrogels and Loading of hAβ42 and P301S aggTau
2.5. Immunohistochemistry
2.6. Processing of Human Post-Mortem Tissue Sections
2.7. Propidium Iodide Live Staining
2.8. Western Blot
2.9. Data Analysis and Statistics
3. Results
3.1. Culturing of Organotypic Brain Slices and Collagen Hydrogel Application

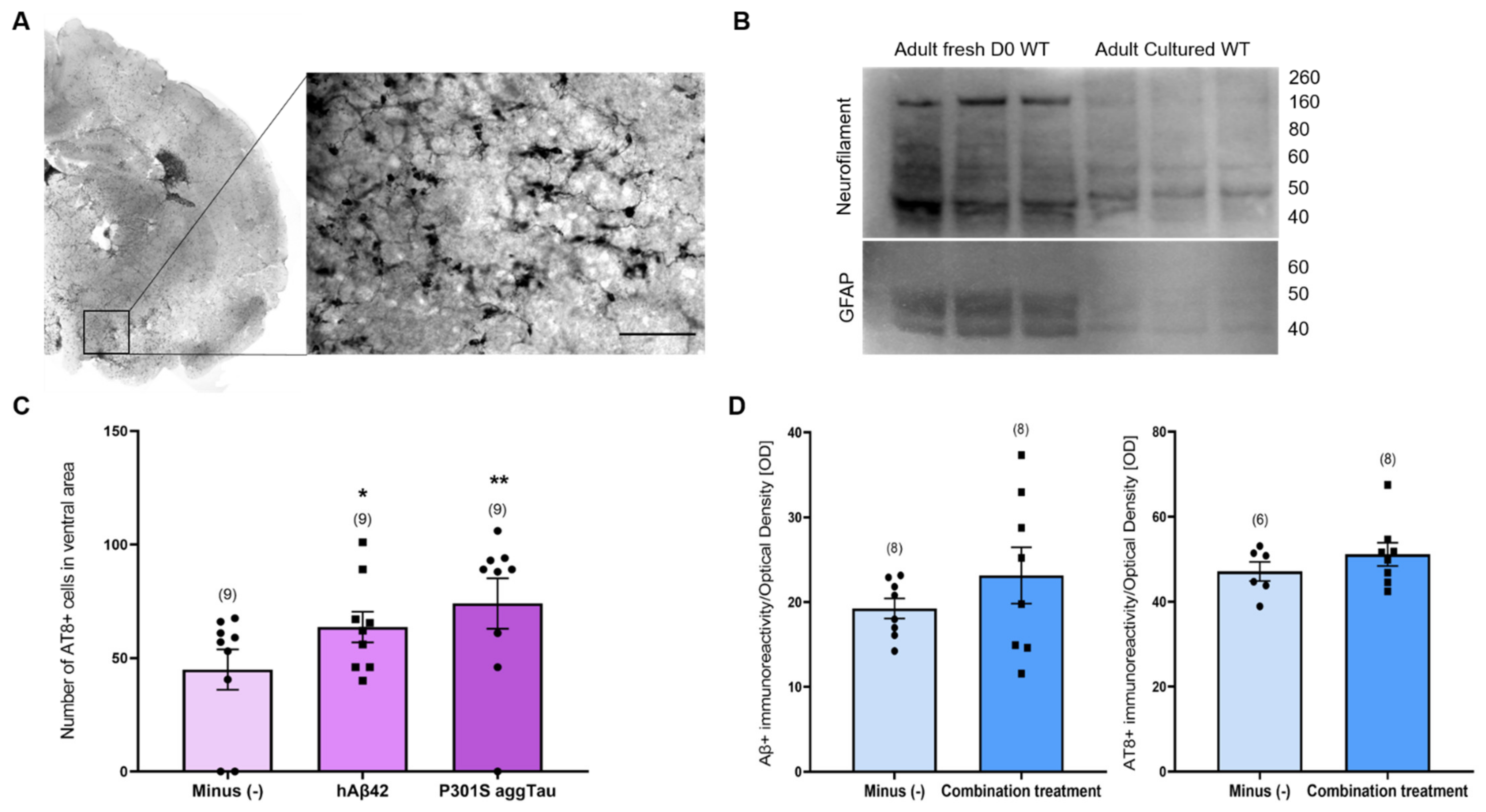
3.2. Spreading of hAβ42 and P301S aggTau to Ventral Areas
3.3. Aβ Plaques and Tau NFTs in Postnatal WT and TG APP_SDI Slices
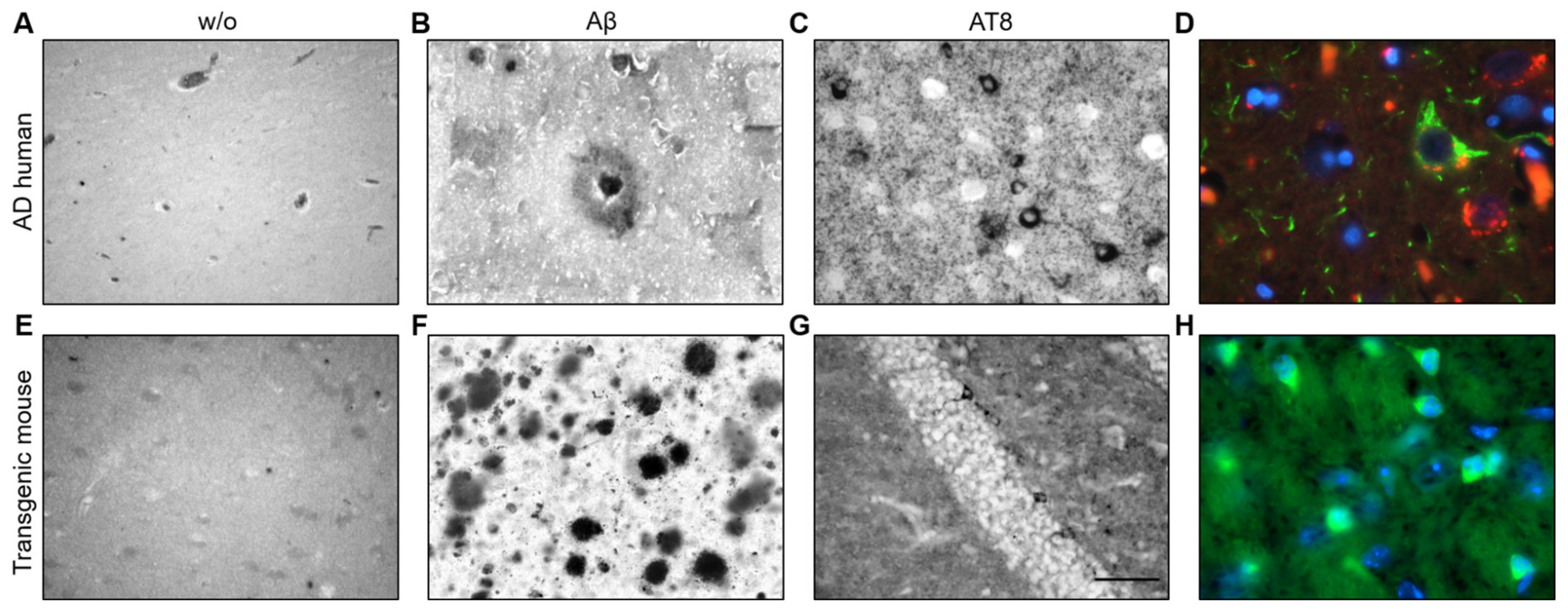
3.4. Aβ Plaques and Tau NFTs in Post-Mortem Human and TG Mice Slices
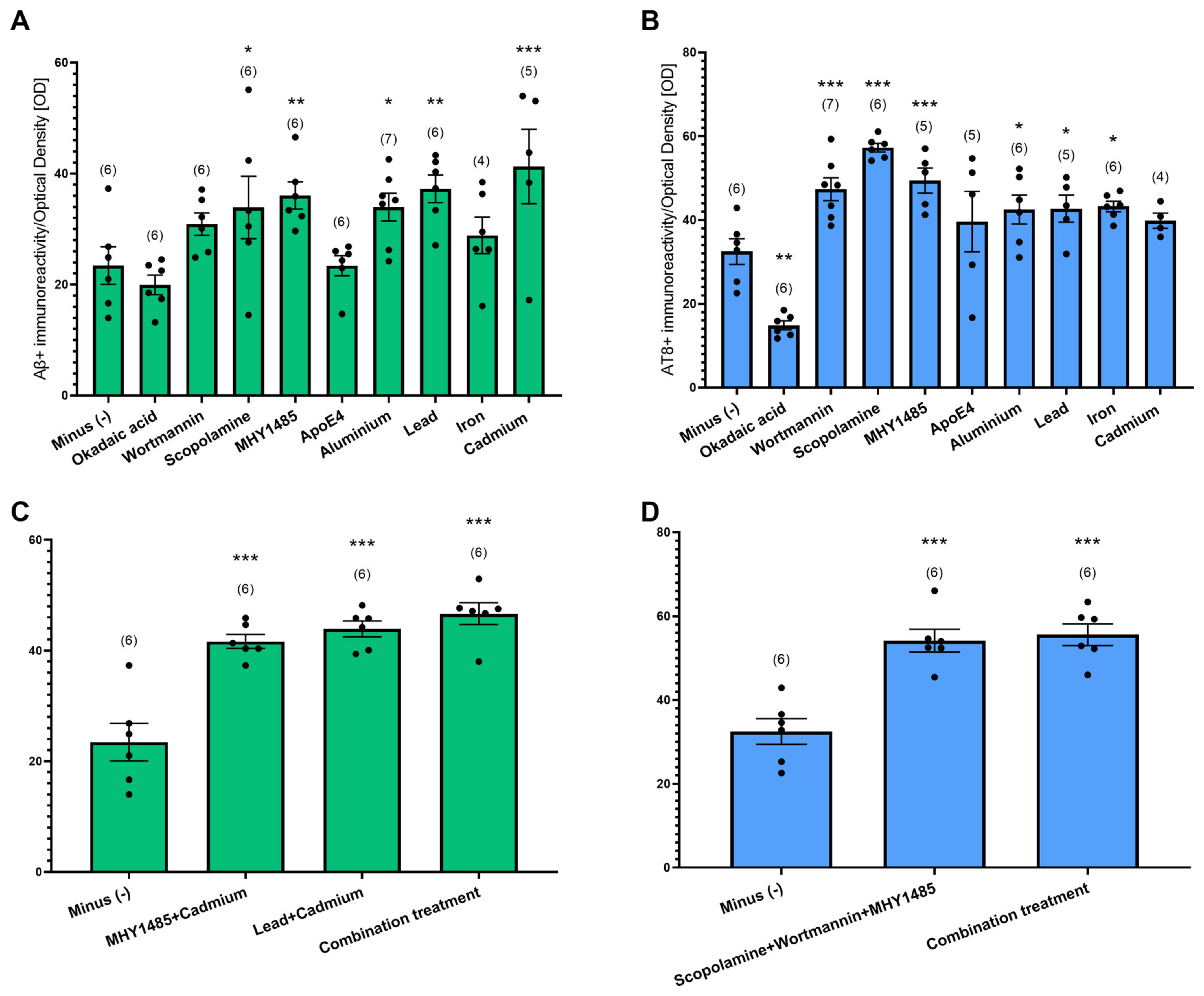
3.5. Pharmacological Manipulation of Slices to Generate Aβ Plaques and Tau NFTs
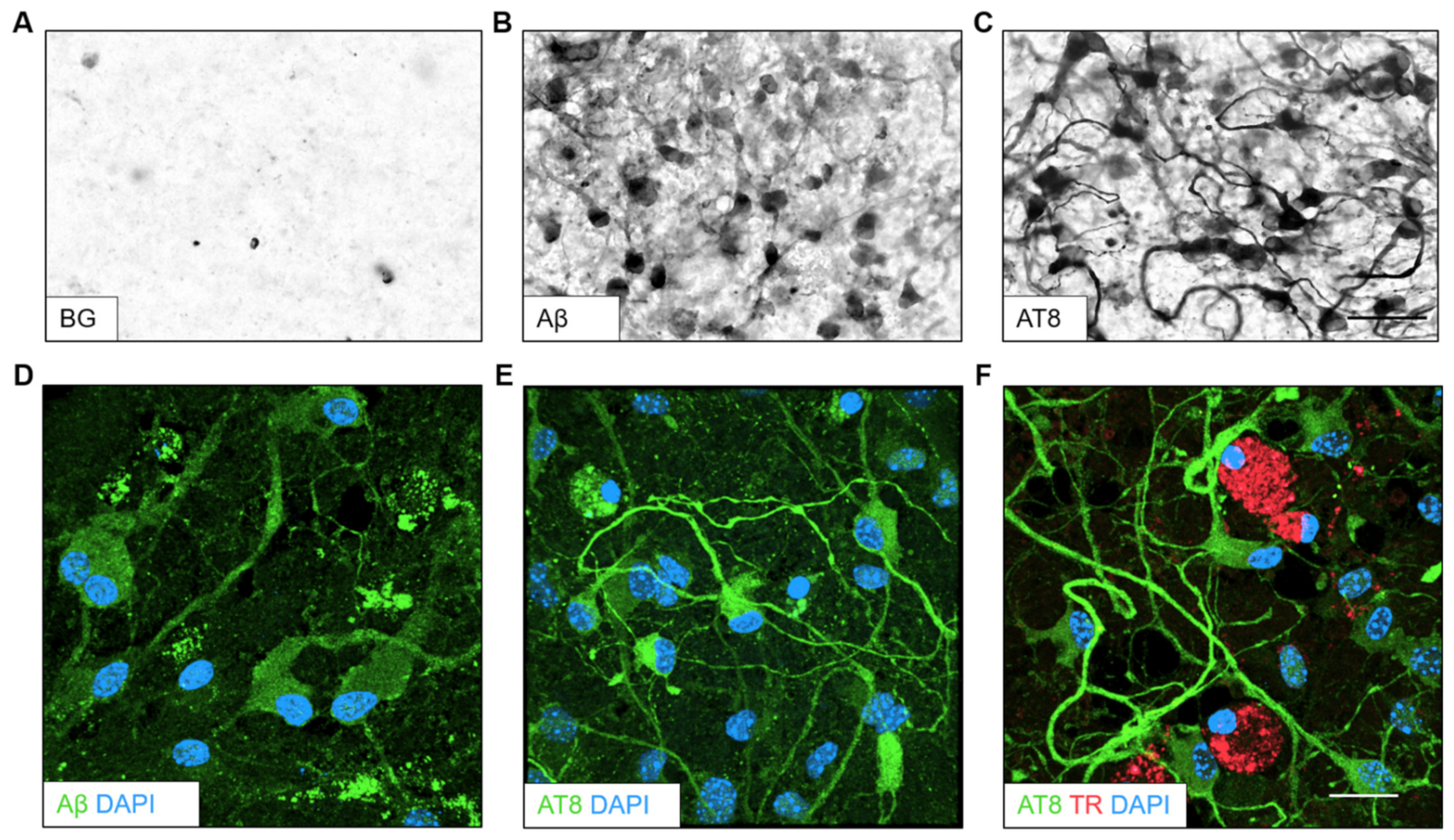
3.6. Plaque-Like and NFT-Like Features upon Treatment with Pharmacological Agents
3.7. Aβ Plaques Develop Intracellularly Prior to Cell Death
3.8. Translation to Adult WT and TG APP_SDI Slices
4. Discussion
4.1. Spreading of hAβ42 and P301S aggTau in Postnatal Slices
4.2. Aβ Plaque- and Tau Tangle-Like Pathologies Develop Independently
4.3. Heavy Metals Augment Aβ Plaque-Like Pathology
4.4. Intracellular Pathway Modulators Boosts tau NFT-Like Pathology
4.5. Combined Model of AD Neuropathologies
4.6. Aβ Plaques Develop Intracellularly before Cell Death
4.7. Translation to Adult Slices
4.8. Translation to Humans and Outlook for Therapeutic Strategies
4.9. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APP_SDI | Amyloid precursor protein with Swedish–Dutch–Iowa mutations |
| Aβ | Amyloid-β |
| BSA | Bovine serum albumin |
| DAB | 3,3′-diaminobenzidine |
| FFPE | Formalin fixed paraffin embedded |
| GFAP | Glial fibrillary acidic protein |
| GSK-3β | Glycogen synthase kinase-3 β |
| hTau | human tau |
| M.O.M | Mouse on Mouse |
| mTOR | Mammalian target of rapamycin |
| NFT | Neurofibrillary tangle |
| P301S aggTau | P301S aggregated tau (active) |
| PBS | Phosphate-buffered saline |
| PI3K | Phosphatidylinositol 3-kinase |
| PFA | Paraformaldehyde |
| RT | Room temperature |
| SEM | Standard error of mean |
| TBS | Tris-buffered saline |
| TG | Transgenic |
| WT | Wild-type |
| α-Syn | α-Synuclein |
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Guell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.; McGonigle, P. Overview of Transgenic Mouse Models for Alzheimer’s Disease. Curr. Protoc. Neurosci. 2019, 89, e81. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Xu, F.; Deane, R.; Romanov, G.; Previti, M.L.; Zeigler, K.; Zlokovic, B.V.; Van Nostrand, W.E. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J. Biol. Chem. 2004, 279, 20296–20306. [Google Scholar] [CrossRef] [PubMed]
- Andorfer, C.; Kress, Y.; Espinoza, M.; de Silva, R.; Tucker, K.L.; Barde, Y.A.; Duff, K.; Davies, P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003, 86, 582–590. [Google Scholar] [CrossRef]
- Polydoro, M.; Acker, C.M.; Duff, K.; Castillo, P.E.; Davies, P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J. Neurosci. 2009, 29, 10741–10749. [Google Scholar] [CrossRef] [PubMed]
