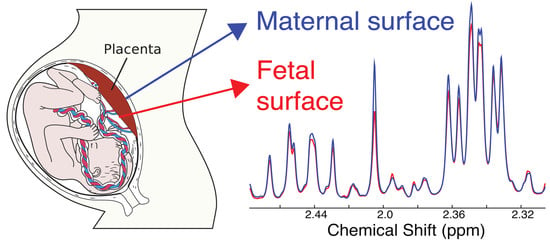
Global Metabolomics of the Placenta Reveals Distinct Metabolic Profiles between Maternal and Fetal Placental Tissues Following Delivery in Non-Labored Women
Abstract
:1. Introduction
2. Results
2.1. Metabolic Alterations between the Maternal and Fetal Surfaces of the Placenta
2.2. The Maternal Surface of the Placenta Reveals Metabolites That Are Sensitive to the Timing of Collection Following Delivery
2.3. The Fetal Surface of the Placenta Shows Less Sensitivity to Timing of Collection Following Delivery
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Tissue Preparation
4.3. Metabolomic Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carter, A.M. Placental oxygen consumption. Part I: In vivo studies—A review. Placenta 2000, 21, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Kennaugh, J.M.; Battaglia, F.C.; Makowski, E.L.; Meschia, G. Metabolic and circulatory studies of fetal lamb at midgestation. Am. J. Physiol. 1986, 250, E538–E544. [Google Scholar] [CrossRef] [PubMed]
- Aldoretta, P.W.; Hay, W.W. Effect of glucose supply on ovine uteroplacental glucose metabolism. Am. J. Physiol. 1999, 277, R947–R958. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, M.; Chung, M.; Wilkening, R.B.; Fennessey, P.V.; Battaglia, F.C.; Meschia, G. Relationship of fetal alanine uptake and placental alanine metabolism to maternal plasma alanine concentration. Am. J. Physiol. 1998, 275, E942–E950. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Marconi, A.M.; Corbetta, C.; Lanfranchi, A.; Baggiani, A.M.; Battaglia, F.C.; Pardi, G. Fetal amino-acids in normal pregnancies and in pregnancies complicated by intrauterine growth-retardation. Early Hum. Dev. 1992, 29, 183–186. [Google Scholar] [CrossRef]
- Chung, M.; Teng, C.; Timmerman, M.; Meschia, G.; Battaglia, F.C. Production and utilization of amino acids by ovine placenta in vivo. Am. J. Physiol. 1998, 274, E13–E22. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.S.; Moores, R.R.; Teng, C.; Meschia, G.; Battaglia, F.C. Main routes of plasma lactate carbon disposal in the midgestation fetal lamb. Biol. Neonate 1995, 67, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Ronzoni, S.; Marconi, A.M.; Perugino, G.; Corbetta, C.; Battaglia, F.C.; Pardi, G. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am. J. Obstet. Gynecol. 1996, 174, 1575–1583. [Google Scholar] [CrossRef]
- Chien, P.F.W.; Smith, K.; Watt, P.W.; Scrimgeour, C.M.; Taylor, D.J.; Rennie, M.J. Protein-turnover in the human fetus studied at term using stable-isotope tracer amino-acids. Am. J. Physiol. 1993, 265, E31–E35. [Google Scholar] [CrossRef] [PubMed]
