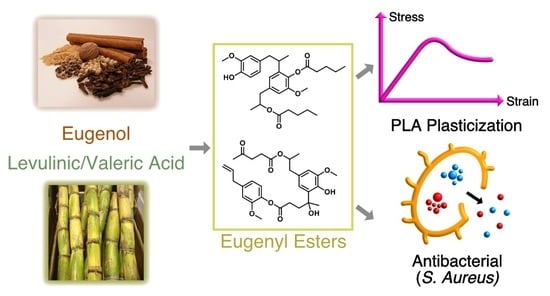
Dual-Functioning Antibacterial Eugenol-Derived Plasticizers for Polylactide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Plasticizers
2.3. Nuclear Magnetic Resonance (NMR)
2.4. Electrospray Ionization Mass Spectrometry (ESI-MS)
2.5. Preparation of PLA Films
2.6. Differential Scanning Calorimetry (DSC)
2.7. Thermal Gravimetric Analysis (TGA)
2.8. Tensile Test
2.9. Zone of Inhibition Test
3. Results & Discussion
3.1. Synthesis and Chemical Structure of the Plasticizer Candidates
3.2. Miscibility of PLA with Plasticizer Candidates
3.3. Thermal Stability of PLA Blends with Plasticizer Candidates
3.4. Mechanical Performance of PLA Blends with Plasticizer Candidates
3.5. Antibacterial Properties of The Synthesized Plasticizers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haugaard, V.K.; Frederiksen, C.S.; Poll, L.; Miquel Becker, E. Light-induced quality changes in plain yoghurt packed in polylactate and polystyrene. Eur. Food Res. Technol. 2003, 217, 61–69. [Google Scholar] [CrossRef]
- Haugaard, V.; Weber, C.; Danielsen, B.; Bertelsen, G. Quality changes in orange juice packed in materials based on polylactate. Eur. Food Res. Technol. 2002, 214, 423–428. [Google Scholar] [CrossRef]
- Koide, S.; Shi, J. Microbial and quality evaluation of green peppers stored in biodegradable film packaging. Food Control 2007, 18, 1121–1125. [Google Scholar] [CrossRef]
- Haugaard, V.K.; Danielsen, B.; Bertelsen, G. Impact of polylactate and poly(hydroxybutyrate) on food quality. Eur. Food Res. Technol. 2003, 216, 233–240. [Google Scholar] [CrossRef]
- Holm, V.; Mortensen, G.; Risbo, J. Quality changes in semi-hard cheese packaged in a poly(lactic acid) material. Food Chem. 2006, 97, 401–410. [Google Scholar] [CrossRef]
- Joo, M.; Lewandowski, N.; Auras, R.; Harte, J.; Almenar, E. Comparative shelf life study of blackberry fruit in bio-based and petroleum-based containers under retail storage conditions. Food Chem. 2011, 126, 1734–1740. [Google Scholar] [CrossRef]
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J.; Ladu, B.N.; Pariza, M.W. Safety assessment of polylactide (PLA) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner, D.A.; Springs, B.; O’Connor, R.P.; Kolstad, J.; Gruber, P.R. The Sustainability of NatureWorksTM Polylactide Polymers and IngeoTM Polylactide Fibers: An Update of the Future. Macromol. Biosci. 2004, 4, 551–564. [Google Scholar] [CrossRef]
- Catalá, R.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R. PLA and Active Packaging. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; RSC Publishing: Cambridge, UK, 2014; Chapter 10; pp. 243–265. ISBN 9781782624806. [Google Scholar]
- Arrieta, M.P.; Castro-López, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized Poly(lactic acid)–Poly(hydroxybutyrate) (PLA–PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef]
- Jin, T.; Liu, L.; Zhang, H.; Hicks, K. Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. Int. J. Food Sci. Technol. 2009, 44, 322–329. [Google Scholar] [CrossRef]
- Jin, T. Inactivation of Listeria monocytogenes in Skim Milk and Liquid Egg White by Antimicrobial Bottle Coating with Polylactic Acid and Nisin. J. Food Sci. 2010, 75, M83–M88. [Google Scholar] [CrossRef] [PubMed]