- Karmirian, K.; Holubiec, M.; Goto-Silva, L.; Fernandez Bessone, I.; Vitoria, G.; Mello, B.; Alloatti, M.; Vanderborght, B.; Falzone, T.L.; Rehen, S. Modeling Alzheimer’s Disease Using Human Brain Organoids. Methods Mol. Biol. 2023, 2561, 135–158. [Google Scholar] [CrossRef]
- Mungenast, A.E.; Siegert, S.; Tsai, L.H. Modeling Alzheimer’s disease with human induced pluripotent stem (iPS) cells. Mol. Cell. Neurosci. 2016, 73, 13–31. [Google Scholar] [CrossRef]
- Penney, J.; Ralvenius, W.T.; Tsai, L.H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef]
- Ranjan, V.D.; Qiu, L.; Tan, E.K.; Zeng, L.; Zhang, Y. Modelling Alzheimer’s disease: Insights from in vivo to in vitro three-dimensional culture platforms. J. Tissue Eng. Regen. Med. 2018, 12, 1944–1958. [Google Scholar] [CrossRef]
- Croft, C.L.; Futch, H.S.; Moore, B.D.; Golde, T.E. Organotypic brain slice cultures to model neurodegenerative proteinopathies. Mol. Neurodegener. 2019, 14, 45. [Google Scholar] [CrossRef]
- Humpel, C. Organotypic brain slice cultures: A review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef]
- Stoppini, L.; Buchs, P.A.; Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef] [PubMed]
- De Simoni, A.; Griesinger, C.B.; Edwards, F.A. Development of rat CA1 neurones in acute versus organotypic slices: Role of experience in synaptic morphology and activity. J. Physiol. 2003, 550, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Korde, D.S.; Humpel, C. Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures. Biomolecules 2022, 12, 1164. [Google Scholar] [CrossRef]
- McCarthy, J.M.; Virdee, J.; Brown, J.; Ursu, D.; Ahmed, Z.; Cavallini, A.; Nuthall, H.N. Development of P301S tau seeded organotypic hippocampal slice cultures to study potential therapeutics. Sci. Rep. 2021, 11, 10309. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.V.C.; Mukadam, A.S.; Durrant, C.S.; Vaysburd, M.J.; Katsinelos, T.; Tuck, B.J.; Sanford, S.; Sheppard, O.; Knox, C.; Cheng, S.; et al. Tau assemblies do not behave like independently acting prion-like particles in mouse neural tissue. Acta Neuropathol. Commun. 2021, 9, 41. [Google Scholar] [CrossRef]
- Moelgg, K.; Jummun, F.; Humpel, C. Spreading of Beta-Amyloid in Organotypic Mouse Brain Slices and Microglial Elimination and Effects on Cholinergic Neurons. Biomolecules 2021, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Stefanova, N.; Humpel, C. Spreading of Aggregated alpha-Synuclein in Sagittal Organotypic Mouse Brain Slices. Biomolecules 2022, 12, 163. [Google Scholar] [CrossRef]
- Sundstrom, L.; Morrison, B., 3rd; Bradley, M.; Pringle, A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov. Today 2005, 10, 993–1000. [Google Scholar] [CrossRef]
- Bahr, B.A. Long-term hippocampal slices: A model system for investigating synaptic mechanisms and pathologic processes. J. Neurosci. Res. 1995, 42, 294–305. [Google Scholar] [CrossRef]