- Marconi, A.M.; Paolini, C.L.; Stramare, L.; Cetin, I.; Fennessey, P.V.; Pardi, G.; Battaglia, F.C. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr. Res. 1999, 46, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Paolini, C.L.; Marconi, A.M.; Ronzoni, S.; Di Noio, M.; Fennessey, P.V.; Pardi, G.; Battaglia, F.C. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J. Clin. Endocrinol. Metab. 2001, 86, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.E.; Bloxam, D.L. Energy metabolism and glycolysis in human placental trophoblast cells during differentiation. Biochim. Biophys. Acta 1997, 1319, 283–292. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Winn, V.D.; Haimov-Kochman, R.; Paquet, A.C.; Yang, Y.J.; Madhusudhan, M.S.; Gormley, M.; Feng, K.T.V.; Bernlohr, D.A.; McDonagh, S.; Pereira, L.; et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology 2007, 148, 1059–1079. [Google Scholar] [CrossRef] [PubMed]
- Uuskula, L.; Mannik, J.; Rull, K.; Minajeva, A.; Koks, S.; Vaas, P.; Teesalu, P.; Reimand, J.; Laan, M. Mid-gestational gene expression profile in placenta and link to pregnancy complications. PLoS ONE 2012, 7, e49248. [Google Scholar] [CrossRef] [PubMed]
- Horgan, R.P.; Broadhurst, D.I.; Dunn, W.B.; Brown, M.; Heazell, A.E.; Kell, D.B.; Baker, P.N.; Kenny, L.C. Changes in the metabolic footprint of placental explant-conditioned medium cultured in different oxygen tensions from placentas of small for gestational age and normal pregnancies. Placenta 2010, 31, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Brown, M.; Worton, S.A.; Crocker, I.P.; Broadhurst, D.; Horgan, R.; Kenny, L.C.; Baker, P.N.; Kell, D.B.; Heazell, A.E. Changes in the metabolic footprint of placental explant-conditioned culture medium identifies metabolic disturbances related to hypoxia and pre-eclampsia. Placenta 2009, 30, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Van Patot, M.C.T.; Murray, A.J.; Beckey, V.; Cindrova-Davies, T.; Johns, J.; Zwerdlinger, L.; Jauniaux, E.; Burton, G.J.; Serkova, N.J. Human placental metabolic adaptation to chronic hypoxia, high altitude: Hypoxic preconditioning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R166–R172. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.E.P.; Brown, M.; Dunn, W.B.; Worton, S.A.; Crocker, I.P.; Baker, P.N.; Kell, D.B. Analysis of the metabolic footprint and tissue metabolome of placental villous explants cultured at different oxygen tensions reveals novel redox biomarkers. Placenta 2008, 29, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Austdal, M.; Thomsen, L.C.V.; Tangeras, L.H.; Skei, B.; Mathew, S.; Bjorge, L.; Austgulen, R.; Bathen, T.F.; Iversen, A.C. Metabolic profiles of placenta in preeclampsia using HR-MAS MRS metabolomics. Placenta 2015, 36, 1455–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.; Pei, L.; Chen, G.; Song, X.; Zhao, A.; Chen, T.; Su, M.; Zhang, Y.; Liu, J.; Ren, A.; et al. Metabonomic profiling of human placentas reveals different metabolic patterns among subtypes of neural tube defects. J. Proteome Res. 2014, 13, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Mossman, H.W. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Contrib. Embryol. 1937, 26, 129–246. [Google Scholar] [CrossRef]
- Serkova, N.; Bendrick-Peart, J.; Alexander, B.; Van Patot, M.C.T. Metabolite concentrations in human term placentae and their changes due to delayed collection after delivery. Placenta 2003, 24, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Mohlen, K.H.; Challier, J.C.; Dancis, J. Transfer of glutamic-acid across the human-placenta perfused invitro. Br. J. Obstet. Gynaecol. 1979, 86, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sweiry, J.H.; Page, K.R.; Dacke, C.G.; Abramovich, D.R.; Yudilevich, D.L. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human-placenta by rapid paired-tracer dilution—Studies with calcium and choline. J. Dev. Physiol. 1986, 8, 435–445. [Google Scholar] [PubMed]
- Meigs, R.A.; Sheean, L.A. Mitochondria from human term placenta. III. The role of respiration and energy generation in progesterone biosynthesis. Biochim. Biophys. Acta 1977, 489, 225–235. [Google Scholar] [CrossRef]
- Swierczynski, J.; Scislowski, P.; Aleksandrowicz, Z.; Zelewski, L. Stimulation of citrate oxidation and transport in human placental mitochondria by L-malate. Acta Biochim. Pol. 1976, 23, 93–102. [Google Scholar] [PubMed]
- Pinto, J.; Barros, A.S.; Domingues, M.R.; Goodfellow, B.J.; Galhano, E.; Pita, C.; Almeida Mdo, C.; Carreira, I.M.; Gil, A.M. Following healthy pregnancy by NMR metabolomics of plasma and correlation to urine. J. Proteome Res. 2015, 14, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Hellmuth, C.; Uhl, O.; Buss, C.; Wadhwa, P.D.; Koletzko, B.; Entringer, S. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS ONE 2015, 10, e0145794. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.A.; Hardell, L.I. Very low-density lipoproteins in cord blood. Clin. Chim. Acta 1978, 90, 295–296. [Google Scholar] [CrossRef]
- Okada, T.; Yonezawa, R.; Miyashita, M.; Mugishima, H. Triglyceride concentrations in very low-density lipoprotein fraction in cord blood during 32–35 week gestation. Early Hum. Dev. 2011, 87, 451. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, R.; Okada, T.; Kitamura, T.; Fujita, H.; Inami, I.; Makimoto, M.; Hosono, S.; Minato, M.; Takahashi, S.; Mugishima, H.; et al. Very low-density lipoprotein in the cord blood of preterm neonates. Metabolism 2009, 58, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.A. Fatty acid biosynthesis in developing fetal lung. Am. J. Physiol. 1989, 257, L195–L201. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Stover, P.J. Folate-mediated one-carbon metabolism. Vitam. Horm. 2008, 79, 1–44. [Google Scholar] [PubMed]
