Non-Targeted UHPLC-Q-TOF/MS-Based Metabolomics Reveals a Metabolic Shift from Glucose to Glutamine in CPB Cells during ISKNV Infection Cycle
Abstract
:1. Introduction
2. Results
2.1. Quality Control of Untargeted Metabolomics Analysis
2.2. Identification of Differential Metabolites from ISKNV-Infected CPB Cells
2.3. Functional Annotation and Enrichment Analysis of Differential Metabolites
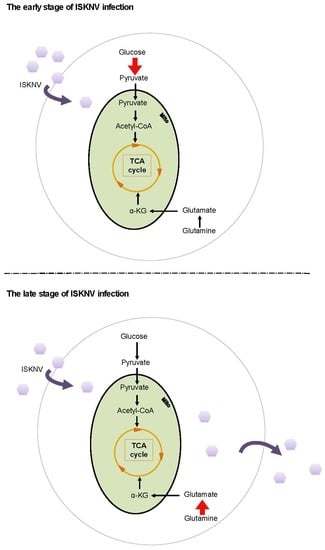
2.4. Analysis of Differential Metabolites Related to Glucose Metabolism
2.5. Analysis of Differential Metabolites Related to Amino Acid Metabolism
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Sample Acquisition
4.3. Sample Preparation
4.4. UHPLC-Q-TOF/MS Analysis
4.5. Data Analysis
4.6. Determination of Glucose Consumption in Supernatant
4.7. L-Glutamine and D-Asparate Replenishing Experiment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Q.S.; Li, H.X.; Pan, J.H.; Huang, B.Z. Research on the pathogen of the outbreak-infective disease of Siniperca chuatsi. J. Fish. China 1997, 21, 56–60. [Google Scholar]
- He, J.; Wong, S.; Huang, Z.; Kang, Z. Identifcation of outbreak and infectious diseases pathogen of Siniperca chuatsi. Acta Sci. Nat. Univ. Sunyatseni 1998, 5, 25–31. [Google Scholar]
- Chinchar, V.G.; Essbauer, S.; He, J.G.; Hyatt, A.; Miyazaki, T.; Seligy, V.; Williams, T. VIIIth Report of the International Committee on Taxonomy of Viruses; Elsevier/Academic Press: London, UK, 2005. [Google Scholar]
- Fu, X.; Li, N.; Liu, L.; Lin, Q.; Wang, F.; Lai, Y.; Jiang, H.; Pan, H.; Shi, C.; Wu, S. Genotype and host range analysis of infectious spleen and kidney necrosis virus (ISKNV). Virus Genes 2011, 42, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lu, L.; Weng, S.P.; Huang, J.N.; Chan, S.M.; He, J.G. Molecular epidemiology and phylogenetic analysis of a marine fish infectious spleen and kidney necrosis virus-like (ISKNV-like) virus. Arch. Virol. 2007, 152, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Lai, Y.; Luo, X.; Wang, Y.; Shi, C.; Huang, Z.; Wu, S.; Su, J. A novel fish cell line derived from the brain of Chinese perch Siniperca chuatsi: Development and characterization. J. Fish Biol. 2015, 86, 32–45. [Google Scholar] [CrossRef]
- Dong, C.; Weng, S.; Shi, X.; Xu, X.; Shi, N.; He, J. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef]
- Hu, X.; Fu, X.; Li, N.; Dong, X.; Zhao, L.; Lan, J.; Ji, W.; Zhou, W.; Ai, T.; Wu, S.; et al. Transcriptomic analysis of Mandarin fish brain cells infected with infectious spleen and kidney necrosis virus with an emphasis on retinoic acid-inducible gene 1-like receptors and apoptosis pathways. Fish Shellfish Immunol. 2015, 45, 619–629. [Google Scholar] [CrossRef]
- Wu, S.; Yu, L.; Fu, X.; Yan, X.; Lin, Q.; Liu, L.; Liang, H.; Li, N. iTRAQ-based proteomic profile analysis of ISKNV-infected CPB cells with emphasizing on glucose metabolism, apoptosis and autophagy pathways. Fish Shellfish Immunol. 2018, 79, 102–111. [Google Scholar] [CrossRef]