- Malouf, A.T. Effect of beta amyloid peptides on neurons in hippocampal slice cultures. Neurobiol. Aging 1992, 13, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Marksteiner, J.; Humpel, C. Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol. Psychiatry 2008, 13, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, S.P.; Schmid, R.S.; He, D.N.; Sung, M.L.; Cho, S.; Resnick, L.; Monaghan, M.M.; Hirst, W.D.; Essrich, C.; Reinhart, P.H.; et al. Inhibition of c-Jun kinase provides neuroprotection in a model of Alzheimer’s disease. Neurobiol. Dis. 2010, 39, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.L.; Noble, W. Preparation of organotypic brain slice cultures for the study of Alzheimer’s disease. F1000Research 2018, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Harwell, C.S.; Coleman, M.P. Synaptophysin depletion and intraneuronal Abeta in organotypic hippocampal slice cultures from huAPP transgenic mice. Mol. Neurodegener. 2016, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Novotny, R.; Langer, F.; Mahler, J.; Skodras, A.; Vlachos, A.; Wegenast-Braun, B.M.; Kaeser, S.A.; Neher, J.J.; Eisele, Y.S.; Pietrowski, M.J.; et al. Conversion of Synthetic Abeta to In Vivo Active Seeds and Amyloid Plaque Formation in a Hippocampal Slice Culture Model. J. Neurosci. 2016, 36, 5084–5093. [Google Scholar] [CrossRef]
- Croft, C.L.; Cruz, P.E.; Ryu, D.H.; Ceballos-Diaz, C.; Strang, K.H.; Woody, B.M.; Lin, W.L.; Deture, M.; Rodriguez-Lebron, E.; Dickson, D.W.; et al. rAAV-based brain slice culture models of Alzheimer’s and Parkinson’s disease inclusion pathologies. J. Exp. Med. 2019, 216, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.L.; Wade, M.A.; Kurbatskaya, K.; Mastrandreas, P.; Hughes, M.M.; Phillips, E.C.; Pooler, A.M.; Perkinton, M.S.; Hanger, D.P.; Noble, W. Membrane association and release of wild-type and pathological tau from organotypic brain slice cultures. Cell Death Dis. 2017, 8, e2671. [Google Scholar] [CrossRef]
- Duff, K.; Noble, W.; Gaynor, K.; Matsuoka, Y. Organotypic slice cultures from transgenic mice as disease model systems. J. Mol. Neurosci. 2002, 19, 317–320. [Google Scholar] [CrossRef]
- Li, L.; Sengupta, A.; Haque, N.; Grundke-Iqbal, I.; Iqbal, K. Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett. 2004, 566, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Messing, L.; Decker, J.M.; Joseph, M.; Mandelkow, E.; Mandelkow, E.M. Cascade of tau toxicity in inducible hippocampal brain slices and prevention by aggregation inhibitors. Neurobiol. Aging 2013, 34, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.W.; Chen, Y.C.; Hsieh, W.L.; Chiou, S.H.; Kao, C.L. Ageing and neurodegenerative diseases. Ageing Res. Rev. 2010, 9 (Suppl. S1), S36–S46. [Google Scholar] [CrossRef] [PubMed]
- Humpel, C. Organotypic vibrosections from whole brain adult Alzheimer mice (overexpressing amyloid-precursor-protein with the Swedish-Dutch-Iowa mutations) as a model to study clearance of beta-amyloid plaques. Front. Aging Neurosci. 2015, 7, 47. [Google Scholar] [CrossRef]