- Washburn, S.E.; Caudill, M.A.; Malysheva, O.; MacFarlane, A.J.; Behan, N.A.; Harnett, B.; MacMillan, L.; Pongnopparat, T.; Brosnan, J.T.; Brosnan, M.E. Formate metabolism in fetal and neonatal sheep. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E921–E927. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Mischel, W. Biogenic amines of the placenta. III. Nicotinic acid and nicotinamide content of mature, immature, normal and pathological human placenta. Z. Geburtshilfe Gynakol. 1957, 148, 149–160. [Google Scholar] [PubMed]
- Dona, A.C.; Jimenez, B.; Schafer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
- Bingol, K.; Li, D.W.; Zhang, B.; Bruschweiler, R. Comprehensive metabolite identification strategy using multiple two-dimensional NMR spectra of a complex mixture implemented in the COLMARm web server. Anal. Chem. 2016, 88, 12411–12418. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1 h NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Wold, S. Multi- and Megavariate Data Analysis. Principles and Applications, 3rd ed.; Umetrics AB: Umea, Sweden, 2001. [Google Scholar]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. Pls-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar]
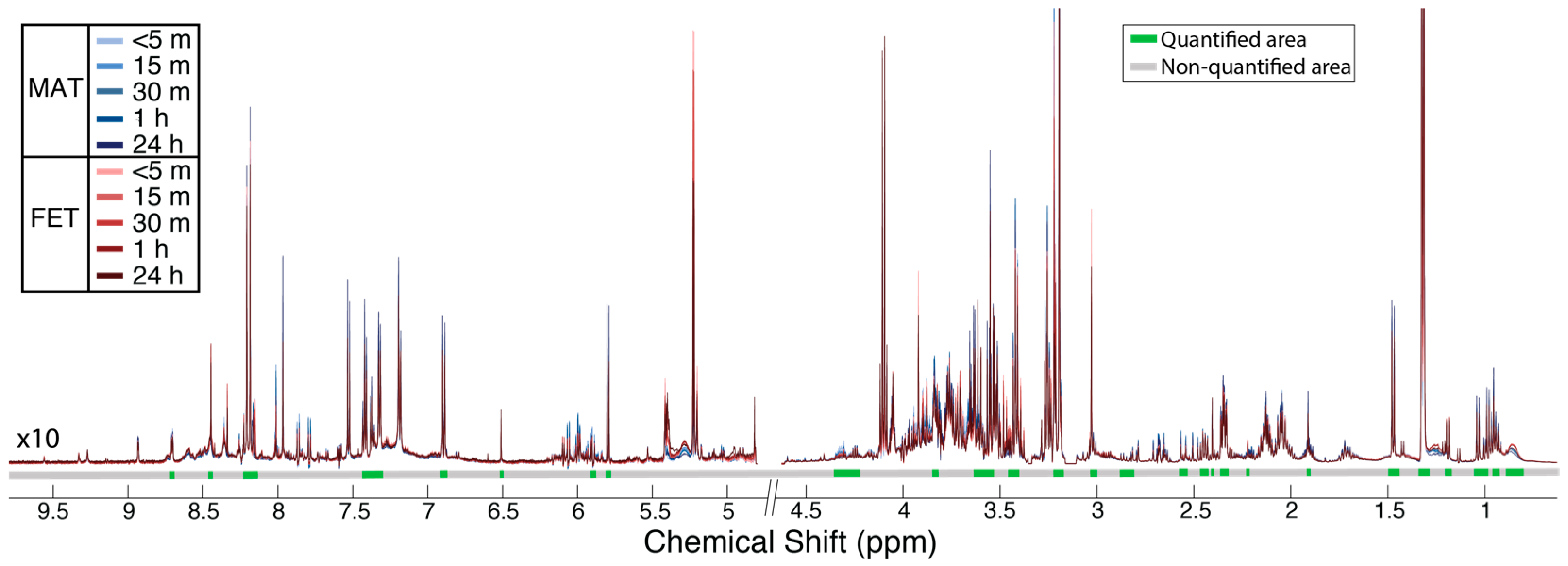

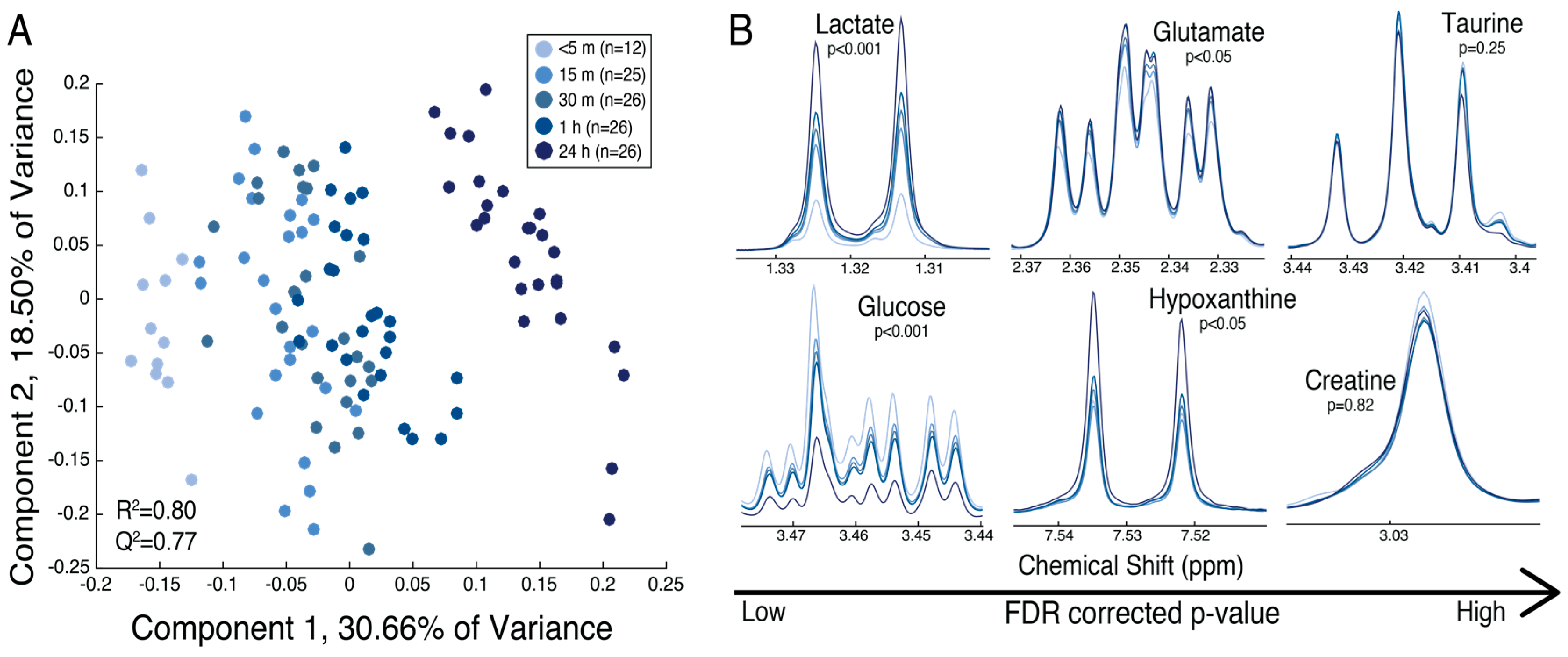
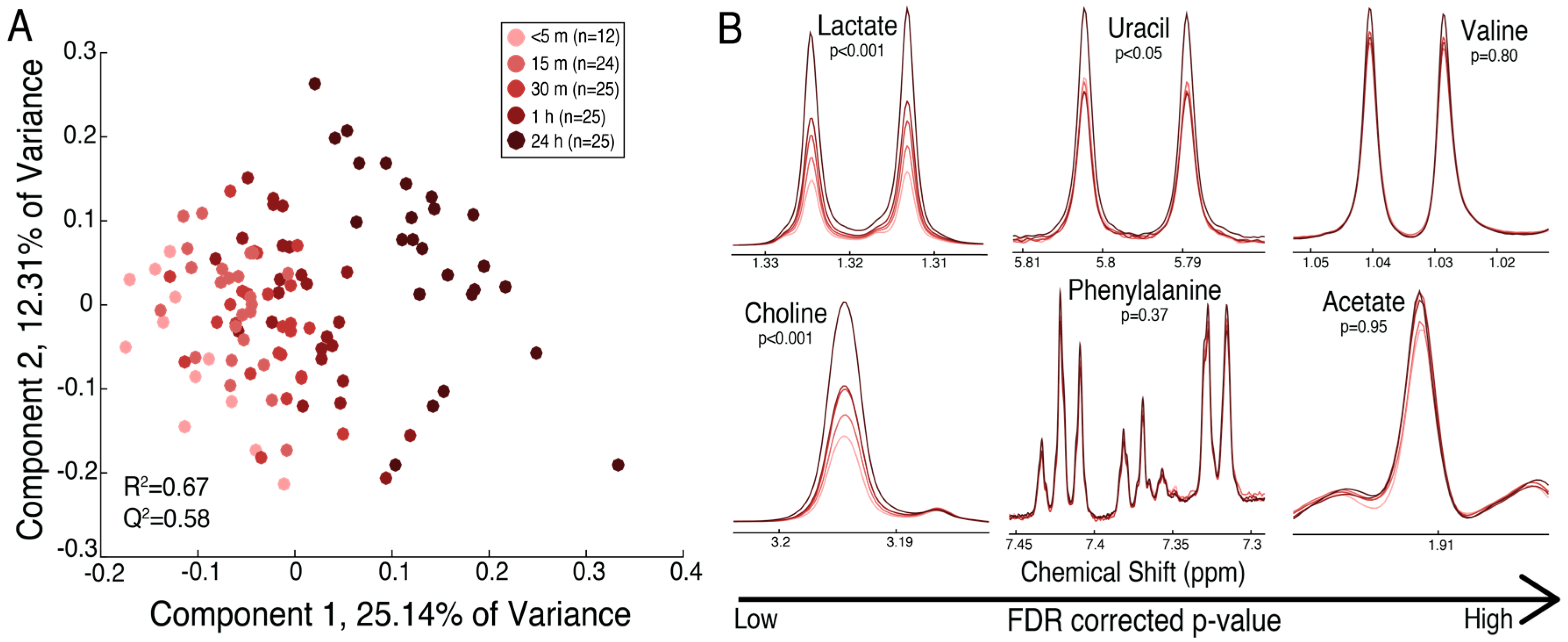
| Metabolite | Mean (SE) a | FDR-Corrected p-Value | Fold Change: Maternal vs. Fetal | |
|---|---|---|---|---|
| Maternal | Fetal | |||
| Choline | 3.372 (0.113) | 2.142 (0.165) | 3.6 × 10−4 | 1.57 |
| Serine | 1.917 (0.070) | 1.409 (0.052) | 4.0 × 10−4 | 1.36 |
| Threonine | 0.395 (0.007) | 0.349 (0.004) | 4.0 × 10−4 | 1.13 |
| Citrate | 0.475 (0.019) | 0.356 (0.012) | 9.9 × 10−4 | 1.34 |
| Glycerol | 0.210 (0.008) | 0.162 (0.006) | 4.3 × 10−3 | 1.29 |
| Acetate | 0.335 (0.008) | 0.278 (0.010) | 5.2 × 10−3 | 1.21 |
| Succinate | 0.230 (0.011) | 0.174 (0.007) | 8.5 × 10−3 | 1.32 |
| Formate | 0.037 (0.001) | 0.048 (0.002) | 9.9 × 10−3 | 0.78 |
| Aspartate | 0.152 (0.003) | 0.128 (0.006) | 0.01 | 1.19 |
| VLDL b | 1.333 (0.049) | 1.593 (0.059) | 0.03 | 0.84 |
| Taurine | 2.787 (0.099) | 2.387 (0.070) | 0.04 | 1.17 |
| Niacinamide | 0.040 (0.001) | 0.035 (0.001) | 0.04 | 1.16 |
| Alanine | 1.171 (0.029) | 1.026 (0.037) | 0.05 | 1.14 |
| GPC c | 2.769 (0.207) | 1.960 (0.160) | 0.05 | 1.41 |
| PC d | 0.987 (0.034) | 0.847 (0.036) | 0.07 | 1.17 |
| Uridine | 0.056 (0.009) | 0.031 (0.004) | 0.12 | 1.79 |