- He, J.G.; Deng, M.; Weng, S.P.; Li, Z.; Zhou, S.Y.; Long, Q.X.; Wang, X.Z.; Chan, S.-M. Complete Genome Analysis of the Mandarin Fish Infectious Spleen and Kidney Necrosis Iridovirus. Virology 2001, 291, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Fu, X.; Li, N.; Lin, Q.; Liu, L.; Wu, S. Molecular characterization and expression pattern of tumor suppressor protein p53 in mandarin fish, Siniperca chuatsi following virus challenge. Fish Shellfish Immunol. 2016, 51, 392–400. [Google Scholar] [CrossRef]
- Xu, X.; Huang, L.; Weng, S.; Wang, J.; Lin, T.; Tang, J.; Li, Z.; Lu, Q.; Xia, Q.; Yu, X.; et al. Tetraodon nigroviridis as a nonlethal model of infectious spleen and kidney necrosis virus (ISKNV) infection. Virology 2010, 406, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Tang, J.; Li, M.; Fu, Y.; Dong, C.; Zhong, J.F.; He, J. Immunological evaluation of Vibrio alginolyticus, Vibrio harveyi, Vibrio vulnificus and infectious spleen and kidney necrosis virus (ISKNV) combined-vaccine efficacy in Epinephelus coioides. Vet. Immunol. Immunopathol. 2012, 150, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Weng, S.; Huang, Z.; Lu, J.; Lan, D.; Zhong, X.; Yu, X.; Xu, A.; He, J. A zebrafish (Danio rerio) model of infectious spleen and kidney necrosis virus (ISKNV) infection. Virology 2008, 376, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Lai, Y.; Liu, L.; Lin, Q.; Shi, C.; Huang, Z.; Wu, S. Protective immunity against iridovirus disease in mandarin fish, induced by recombinant major capsid protein of infectious spleen and kidney necrosis virus. Fish Shellfish Immunol. 2012, 33, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Hayton, S.; Maker, G.L.; Mullaney, I.; Trengove, R.D. Experimental design and reporting standards for metabolomics studies of mammalian cell lines. Cell. Mol. Life Sci. 2017, 74, 4421–4441. [Google Scholar] [CrossRef] [PubMed]
- León, Z.; García-Cañaveras, J.C.; Donato, M.T.; Lahoz, A.; García-Cañaveras, J.C. Mammalian cell metabolomics: Experimental design and sample preparation. Electrophoresis 2013, 34, 2762–2775. [Google Scholar] [CrossRef]
- Blow, N. Metabolomics: Biochemistry’s new look. Nature 2008, 455, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Semmo, N.; Weber, T.; Idle, J.R.; Beyoglu, D. Metabolomics reveals that aldose reductase activity due to AKR1B10 is upregulated in hepatitis C virus infection. J. Viral Hepat. 2015, 22, 617–624. [Google Scholar] [CrossRef]
- Delgado, T.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Global Metabolic Profiling of Infection by an Oncogenic Virus: KSHV Induces and Requires Lipogenesis for Survival of Latent Infection. PLoS Pathog. 2012, 8, 1002866. [Google Scholar] [CrossRef]
- Ferrarini, A.; Righetti, L.; Martínez, M.P.; Fernández-López, M.; Mastrangelo, A.; Horcajada, J.P.; Betbesé, A.; Esteban, A.; Ordóñez, J.; Gea, J.; et al. Discriminant biomarkers of acute respiratory distress syndrome associated to H1N1 influenza identified by metabolomics HPLC-QTOF-MS/MS platform. Electrophoresis 2017, 38, 2341–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Hou, J.; Fang, J.; Lee, Y.H.; Costa, V.V.; Wong, L.H.; Chen, Q.; Ooi, E.E.; Tannenbaum, S.R.; Chen, J.; et al. Serum Metabolomics Investigation of Humanized Mouse Model of Dengue Virus Infection. J. Virol. 2017, 91, e00386-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.