- Mewes, A.; Franke, H.; Singer, D. Organotypic brain slice cultures of adult transgenic P301S mice—A model for tauopathy studies. PLoS ONE 2012, 7, e45017. [Google Scholar] [CrossRef] [PubMed]
- Mayerl, S.; Ffrench-Constant, C. Establishing an Adult Mouse Brain Hippocampal Organotypic Slice Culture System that Allows for Tracing and Pharmacological Manipulation of ex vivo Neurogenesis. Bio Protoc. 2021, 11, e3869. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 1995, 16, 271–278; discussion 278–284. [Google Scholar] [CrossRef] [PubMed]
- Brunello, C.A.; Merezhko, M.; Uronen, R.L.; Huttunen, H.J. Mechanisms of secretion and spreading of pathological tau protein. Cell. Mol. Life Sci. 2020, 77, 1721–1744. [Google Scholar] [CrossRef]
- d’Errico, P.; Meyer-Luehmann, M. Mechanisms of Pathogenic Tau and Abeta Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 265. [Google Scholar] [CrossRef]
- Kane, M.D.; Lipinski, W.J.; Callahan, M.J.; Bian, F.; Durham, R.A.; Schwarz, R.D.; Roher, A.E.; Walker, L.C. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J. Neurosci. 2000, 20, 3606–3611. [Google Scholar] [CrossRef]
- Mudher, A.; Colin, M.; Dujardin, S.; Medina, M.; Dewachter, I.; Alavi Naini, S.M.; Mandelkow, E.M.; Mandelkow, E.; Buee, L.; Goedert, M.; et al. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun. 2017, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Stohr, J.; Watts, J.C.; Mensinger, Z.L.; Oehler, A.; Grillo, S.K.; DeArmond, S.J.; Prusiner, S.B.; Giles, K. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc. Natl. Acad. Sci. USA 2012, 109, 11025–11030. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.M.; Schilling, S.; Cynis, H.; Silva, A.; Swanson, E.; Wangsanut, T.; Tayler, K.; Wiltgen, B.; Hatami, A.; Ronicke, R.; et al. Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-beta. Nature 2012, 485, 651–655. [Google Scholar] [CrossRef]
- Rapoport, M.; Dawson, H.N.; Binder, L.I.; Vitek, M.P.; Ferreira, A. Tau is essential to beta -amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 6364–6369. [Google Scholar] [CrossRef] [PubMed]
- Shipton, O.A.; Leitz, J.R.; Dworzak, J.; Acton, C.E.; Tunbridge, E.M.; Denk, F.; Dawson, H.N.; Vitek, M.P.; Wade-Martins, R.; Paulsen, O.; et al. Tau protein is required for amyloid beta-induced impairment of hippocampal long-term potentiation. J. Neurosci. 2011, 31, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; Zhang, K.; Brodbeck, J.; Daub, A.C.; Sharma, P.; Finkbeiner, S.; Cui, B.; Mucke, L. Tau reduction prevents Abeta-induced defects in axonal transport. Science 2010, 330, 198. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Buckner, R.L.; Snyder, A.Z.; Shannon, B.J.; LaRossa, G.; Sachs, R.; Fotenos, A.F.; Sheline, Y.I.; Klunk, W.E.; Mathis, C.A.; Morris, J.C.; et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005, 25, 7709–7717. [Google Scholar] [CrossRef]