| Fumarate | 0.009 (0.001) | 0.011 (0.001) | 0.20 | 0.79 |
| Glutamine | 0.710 (0.018) | 0.652 (0.021) | 0.20 | 1.09 |
| Creatine | 0.519 (0.027) | 0.679 (0.075) | 0.21 | 0.76 |
| Glucose | 0.826 (0.033) | 0.948 (0.050) | 0.21 | 0.87 |
| Uracil | 0.058 (0.002) | 0.050 (0.004) | 0.24 | 1.15 |
| 3-HB e | 0.321 (0.021) | 0.392 (0.032) | 0.25 | 0.82 |
| Asparagine | 0.251 (0.004) | 0.261 (0.003) | 0.29 | 0.96 |
| Myo-inositol | 1.520 (0.050) | 1.378 (0.068) | 0.29 | 1.10 |
| Glycine | 0.653 (0.020) | 0.599 (0.030) | 0.37 | 1.09 |
| Inosine | 0.034 (0.006) | 0.025 (0.002) | 0.38 | 1.37 |
| Glutamate | 1.933 (0.049) | 1.839 (0.053) | 0.40 | 1.05 |
| Histidine | 0.080 (0.002) | 0.076 (0.002) | 0.40 | 1.05 |
| Acetone | 0.102 (0.004) | 0.093 (0.006) | 0.41 | 1.10 |
| Leucine | 1.057 (0.022) | 0.995 (0.047) | 0.43 | 1.06 |
| Lactate | 10.632 (0.411) | 10.033 (0.427) | 0.52 | 1.06 |
| Valine | 0.467 (0.012) | 0.448 (0.015) | 0.53 | 1.04 |
| Tyrosine | 0.118 (0.003) | 0.123 (0.005) | 0.63 | 0.96 |
| Phenylalanine | 0.536 (0.011) | 0.548 (0.021) | 0.79 | 0.98 |
| Hypoxanthine | 0.268 (0.007) | 0.271 (0.009) | 0.88 | 0.99 |
| Glutathione | 0.104 (0.005) | 0.102 (0.005) | 0.88 | 1.02 |
| Lysine | 0.390 (0.008) | 0.387 (0.012) | 0.90 | 1.01 |
| Isoleucine | 0.232 (0.005) | 0.231 (0.007) | 0.93 | 1.00 |
| Metabolite | Mean (SE) a | FDR-Corrected p-Value | Fold Change over 1 h | ||||
|---|---|---|---|---|---|---|---|
| <5 min | 15 min | 30 min | 1 h | 24 h | |||
| Glycerol | 0.147 (0.021) | 0.210 (0.014) | 0.235 (0.014) | 0.302 (0.014) | 0.514 (0.014) | 2.03 × 10−31 | 2.06 |
| Lactate | 5.370 (0.750) | 10.632 (0.520) | 11.876 (0.510) | 13.896 (0.510) | 19.354 (0.510) | 1.57 × 10−28 | 2.59 |
| Choline | 2.558 (0.297) | 3.372 (0.206) | 3.840 (0.202) | 4.459 (0.202) | 5.845 (0.202) | 1.41 × 10−15 | 1.74 |
| GPC b | 4.363 (0.318) | 2.769 (0.220) | 2.763 (0.216) | 2.395 (0.216) | 1.174 (0.216) | 8.25 × 10−11 | 0.55 |
| Glucose | 1.031 (0.059) | 0.826 (0.041) | 0.773 (0.040) | 0.741 (0.040) | 0.472 (0.040) | 8.48 × 10−11 | 0.72 |
| Glycine | 0.603 (0.047) | 0.653 (0.033) | 0.700 (0.032) | 0.772 (0.032) | 0.958 (0.032) | 1.66 × 10−9 | 1.28 |
| PC c | 1.109 (0.068) | 0.987 (0.047) | 1.050 (0.046) | 0.934 (0.046) | 0.606 (0.046) | 1.73 × 10−9 | 0.84 |
| Uracil | 0.058 (0.009) | 0.058 (0.006) | 0.063 (0.006) | 0.072 (0.006) | 0.112 (0.006) | 1.81 × 10−8 | 1.25 |
| Formate | 0.046 (0.003) | 0.037 (0.002) | 0.035 (0.002) | 0.030 (0.002) | 0.026 (0.002) | 1.81 × 10−8 | 0.66 |
| Tyrosine | 0.112 (0.009) | 0.118 (0.006) | 0.127 (0.006) | 0.141 (0.006) | 0.164 (0.006) | 8.43 × 10−7 | 1.26 |
| Phenylalanine | 0.506 (0.032) | 0.536 (0.022) | 0.563 (0.022) | 0.615 (0.022) | 0.699 (0.022) | 1.49 × 10−6 | 1.22 |