- Longano, D.; Ditaranto, N.; Cioffi, N.; Di Niso, F.; Sibillano, T.; Ancona, A.; Conte, A.; Del Nobile, M.A.; Sabbatini, L.; Torsi, L. Analytical characterization of laser-generated copper nanoparticles for antibacterial composite food packaging. Anal. Bioanal. Chem. 2012, 403, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Busolo, M.A.; Fernandez, P.; Ocio, M.J.; Lagaron, J.M. Novel silver-based nanoclay as an antimicrobial in polylactic acid food packaging coatings. Food Addit. Contam. Part A 2010, 27, 1617–1626. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, H.; Boyd, G. Incorporation of Preservatives in Polylactic Acid Films for Inactivating Escherichia coli O157:H7 and Extending Microbiological Shelf Life of Strawberry Puree. J. Food Prot. 2010, 73, 812–818. [Google Scholar] [CrossRef]
- Theinsathid, P.; Visessanguan, W.; Kruenate, J.; Kingcha, Y.; Keeratipibul, S. Antimicrobial Activity of Lauric Arginate-Coated Polylactic Acid Films against Listeria monocytogenes and Salmonella Typhimurium on Cooked Sliced Ham. J. Food Sci. 2012, 77, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial nanocomposite films made of poly(lactic acid)–cellulose nanocrystals (PLA–CNC) in food applications—Part B: Effect of oregano essential oil release on the inactivation of Listeria monocytogenes in mixed vegetables. Cellulose 2014, 21, 4271–4285. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, Y.; Yuan, M.; Li, L.; Chen, H.; Cao, J.; Yang, J. Characterization of an antimicrobial poly(lactic acid) film prepared with poly( ε -caprolactone) and thymol for active packaging. Polym. Adv. Technol. 2014, 25, 948–954. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Soy protein–Poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Kayaci, F.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial Electrospun Poly(lactic acid) (PLA) Nanofibrous Webs Incorporating Triclosan/Cyclodextrin Inclusion Complexes. J. Agric. Food Chem. 2013, 61, 3901–3908. [Google Scholar] [CrossRef]
- Bie, P.; Liu, P.; Yu, L.; Li, X.; Chen, L.; Xie, F. The properties of antimicrobial films derived from poly(lactic acid)/starch/chitosan blended matrix. Carbohydr. Polym. 2013, 98, 959–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Immergut, E.H.; Mark, H.F. Principles of Plasticization. In Plasticization and Plasticizer Processes; ACS Publications: Washington, DC, USA, 1965; pp. 1–26. [Google Scholar]
- Yang, X.; Hakkarainen, M. Migration resistant glucose esters as bioplasticizers for polylactide. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Odelius, K.; Hakkarainen, M. Poly(lactide)-g-poly(butylene succinate-co-adipate) with High Crystallization Capacity and Migration Resistance. Materials (Basel) 2016, 9, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Clénet, J.; Xu, H.; Odelius, K.; Hakkarainen, M. Two Step Extrusion Process: From Thermal Recycling of PHB to Plasticized PLA by Reactive Extrusion Grafting of PHB Degradation Products onto PLA Chains. Macromolecules 2015, 48, 2509–2518. [Google Scholar] [CrossRef]
- Azwar, E.; Yin, B.; Hakkarainen, M. Liquefied biomass derived plasticizer for polylactide. J. Chem. Technol. Biotechnol. 2013, 88, 897–903. [Google Scholar] [CrossRef]
- Xuan, W.; Hakkarainen, M.; Odelius, K. Levulinic Acid as a Versatile Building Block for Plasticizer Design. ACS Sustain. Chem. Eng. 2019, 7, 12552–12562. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Title 21: Food and Drugs; PART 181—PRIOR-SANCTIONED FOOD INGREDIENTS; Subpart B—Specific Prior-Sanctioned Food Ingredients; §181.27 Plasticizers; U.S. Department of Health and Human Services: Washington, DC, USA, 2020.
- U.S. Food and Drug Administration. Inventory of Effective Food Contact Substance (FCS) Notifications. Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=FCN&sort=FCN_No&order=DESC&startrow=1&type=advanced&search=¤¤plasticizer¤ (accessed on 22 April 2020).