- Grothe, M.J.; Barthel, H.; Sepulcre, J.; Dyrba, M.; Sabri, O.; Teipel, S.J.; Alzheimer’s Disease Neuroimaging Initiative. In vivo staging of regional amyloid deposition. Neurology 2017, 89, 2031–2038. [Google Scholar] [CrossRef]
- Johnson, K.A.; Schultz, A.; Betensky, R.A.; Becker, J.A.; Sepulcre, J.; Rentz, D.; Mormino, E.; Chhatwal, J.; Amariglio, R.; Papp, K.; et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 2016, 79, 110–119. [Google Scholar] [CrossRef]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Crary, J.F.; Trojanowski, J.Q.; Schneider, J.A.; Abisambra, J.F.; Abner, E.L.; Alafuzoff, I.; Arnold, S.E.; Attems, J.; Beach, T.G.; Bigio, E.H.; et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol. 2014, 128, 755–766. [Google Scholar] [CrossRef]
- Schonheit, B.; Zarski, R.; Ohm, T.G. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol. Aging 2004, 25, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Bakulski, K.M.; Seo, Y.A.; Hickman, R.C.; Brandt, D.; Vadari, H.S.; Hu, H.; Park, S.K. Heavy Metals Exposure and Alzheimer’s Disease and Related Dementias. J. Alzheimers Dis. 2020, 76, 1215–1242. [Google Scholar] [CrossRef] [PubMed]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed]
- Babic Leko, M.; Langer Horvat, L.; Spanic Popovacki, E.; Zubcic, K.; Hof, P.R.; Simic, G. Metals in Alzheimer’s Disease. Biomedicines 2023, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Bolognin, S.; Messori, L.; Drago, D.; Gabbiani, C.; Cendron, L.; Zatta, P. Aluminum, copper, iron and zinc differentially alter amyloid-Abeta(1-42) aggregation and toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Niu, F.; Sun, Z.; Cao, W.; Zhang, X.; Guan, D.; Lv, Z.; Zhang, B.; Xu, Y. Altered expression of Abeta metabolism-associated molecules from D-galactose/AlCl(3) induced mouse brain. Mech. Ageing Dev. 2009, 130, 248–252. [Google Scholar] [CrossRef]
- Mantyh, P.W.; Ghilardi, J.R.; Rogers, S.; DeMaster, E.; Allen, C.J.; Stimson, E.R.; Maggio, J.E. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J. Neurochem. 1993, 61, 1171–1174. [Google Scholar] [CrossRef]
- Sakamoto, T.; Saito, H.; Ishii, K.; Takahashi, H.; Tanabe, S.; Ogasawara, Y. Aluminum inhibits proteolytic degradation of amyloid beta peptide by cathepsin D: A potential link between aluminum accumulation and neuritic plaque deposition. FEBS Lett. 2006, 580, 6543–6549. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, L.; Fu, Y.; Sun, H.; Wang, J. Evaluating effect of metallic ions on aggregation behavior of beta-amyloid peptides by atomic force microscope and surface-enhanced Raman Scattering. Biomed. Eng. Online 2021, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.R.; Murali, M.; Siddiqi, H.K.; Ghosal, K.; Siddiqi, O.K.; Lashuel, H.A.; Ge, Y.W.; Lahiri, D.K.; Zawia, N.H. Lead (Pb) exposure and its effect on APP proteolysis and Abeta aggregation. FASEB J. 2005, 19, 2083–2084. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Basha, M.R.; Brock, B.; Cox, D.P.; Cardozo-Pelaez, F.; McPherson, C.A.; Harry, J.; Rice, D.C.; Maloney, B.; Chen, D.; et al. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): Evidence for a developmental origin and environmental link for AD. J. Neurosci. 2008, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Gao, Z.Y.; Wang, J.; Wu, M.Q.; Hu, S.; Chen, F.; Liu, J.X.; Pan, H.; Yan, C.H. Lead exposure induces Alzheimers’s disease (AD)-like pathology and disturbes cholesterol metabolism in the young rat brain. Toxicol. Lett. 2018, 296, 173–183. [Google Scholar] [CrossRef]
- Li, X.; Lv, Y.; Yu, S.; Zhao, H.; Yao, L. The effect of cadmium on Abeta levels in APP/PS1 transgenic mice. Exp. Ther. Med. 2012, 4, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Notarachille, G.; Arnesano, F.; Calo, V.; Meleleo, D. Heavy metals toxicity: Effect of cadmium ions on amyloid beta protein 1-42. Possible implications for Alzheimer’s disease. Biometals 2014, 27, 371–388. [Google Scholar] [CrossRef]
- Broetto, N.; Hansen, F.; Brolese, G.; Batassini, C.; Lirio, F.; Galland, F.; Dos Santos, J.P.; Dutra, M.F.; Goncalves, C.A. Intracerebroventricular administration of okadaic acid induces hippocampal glucose uptake dysfunction and tau phosphorylation. Brain Res. Bull. 2016, 124, 136–143. [Google Scholar] [CrossRef]
- Costa, A.P.; Tramontina, A.C.; Biasibetti, R.; Batassini, C.; Lopes, M.W.; Wartchow, K.M.; Bernardi, C.; Tortorelli, L.S.; Leal, R.B.; Goncalves, C.A. Neuroglial alterations in rats submitted to the okadaic acid-induced model of dementia. Behav. Brain Res. 2012, 226, 420–427. [Google Scholar] [CrossRef]
- Kamat, P.K.; Tota, S.; Rai, S.; Swarnkar, S.; Shukla, R.; Nath, C. A study on neuroinflammatory marker in brain areas of okadaic acid (ICV) induced memory impaired rats. Life Sci. 2012, 90, 713–720. [Google Scholar] [CrossRef]
- Kamat, P.K.; Tota, S.; Saxena, G.; Shukla, R.; Nath, C. Okadaic acid (ICV) induced memory impairment in rats: A suitable experimental model to test anti-dementia activity. Brain Res. 2010, 1309, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Konoki, K.; Tachibana, K. Isolation and characterization of okadaic acid binding proteins from the marine sponge Halichondria okadai. Biochemistry 2007, 46, 11410–11420. [Google Scholar] [CrossRef]
- Foidl, B.M.; Humpel, C. Differential Hyperphosphorylation of Tau-S199, -T231 and -S396 in Organotypic Brain Slices of Alzheimer Mice. A Model to Study Early Tau Hyperphosphorylation Using Okadaic Acid. Front. Aging Neurosci. 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Powis, G.; Bonjouklian, R.; Berggren, M.M.; Gallegos, A.; Abraham, R.; Ashendel, C.; Zalkow, L.; Matter, W.F.; Dodge, J.; Grindey, G.; et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994, 54, 2419–2423. [Google Scholar] [PubMed]