-A.; Huang, Y.-T.; Chen, I.-T.; Lee, D.-Y.; Hsieh, Y.-C.; Li, C.-Y.; Ng, T.H.; Liang, S.-Y.; Lin, S.-Y.; Huang, S.-W.; et al. An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway. PLoS Pathog. 2014, 10, e1004196. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hu, X.; Li, N.; Zheng, F.; Dong, X.; Duan, J.; Lin, Q.; Tu, J.; Zhao, L.; Huang, Z.; et al. Glutamine and glutaminolysis are required for efficient replication of infectious spleen and kidney necrosis virus in Chinese perch brain cells. Oncotarget 2017, 8, 2400–2412. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis_Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Chen, Y.M.; Li, C.Y.; Chang, Y.H.; Liang, S.Y.; Lin, S.Y.; Lin, C.Y.; Chang, S.H.; Wang, Y.J.; Khoo, K.H.; et al. To complete its replication cycle, a shrimp virus changes the population of long chain fatty acids during infection via the PI3K-Akt-mTOR-HIF1alpha pathway. Dev. Comp. Immunol. 2015, 53, 85–95. [Google Scholar] [CrossRef]
- Guo, C.-H.; Sun, T.-T.; Weng, X.-D.; Zhang, J.-C.; Chen, J.-X.; Deng, G.-J. The investigation of glucose metabolism and insulin secretion in subjects of chronic hepatitis B with cirrhosis. Int. J. Clin. Exp. Pathol. 2015, 8, 13381–13386. [Google Scholar]
- Tso, F.Y.; Kossenkov, A.V.; Lidenge, S.J.; Ngalamika, O.; Ngowi, J.R.; Mwaiselage, J.; Wickramasinghe, J.; Kwon, E.H.; West, J.T.; Lieberman, P.M.; et al. RNA-Seq of Kaposi’s sarcoma reveals alterations in glucose and lipid metabolism. PLoS Pathog. 2018, 14, e1006844. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Siqueira, L.O.; Zeidler, J.D.; Sousa, B.G.; Ferreira, T.; Da Poian, A.T. Anaplerotic Role of Glucose in the Oxidation of Endogenous Fatty Acids during Dengue Virus Infection. MSphere 2018, 3, e00458-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerat, H.; Imache, M.R.; Polyte, J.; Gaudin, A.; Mercey, M.; Donati, F.; Baudesson, C.; Higgs, M.R.; Picard, A.; Magnan, C.; et al. Hepatitis C virus induces a prediabetic state by directly impairing hepatic glucose metabolism in mice. J. Boil. Chem. 2017, 292, 12860–12873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcheray, F.; Léone, C.; Samah, B.; Rimaniol, A.-C.; Dereuddre-Bosquet, N.; Gras, G. Glutamate metabolism in HIV-infected macrophages: Implications for the CNS. Am. J. Physiol. Physiol. 2006, 291, C618–C626. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Maguire, T.G.; Alwine, J.C. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 2010, 84, 1867–1873. [Google Scholar] [CrossRef]
- Fontaine, K.A.; Camarda, R.; Lagunoff, M. Vaccinia Virus Requires Glutamine but Not Glucose for Efficient Replication. J. Virol. 2014, 88, 4366–4374. [Google Scholar] [CrossRef] [Green Version]