- Jamshidian, M.; Arab Tehrany, E.; Cleymand, F.; Leconte, S.; Falher, T.; Desobry, S. Effects of synthetic phenolic antioxidants on physical, structural, mechanical and barrier properties of poly lactic acid film. Carbohydr. Polym. 2012, 87, 1763–1773. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Desobry, S. Antioxidants Release from Solvent-Cast PLA Film: Investigation of PLA Antioxidant-Active Packaging. Food Bioprocess Technol. 2013, 6, 1450–1463. [Google Scholar] [CrossRef]
- Rigoussen, A.; Verge, P.; Raquez, J.-M.; Dubois, P. Natural Phenolic Antioxidants As a Source of Biocompatibilizers for Immiscible Polymer Blends. ACS Sustain. Chem. Eng. 2018, 6, 13349–13357. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girão Da Cruz, M.T.; Cordeiro, M.N.D.S.; Milhazes, N.; Borges, F.; Marques, M.P.M. Phenolic acid derivatives with potential anticancer properties-A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorganic Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, H.K.; Plewa, M.J.; Wagner, E.D.; VanBriesen, J.M.; Burnett, D.B.; Cizmas, L.H.; Richardson, S.D. Identification and Comparative Mammalian Cell Cytotoxicity of New Iodo-Phenolic Disinfection Byproducts in Chloraminated Oil and Gas Wastewaters. Environ. Sci. Technol. Lett. 2017, 4, 475–480. [Google Scholar] [CrossRef]
- Jamarani, R.; Erythropel, H.; Nicell, J.; Leask, R.; Marić, M. How Green is Your Plasticizer? Polymers 2018, 10, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.; Brazel, C. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods-A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Gazzotti, S.; Hakkarainen, M.; Adolfsson, K.H.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. One-Pot Synthesis of Sustainable High-Performance Thermoset by Exploiting Eugenol Functionalized 1,3-Dioxolan-4-one. ACS Sustain. Chem. Eng. 2018, 6, 15201–15211. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Martino, V.P.; Jiménez, A.; Ruseckaite, R.A. Processing and characterization of poly(lactic acid) films plasticized with commercial adipates. J. Appl. Polym. Sci. 2009, 112, 2010–2018. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermúdez, J.M.; Baños, A.; Núñez, C.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Development of PLA films containing oregano essential oil ( Origanum vulgare L. virens ) intended for use in food packaging. Food Addit. Contam. Part A 2016, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Oliveira, R.V.B.; Reiznautt, Q.B.; Samios, D.; Nachtigall, S.M.B. Sunflower-oil biodiesel-oligoesters/polylactide blends: Plasticizing effect and ageing. Polym. Test. 2014, 39, 23–29. [Google Scholar] [CrossRef]
- Orue, A.; Eceiza, A.; Arbelaiz, A. Preparation and characterization of poly(lactic acid) plasticized with vegetable oils and reinforced with sisal fibers. Ind. Crop. Prod. 2018, 112, 170–180. [Google Scholar] [CrossRef]
- Greco, A.; Ferrari, F.; Maffezzoli, A. Mechanical properties of poly(lactid acid) plasticized by cardanol derivatives. Polym. Degrad. Stab. 2019, 159, 199–204. [Google Scholar] [CrossRef]
- Höglund, A.; Hakkarainen, M.; Albertsson, A.C. Migration and hydrolysis of hydrophobic polylactide plasticizer. Biomacromolecules 2010, 11, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, M. Migration of Monomeric and Polymeric PVC Plasticizers. In Chromatography for Sustainable Polymeric Materials; Springer Berlin Heidelberg: Berlin, Heidelberg, Germany, 2008; Volume 211, pp. 159–185. ISBN 9783540787624. [Google Scholar]
- Lindström, A.; Hakkarainen, M. Designed chain architecture for enhanced migration resistance and property preservation in poly(vinyl chloride)/polyester blends. Biomacromolecules 2007, 8, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.R.; Hakkarainen, M.; Albertsson, A.C. Tuning the polylactide hydrolysis rate by plasticizer architecture and hydrophilicity without introducing new migrants. Biomacromolecules 2010, 11, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Bruni, R.; Medici, A.; Andreotti, E.; Fantin, C.; Muzzoli, M.; Dehesa, M.; Romagnoli, C.; Sacchetti, G. Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chem. 2004, 85, 415–421. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, W.; Odelius, K.; Hakkarainen, M. Dual-Functioning Antibacterial Eugenol-Derived Plasticizers for Polylactide. Biomolecules 2020, 10, 1077. https://doi.org/10.3390/biom10071077
Xuan W, Odelius K, Hakkarainen M. Dual-Functioning Antibacterial Eugenol-Derived Plasticizers for Polylactide. Biomolecules. 2020; 10(7):1077. https://doi.org/10.3390/biom10071077
Chicago/Turabian StyleXuan, Wenxiang, Karin Odelius, and Minna Hakkarainen. 2020. "Dual-Functioning Antibacterial Eugenol-Derived Plasticizers for Polylactide" Biomolecules 10, no. 7: 1077. https://doi.org/10.3390/biom10071077