- Liu, S.J.; Zhang, A.H.; Li, H.L.; Wang, Q.; Deng, H.M.; Netzer, W.J.; Xu, H.; Wang, J.Z. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J. Neurochem. 2003, 87, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qureshi, H.Y.; Cafferty, P.W.; Sobue, K.; Agarwal-Mawal, A.; Neufield, K.D.; Paudel, H.K. Glycogen synthase kinase-3beta is complexed with tau protein in brain microtubules. J. Biol. Chem. 2002, 277, 11933–11940. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, F.; Tian, Q.; Yang, Y.; Wang, Q.; Wang, J.Z. Activation of glycogen synthase kinase-3 induces Alzheimer-like tau hyperphosphorylation in rat hippocampus slices in culture. J. Neural Transm. 2006, 113, 93–102. [Google Scholar] [CrossRef]
- Liu, S.J.; Wang, J.Z. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol. Sin. 2002, 23, 183–187. [Google Scholar] [PubMed]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Safar, M.M.; Arab, H.H.; Rizk, S.M.; El-Maraghy, S.A. Bone Marrow-Derived Endothelial Progenitor Cells Protect Against Scopolamine-Induced Alzheimer-Like Pathological Aberrations. Mol. Neurobiol. 2016, 53, 1403–1418. [Google Scholar] [CrossRef]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S. The role of mTOR signaling in Alzheimer disease. Front. Biosci. (Schol. Ed.) 2012, 4, 941–952. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Di Domenico, F. Targeting mTOR to reduce Alzheimer-related cognitive decline: From current hits to future therapies. Expert Rev. Neurother. 2017, 17, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, H.C.; Pinky, P.D.; Smith, W.; Suppiramaniam, V.; Reed, M.N. The role of APOE4 in Alzheimer’s disease: Strategies for future therapeutic interventions. Neuronal Signal. 2019, 3, NS20180203. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.; Hoppe, J.; Santin, K.; Frozza, R.; Zamin, L.L.; Simao, F.; Horn, A.P.; Salbego, C. Beta-amyloid peptide toxicity in organotypic hippocampal slice culture involves Akt/PKB, GSK-3beta, and PTEN. Neurochem. Int. 2007, 50, 229–235. [Google Scholar] [CrossRef]
- Pischiutta, F.; Cavaleiro, H.; Caruso, E.; Tribuzio, F.; Di Marzo, N.; Moro, F.; Kobeissy, F.; Wang, K.K.; Salgado, A.J.; Zanier, E.R. A novel organotypic cortical slice culture model for traumatic brain injury: Molecular changes induced by injury and mesenchymal stromal cell secretome treatment. Front. Cell. Neurosci. 2023, 17, 1217987. [Google Scholar] [CrossRef]
- Knobloch, M.; Konietzko, U.; Krebs, D.C.; Nitsch, R.M. Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol. Aging 2007, 28, 1297–1306. [Google Scholar] [CrossRef]
- Rajendran, L.; Knobloch, M.; Geiger, K.D.; Dienel, S.; Nitsch, R.; Simons, K.; Konietzko, U. Increased Abeta production leads to intracellular accumulation of Abeta in flotillin-1-positive endosomes. Neurodegener. Dis. 2007, 4, 164–170. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Milner, T.A.; Li, F.; Nam, E.E.; Edgar, M.A.; Yamaguchi, H.; Beal, M.F.; Xu, H.; Greengard, P.; Gouras, G.K. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 2002, 161, 1869–1879. [Google Scholar] [CrossRef]