- Wise, D.R.; Ward, P.S.; Shay, J.E.; Cross, J.R.; Gruber, J.J.; Sachdeva, U.M.; Platt, J.M.; DeMatteo, R.G.; Simon, M.C.; Thompson, C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 2011, 108, 19611–19616. [Google Scholar] [CrossRef]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2011, 481, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2011, 481, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Filipp, F.V.; Ratnikov, B.; De Ingeniis, J.; Smith, J.W.; Osterman, A.L.; Scott, D.A. Glutamine-fueled mitochondrial metabolism is decoupled from glycolysis in melanoma. Pigment. Cell Melanoma Res. 2012, 25, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Fendt, S.-M.; Bell, E.L.; Keibler, M.A.; Olenchock, B.A.; Mayers, J.R.; Wasylenko, T.M.; Vokes, N.I.; Guarente, L.; Heiden, M.G.V.; Stephanopoulos, G. Reductive glutamine metabolism is a function of the α-ketoglutarate to citrate ratio in cells. Nat. Commun. 2013, 4, 2236. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.L.; Ulanet, D.B.; Pirman, D.; Mahoney, C.E.; Coco, J.; Si, Y.; Chen, Y.; Huang, L.; Ren, J.; Choe, S.; et al. Differential Aspartate Usage Identifies a Subset of Cancer Cells Particularly Dependent on OGDH. Cell Rep. 2016, 17, 876–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fan, J.; Venneti, S.; Cross, J.R.; Takagi, T.; Bhinder, B.; Djaballah, H.; Kanai, M.; Cheng, E.H.; Judkins, A.R.; et al. Asparagine Plays a Critical Role in Regulating Cellular Adaptation to Glutamine Depletion. Mol. Cell 2014, 56, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.; Menon, D.; Bernfeld, E.; Mroz, V.; Kalan, S.; Loayza, D.; Foster, D.A. Aspartate Rescues S-phase Arrest Caused by Suppression of Glutamine Utilization in KRas-driven Cancer Cells*. J. Boil. Chem. 2016, 291, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Gui, D.Y.; Sullivan, L.B.; Luengo, A.; Hosios, A.M.; Bush, L.N.; Gitego, N.; Davidson, S.M.; Freinkman, E.; Thomas, C.J.; Vander Heiden, M.G. Environment Dictates Dependence on Mitochondrial Complex I for NAD+ and Aspartate Production and Determines Cancer Cell Sensitivity to Metformin. Cell Metab. 2016, 24, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.-I.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, D.; Zhang, X.; Wang, H. UHPLC-Q-TOF/MS based plasma metabolomics reveals the metabolic perturbations by manganese exposure in rat models. Metallomics 2017, 9, 192–203. [Google Scholar]
- Gao, Y.; Fang, J.; Li, W.; Wang, X.; Li, F.; Du, M.; Fang, J.; Lin, F.; Jiang, W.; Jiang, Z. Effects of atrazine on the physiology, sexual reproduction, and metabolism of eelgrass (Zostera marina L.). Aquat. Bot. 2019, 153, 8–14. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Lin, Q.; Fu, X.; Liu, L.; Liang, H.; Guo, H.; Yin, S.; Kumaresan, V.; Huang, Z.; Li, N. Application and development of a TaqMan real-time PCR for detecting infectious spleen and kidney necrosis virus in Siniperca chuatsi. Microb. Pathog. 2017, 107, 98–105. [Google Scholar] [CrossRef]










| Detection Mode | HILIC | HSS T3 |
|---|---|---|
| Positive ion | 8408 | 13,027 |
| Negative ion | 4438 | 13,285 |
| Total | 12,846 | 26,312 |
| Detection Mode | HILIC | HSS T3 | ||||
|---|---|---|---|---|---|---|
| Number of PC | R2 (cum) | Q2 (cum) | Number of PC | R2 (cum) | Q2 (cum) | |
| Positive ion | 3 | 0.9811 | 0.58625 | 4 | 0.99866 | 0.60266 |
| Negative ion | 4 | 0.99984 | 0.49266 | 5 | 0.91667 | 0.6841 |
| Time | Name | VIP | Fold Change | p-Value |
|---|---|---|---|---|
| 24 h | Eicosapentaenoic acid | 2.535 | 6.723 | 0.0058 * |