| Isoleucine | 0.209 (0.013) | 0.232 (0.009) | 0.244 (0.009) | 0.265 (0.009) | 0.292 (0.009) | 4.20 × 10−6 | 1.27 |
| Leucine | 0.925 (0.070) | 1.057 (0.048) | 1.117 (0.048) | 1.237 (0.048) | 1.346 (0.048) | 1.23 × 10−5 | 1.34 |
| Acetate | 0.322 (0.019) | 0.335 (0.013) | 0.366 (0.013) | 0.392 (0.013) | 0.423 (0.013) | 1.24 × 10−5 | 1.22 |
| Valine | 0.441 (0.032) | 0.467 (0.022) | 0.499 (0.021) | 0.544 (0.021) | 0.604 (0.021) | 6.16 × 10−5 | 1.23 |
| Citrate | 0.421 (0.028) | 0.475 (0.019) | 0.487 (0.019) | 0.450 (0.019) | 0.360 (0.019) | 9.97 × 10−5 | 1.07 |
| Alanine | 1.051 (0.081) | 1.171 (0.056) | 1.229 (0.055) | 1.324 (0.055) | 1.452 (0.055) | 8.05 × 10−4 | 1.26 |
| Glutamine | 0.615 (0.029) | 0.710 (0.020) | 0.739 (0.020) | 0.756 (0.020) | 0.707 (0.020) | 4.71 × 10−3 | 1.23 |
| Threonine | 0.381 (0.009) | 0.395 (0.006) | 0.393 (0.006) | 0.388 (0.006) | 0.364 (0.006) | 0.01 | 1.02 |
| Glutathione | 0.085 (0.009) | 0.104 (0.006) | 0.114 (0.006) | 0.099 (0.006) | 0.086 (0.006) | 0.01 | 1.16 |
| Hypoxanthine | 0.288 (0.016) | 0.268 (0.011) | 0.278 (0.011) | 0.280 (0.011) | 0.319 (0.011) | 0.02 | 0.97 |
| Succinate | 0.168 (0.021) | 0.230 (0.014) | 0.239 (0.014) | 0.252 (0.014) | 0.248 (0.014) | 0.03 | 1.50 |
| Niacinamide | 0.048 (0.002) | 0.040 (0.001) | 0.039 (0.001) | 0.040 (0.001) | 0.042 (0.001) | 0.03 | 0.84 |
| VLDL d | 1.293 (0.097) | 1.333 (0.067) | 1.250 (0.066) | 1.225 (0.066) | 1.033 (0.066) | 0.04 | 0.95 |
| Histidine | 0.075 (0.005) | 0.080 (0.003) | 0.078 (0.003) | 0.083 (0.003) | 0.068 (0.003) | 0.04 | 1.11 |
| Myo-inositol | 1.521 (0.081) | 1.520 (0.056) | 1.568 (0.055) | 1.559 (0.055) | 1.342 (0.055) | 0.04 | 1.02 |
| Glutamate | 1.820 (0.089) | 1.933 (0.062) | 2.001 (0.061) | 2.123 (0.061) | 2.080 (0.061) | <0.05 | 1.17 |
| Lysine | 0.375 (0.023) | 0.390 (0.016) | 0.407 (0.015) | 0.422 (0.015) | 0.448 (0.015) | >0.05 | 1.13 |
| Acetone | 0.103 (0.007) | 0.102 (0.005) | 0.105 (0.005) | 0.096 (0.005) | 0.087 (0.005) | 0.09 | 0.93 |
| Aspartate | 0.137 (0.008) | 0.152 (0.006) | 0.155 (0.005) | 0.160 (0.005) | 0.163 (0.005) | 0.12 | 1.17 |
| Inosine | 0.028 (0.006) | 0.034 (0.004) | 0.022 (0.004) | 0.021 (0.004) | 0.024 (0.004) | 0.14 | 0.72 |
| Serine | 1.784 (0.107) | 1.917 (0.074) | 1.923 (0.073) | 1.966 (0.073) | 1.736 (0.073) | 0.19 | 1.10 |
| Taurine | 2.803 (0.141) | 2.787 (0.098) | 2.792 (0.096) | 2.844 (0.096) | 2.549 (0.096) | 0.25 | 1.02 |
| Fumarate | 0.008 (0.001) | 0.009 (0.001) | 0.009 (0.001) | 0.009 (0.001) | 0.011 (0.001) | 0.29 | 1.11 |
| Asparagine | 0.259 (0.008) | 0.251 (0.005) | 0.259 (0.005) | 0.265 (0.005) | 0.263 (0.005) | 0.44 | 1.03 |
| Uridine | 0.050 (0.010) | 0.056 (0.007) | 0.040 (0.007) | 0.042 (0.007) | 0.041 (0.007) | 0.44 | 0.84 |