- Cuello, A.C.; Allard, S.; Ferretti, M.T. Evidence for the accumulation of Abeta immunoreactive material in the human brain and in transgenic animal models. Life Sci. 2012, 91, 1141–1147. [Google Scholar] [CrossRef]
- D’Andrea, M.R.; Nagele, R.G.; Wang, H.Y.; Peterson, P.A.; Lee, D.H.S. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology 2001, 38, 120–134. [Google Scholar] [CrossRef]
- Gouras, G.K.; Tsai, J.; Naslund, J.; Vincent, B.; Edgar, M.; Checler, F.; Greenfield, J.P.; Haroutunian, V.; Buxbaum, J.D.; Xu, H.; et al. Intraneuronal Abeta42 accumulation in human brain. Am. J. Pathol. 2000, 156, 15–20. [Google Scholar] [CrossRef]
- Kobro-Flatmoen, A.; Hormann, T.M.; Gouras, G. Intracellular Amyloid-beta in the Normal Rat Brain and Human Subjects and Its relevance for Alzheimer’s Disease. J. Alzheimers Dis. 2023, 95, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Pensalfini, A.; Albay, R., 3rd; Rasool, S.; Wu, J.W.; Hatami, A.; Arai, H.; Margol, L.; Milton, S.; Poon, W.W.; Corrada, M.M.; et al. Intracellular amyloid and the neuronal origin of Alzheimer neuritic plaques. Neurobiol. Dis. 2014, 71, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Staal, J.A.; Alexander, S.R.; Liu, Y.; Dickson, T.D.; Vickers, J.C. Characterization of cortical neuronal and glial alterations during culture of organotypic whole brain slices from neonatal and mature mice. PLoS ONE 2011, 6, e22040. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Paradiso, B.; Long, Y.S.; Liao, W.P.; Simonato, M. Evaluation of cell damage in organotypic hippocampal slice culture from adult mouse: A potential model system to study neuroprotection. Brain Res. 2011, 1385, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.L.; Kurbatskaya, K.; Hanger, D.P.; Noble, W. Inhibition of glycogen synthase kinase-3 by BTA-EG(4) reduces tau abnormalities in an organotypic brain slice culture model of Alzheimer’s disease. Sci. Rep. 2017, 7, 7434. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, J.B.; Haag, M.; Whalley, B.J.; Salbego, C.G.; Cimarosti, H. Curcumin protects organotypic hippocampal slice cultures from Abeta1-42-induced synaptic toxicity. Toxicol. In Vitro 2013, 27, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korde, D.S.; Humpel, C. A Combination of Heavy Metals and Intracellular Pathway Modulators Induces Alzheimer Disease-like Pathologies in Organotypic Brain Slices. Biomolecules 2024, 14, 165. https://doi.org/10.3390/biom14020165
Korde DS, Humpel C. A Combination of Heavy Metals and Intracellular Pathway Modulators Induces Alzheimer Disease-like Pathologies in Organotypic Brain Slices. Biomolecules. 2024; 14(2):165. https://doi.org/10.3390/biom14020165
Chicago/Turabian StyleKorde, Dhwani S., and Christian Humpel. 2024. "A Combination of Heavy Metals and Intracellular Pathway Modulators Induces Alzheimer Disease-like Pathologies in Organotypic Brain Slices" Biomolecules 14, no. 2: 165. https://doi.org/10.3390/biom14020165