| Phenol | 1.850 | 4.428 | 0.0203 * | |
| Rutin | 2.019 | 4.307 | 0.0137 * | |
| Tyramine | 2.083 | 4.233 | 0.0041 * | |
| L-Phenylalanine | 1.783 | 3.481 | 0.0529 | |
| Hypoxanthine | 2.320 | 2.949 | 0.0000 * | |
| L-Glutamate | 1.154 | 2.721 | 0.0750 | |
| Creatinine | 2.120 | 2.470 | 0.0000 * | |
| N-Formylmethionine | 1.299 | 2.272 | 0.0111 * | |
| 2′-Deoxyguanosine 5′-monophosphate (dGMP) | 1.786 | 2.217 | 0.0361 * | |
| alpha-Linolenic acid | 1.134 | 2.114 | 0.0164 * | |
| L-Tryptophan | 1.677 | 2.102 | 0.0149 * | |
| 1.795 | 1.923 | 0.0023 * | ||
| Phosphocreatine | 1.106 | 2.100 | 0.0914 | |
| Inosine | 1.930 | 2.003 | 0.0010 * | |
| Linoleic acid | 1.155 | 1.974 | 0.0030 * | |
| Succinate | 1.745 | 1.939 | 0.0112 * | |
| L-Tyrosine | 1.608 | 1.904 | 0.0082 * | |
| 1.870 | 3.430 | 0.0472 * | ||
| Adenosine | 1.478 | 1.800 | 0.0144 * | |
| L-Asparagine | 1.448 | 1.775 | 0.0229 * | |
| Betaine | 1.574 | 1.772 | 0.0287 * | |
| L-Histidine | 1.906 | 1.657 | 0.0573 | |
| Acetyl-DL-Leucine | 1.691 | 1.643 | 0.0014 * | |
| L-Norleucine | 1.670 | 1.624 | 0.0006 * | |
| S-Methyl-5′-thioadenosine | 1.387 | 1.606 | 0.0008 * | |
| Dopamine | 1.272 | 1.605 | 0.0326 * | |
| Taurine | 1.268 | 1.582 | 0.0323 * | |
| Indole | 1.236 | 1.536 | 0.0630 | |
| Adenine | 1.053 | 1.423 | 0.0313 * | |
| Maltotriose | 1.065 | 1.417 | 0.0365 * | |
| N-Acetyl-L-aspartic acid | 1.084 | 1.369 | 0.0036 * | |
| 1.833 | 2.434 | 0.0000 * | ||
| D-Aspartic acid | 1.117 | 1.291 | 0.0359 * | |
| Spermidine | 1.217 | 0.786 | 0.0345 * | |
| GDP-mannose | 1.097 | 0.735 | 0.0220 * | |
| UDP-N-acetylglucosamine | 1.300 | 0.667 | 0.0072 * | |
| Cytidine triphosphate (CTP) | 1.127 | 0.666 | 0.0835 | |
| Uridine 5′-diphosphoglucuronic acid (UDP-D-glucuronate) | 1.372 | 0.646 | 0.0052 * | |
| Uridine 5′-triphosphate (UTP) | 1.252 | 0.639 | 0.0365 * | |
| myo-Inositol | 1.204 | 0.636 | 0.0001 * | |
| sn-Glycerol 3-phosphoethanolamine | 1.285 | 0.607 | 0.0003 * | |
| UDP-D-Galactose | 1.573 | 0.592 | 0.0012 * | |
| Guanosine 5′-monophosphate (GMP) | 1.325 | 0.567 | 0.0386 * | |
| 5′-Phosphoribosyl-5-amino-4-imidazolecarboxamide (AICAR) | 1.044 | 0.561 | 0.0481 * | |
| alpha-D-Glucose 1-phosphate | 1.301 | 0.529 | 0.0181 * | |
| D-Mannose 1-phosphate | 1.188 | 0.517 | 0.0252 * | |
| S-Adenosylmethionine | 1.795 | 0.488 | 0.0046 * | |
| cis-Aconitate | 1.115 | 0.473 | 0.0913 | |
| Cytidine 5′-diphosphocholine (CDP-choline) | 1.997 | 0.392 | 0.0057 * | |
| Citrate | 1.463 | 0.371 | 0.0649 | |
| Gutathione | 1.980 | 0.314 | 0.0326 * | |
| 72 h | D-Mannitol | 2.611 | 2.182 | 0.0021 * |
| S-Methyl-5′-thioadenosine | 2.143 | 2.135 | 0.0142 * | |
| 1.627 | 1.411 | 0.0817 | ||
| Guanosine 5′-triphosphate (GTP) | 2.066 | 2.072 | 0.0011 * | |
| D-Proline | 2.028 | 2.051 | 0.0324 * | |
| 1.230 | 1.175 | 0.0232 * | ||
| N-Acetyl-L-aspartic acid | 2.262 | 1.946 | 0.0008 * | |
| 1.923 | 1.550 | 0.0005 * | ||
| 1.955 | 1.854 | 0.0036 * | ||
| Pantothenate | 2.257 | 1.868 | 0.0202 * | |
| 1.252 | 1.211 | 0.0070 * | ||
| UDP-N-acetylglucosamine | 1.970 | 1.709 | 0.0059 * | |
| 1.419 | 1.230 | 0.0936 | ||
| S-Adenosylmethionine | 1.548 | 1.593 | 0.0596 | |
| UDP-D-Galactose | 1.728 | 1.561 | 0.0164 * | |