| Creatine | 0.560 (0.043) | 0.519 (0.030) | 0.500 (0.029) | 0.513 (0.029) | 0.535 (0.029) | 0.82 | 0.92 |
| 3-HB e | 0.297 (0.042) | 0.321 (0.029) | 0.320 (0.029) | 0.306 (0.029) | 0.318 (0.029) | 0.99 | 1.03 |
| Metabolite | Mean (SE) a | FDR-Corrected p-Value | Fold Change over 1 h | ||||
|---|---|---|---|---|---|---|---|
| <5 min | 15 min | 30 min | 1 h | 24 h | |||
| Glycerol | 0.140 (0.019) | 0.162 (0.013) | 0.184 (0.013) | 0.193 (0.013) | 0.381 (0.013) | 4.4 × 10−22 | 1.38 |
| Lactate | 7.824 (1.109) | 10.033 (0.784) | 12.127 (0.768) | 14.392 (0.768) | 22.069 (0.768) | 3.4 × 10−20 | 1.84 |
| Choline | 1.730 (0.282) | 2.142 (0.200) | 2.594 (0.196) | 2.683 (0.196) | 4.158 (0.196) | 1.5 × 10−10 | 1.55 |
| PC b | 1.089 (0.069) | 0.847 (0.049) | 0.886 (0.048) | 0.891 (0.048) | 0.589 (0.048) | 2.6 × 10−6 | 0.82 |
| GPC c | 2.443 (0.221) | 1.960 (0.157) | 1.949 (0.153) | 1.640 (0.153) | 0.980 (0.153) | 7.7 × 10−6 | 0.67 |
| Succcinate | 0.175 (0.016) | 0.174 (0.012) | 0.190 (0.011) | 0.218 (0.011) | 0.259 (0.011) | 2.3 × 10−5 | 1.24 |
| Threonine | 0.346 (0.009) | 0.349 (0.006) | 0.354 (0.006) | 0.348 (0.006) | 0.311 (0.006) | 1.2 × 10−4 | 1.00 |
| Citrate | 0.363 (0.021) | 0.356 (0.015) | 0.380 (0.014) | 0.389 (0.014) | 0.296 (0.014) | 5.9 × 10−4 | 1.07 |
| Uracil | 0.052 (0.006) | 0.050 (0.004) | 0.049 (0.004) | 0.049 (0.004) | 0.071 (0.004) | 4.4 × 10−3 | 0.94 |
| Fumarate | 0.010 (0.001) | 0.011 (0.001) | 0.011 (0.001) | 0.012 (0.001) | 0.016 (0.001) | 6.3 × 10−3 | 1.20 |
| Glycine | 0.605 (0.042) | 0.599 (0.030) | 0.616 (0.029) | 0.607 (0.029) | 0.735 (0.029) | 0.02 | 1.00 |
| Glutathione | 0.092 (0.008) | 0.102 (0.006) | 0.100 (0.006) | 0.107 (0.006) | 0.080 (0.006) | 0.04 | 1.17 |
| Niacinamide | 0.040 (0.002) | 0.035 (0.001) | 0.033 (0.001) | 0.034 (0.001) | 0.037 (0.001) | 0.04 | 0.83 |
| Asparagine | 0.255 (0.010) | 0.261 (0.007) | 0.253 (0.007) | 0.251 (0.007) | 0.232 (0.007) | 0.11 | 0.98 |
| Acetone | 0.101 (0.012) | 0.093 (0.009) | 0.101 (0.009) | 0.094 (0.009) | 0.067 (0.009) | 0.12 | 0.94 |
| Glucose | 1.140 (0.133) | 0.948 (0.094) | 1.022 (0.092) | 0.770 (0.092) | 0.751 (0.092) | 0.12 | 0.68 |
| VLDL d | 1.527 (0.114) | 1.593 (0.081) | 1.528 (0.079) | 1.491 (0.079) | 1.279 (0.079) | 0.16 | 0.98 |
| Glutamate | 1.949 (0.100) | 1.839 (0.071) | 1.924 (0.069) | 1.920 (0.069) | 1.693 (0.069) | 0.19 | 0.98 |
| Taurine | 2.638 (0.136) | 2.387 (0.096) | 2.478 (0.094) | 2.271 (0.094) | 2.269 (0.094) | 0.25 | 0.86 |
| Isoleucine | 0.216 (0.010) | 0.231 (0.007) | 0.226 (0.007) | 0.229 (0.007) | 0.244 (0.007) | 0.29 | 1.06 |
| Alanine | 1.052 (0.061) | 1.026 (0.043) | 1.030 (0.042) | 1.044 (0.042) | 1.156 (0.042) | 0.29 | 0.99 |
| Creatine | 0.997 (0.126) | 0.679 (0.089) | 0.620 (0.087) | 0.700 (0.087) | 0.776 (0.087) | 0.29 | 0.70 |
| Hypoxanthine | 0.294 (0.016) | 0.271 (0.011) | 0.248 (0.011) | 0.272 (0.011) | 0.274 (0.011) | 0.29 | 0.92 |
| Phenylalanine | 0.535 (0.025) | 0.548 (0.018) | 0.511 (0.018) | 0.520 (0.018) | 0.563 (0.018) | 0.37 | 0.97 |
| Formate | 0.053 (0.006) | 0.048 (0.004) | 0.047 (0.004) | 0.054 (0.004) | 0.043 (0.004) | 0.51 | 1.03 |
| Tyrosine | 0.124 (0.007) | 0.123 (0.005) | 0.117 (0.005) | 0.117 (0.005) | 0.129 (0.005) | 0.60 | 0.94 |
| Glutamine | 0.683 (0.033) | 0.652 (0.023) | 0.687 (0.023) | 0.707 (0.023) | 0.662 (0.023) | 0.67 | 1.03 |
| Lysine | 0.379 (0.019) | 0.387 (0.013) | 0.360 (0.013) | 0.358 (0.013) | 0.369 (0.013) | 0.67 | 0.95 |
| Uridine | 0.025 (0.008) | 0.031 (0.006) | 0.040 (0.006) | 0.035 (0.006) | 0.031 (0.006) | 0.75 | 1.43 |
| Valine | 0.451 (0.024) | 0.448 (0.017) | 0.449 (0.017) | 0.444 (0.017) | 0.476 (0.017) | 0.80 | 0.98 |
| Serine | 1.484 (0.100) | 1.409 (0.071) | 1.490 (0.069) | 1.359 (0.069) | 1.458 (0.069) | 0.80 | 0.92 |
| Inosine | 0.022 (0.007) | 0.025 (0.005) | 0.033 (0.005) | 0.029 (0.005) | 0.031 (0.005) | 0.80 | 1.33 |
| Histidine | 0.076 (0.004) | 0.076 (0.003) | 0.074 (0.003) | 0.073 (0.003) | 0.071 (0.003) | 0.87 | 0.97 |
| Leucine | 0.937 (0.057) | 0.995 (0.040) | 0.950 (0.039) | 0.944 (0.039) | 0.991 (0.039) | 0.88 | 1.01 |
| 3-HB e | 0.417 (0.066) | 0.392 (0.047) | 0.432 (0.046) | 0.438 (0.046) | 0.402 (0.046) | 0.95 | 1.05 |
| Acetate | 0.271 (0.016) | 0.278 (0.012) | 0.287 (0.011) | 0.284 (0.011) | 0.284 (0.011) | 0.95 | 1.05 |
| Aspartate | 0.122 (0.009) | 0.128 (0.006) | 0.128 (0.006) | 0.127 (0.006) | 0.123 (0.006) | 0.95 | 1.04 |
| Myo-inositol | 1.363 (0.144) | 1.378 (0.102) | 1.475 (0.100) | 1.466 (0.100) | 1.429 (0.100) | 0.95 | 1.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walejko, J.M.; Chelliah, A.; Keller-Wood, M.; Gregg, A.; Edison, A.S. Global Metabolomics of the Placenta Reveals Distinct Metabolic Profiles between Maternal and Fetal Placental Tissues Following Delivery in Non-Labored Women. Metabolites 2018, 8, 10. https://doi.org/10.3390/metabo8010010
Walejko JM, Chelliah A, Keller-Wood M, Gregg A, Edison AS. Global Metabolomics of the Placenta Reveals Distinct Metabolic Profiles between Maternal and Fetal Placental Tissues Following Delivery in Non-Labored Women. Metabolites. 2018; 8(1):10. https://doi.org/10.3390/metabo8010010
Chicago/Turabian StyleWalejko, Jacquelyn M., Anushka Chelliah, Maureen Keller-Wood, Anthony Gregg, and Arthur S. Edison. 2018. "Global Metabolomics of the Placenta Reveals Distinct Metabolic Profiles between Maternal and Fetal Placental Tissues Following Delivery in Non-Labored Women" Metabolites 8, no. 1: 10. https://doi.org/10.3390/metabo8010010