| S-Lactoylglutathione | 1.620 | 1.555 | 0.0847 | |
| Adenosine 5′-triphosphate (ATP) | 1.586 | 1.490 | 0.0008 * | |
| Acetyl-DL-Leucine | 1.370 | 1.452 | 0.0458 * | |
| 1.214 | 1.257 | 0.0707 | ||
| N-Acetylaspartylglutamate (NAAG) | 1.426 | 1.450 | 0.0657 | |
| Argininosuccinic acid | 1.558 | 1.376 | 0.0000 * | |
| alpha-D-Glucose 1-phosphate | 1.358 | 1.337 | 0.0391 * | |
| Glycerol 3-phosphate | 1.190 | 1.325 | 0.0802 | |
| Glycerophosphocholine | 1.210 | 1.300 | 0.0383 * | |
| 1.823 | 1.514 | 0.0039 * | ||
| L-Phenylalanine | 1.353 | 1.273 | 0.0042 * | |
| L-Methionine | 1.264 | 1.271 | 0.0062 * | |
| 1.100 | 1.144 | 0.0715 | ||
| L-Histidine | 1.208 | 1.267 | 0.0592 | |
| D-Aspartic acid | 1.207 | 1.264 | 0.0298 * | |
| sn-Glycerol 3-phosphoethanolamine | 1.143 | 1.254 | 0.0494 * | |
| 1.086 | 1.221 | 0.0983 | ||
| L-Norleucine | 1.209 | 1.227 | 0.0267 * | |
| Phosphocreatine | 1.343 | 1.215 | 0.0646 | |
| 1.097 | 1.157 | 0.0302 * | ||
| Creatine | 1.239 | 1.212 | 0.0010 * | |
| a-D-Galactose 1-phosphate | 1.188 | 1.169 | 0.0061 * | |
| Sarcosine | 1.178 | 1.168 | 0.0024 * | |
| L-Arginine | 1.098 | 0.784 | 0.0895 | |
| L-Carnosine | 1.010 | 0.776 | 0.0912 | |
| N6,N6,N6-Trimethyl-L-lysine | 1.368 | 0.729 | 0.0064 * | |
| Citrate | 1.517 | 0.711 | 0.0980 | |
| 2′-Deoxyguanosine 5′-monophosphate (dGMP) | 1.160 | 0.703 | 0.0022 * | |
| L-Proline | 1.421 | 0.686 | 0.0194 * | |
| Myristic acid | 1.088 | 0.684 | 0.0370 * | |
| Azelaic acid | 1.131 | 0.682 | 0.0401 * | |
| Inosine | 1.903 | 0.635 | 0.0021 * | |
| NG,NG-dimethyl-L-arginine(ADMA) | 1.691 | 0.630 | 0.0046 * | |
| Creatinine | 1.846 | 0.582 | 0.0149 * | |
| 1.258 | 1.186 | 0.0012 * | ||
| Tyramine | 2.186 | 0.567 | 0.0860 | |
| 2.556 | 0.325 | 0.0102 * | ||
| Eicosapentaenoic acid | 1.496 | 0.552 | 0.0913 | |
| Linoleic acid | 1.519 | 0.540 | 0.0937 | |
| Betaine | 1.490 | 0.540 | 0.0626 | |
| cis-9-Palmitoleic acid | 2.072 | 0.473 | 0.0205 * | |
| Rutin | 1.965 | 0.437 | 0.0737 | |
| Arachidonic Acid (peroxide free) | 1.858 | 0.432 | 0.0791 | |
| trans-Vaccenic acid | 1.908 | 0.418 | 0.0600 | |
| Hypoxanthine | 2.683 | 0.377 | 0.0025 * | |
| Palmitic acid | 2.329 | 0.347 | 0.0214 * | |
| Sphingosine | 2.057 | 0.178 | 0.0890 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Guo, X.; Wu, S.; Lin, Q.; Liu, L.; Liang, H.; Niu, Y.; Li, N. Non-Targeted UHPLC-Q-TOF/MS-Based Metabolomics Reveals a Metabolic Shift from Glucose to Glutamine in CPB Cells during ISKNV Infection Cycle. Metabolites 2019, 9, 174. https://doi.org/10.3390/metabo9090174
Fu X, Guo X, Wu S, Lin Q, Liu L, Liang H, Niu Y, Li N. Non-Targeted UHPLC-Q-TOF/MS-Based Metabolomics Reveals a Metabolic Shift from Glucose to Glutamine in CPB Cells during ISKNV Infection Cycle. Metabolites. 2019; 9(9):174. https://doi.org/10.3390/metabo9090174
Chicago/Turabian StyleFu, Xiaozhe, Xixi Guo, Shiwei Wu, Qiang Lin, Lihui Liu, Hongru Liang, Yinjie Niu, and Ningqiu Li. 2019. "Non-Targeted UHPLC-Q-TOF/MS-Based Metabolomics Reveals a Metabolic Shift from Glucose to Glutamine in CPB Cells during ISKNV Infection Cycle" Metabolites 9, no. 9: 174. https://doi.org/10.3390/metabo9090174