Volatile Metabolite Profiling of Durum Wheat Kernels Contaminated by Fusarium poae
Abstract
:1. Introduction
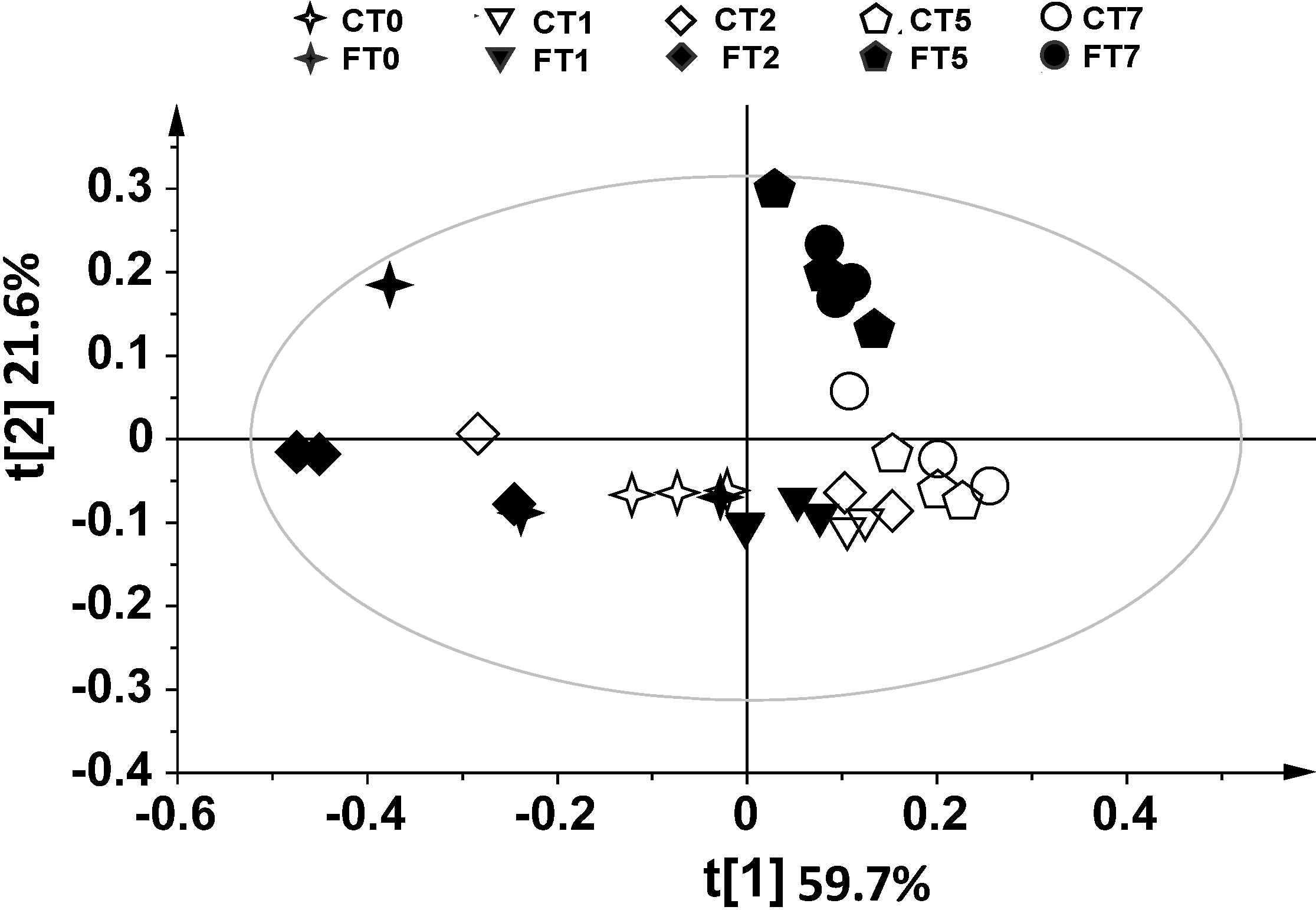
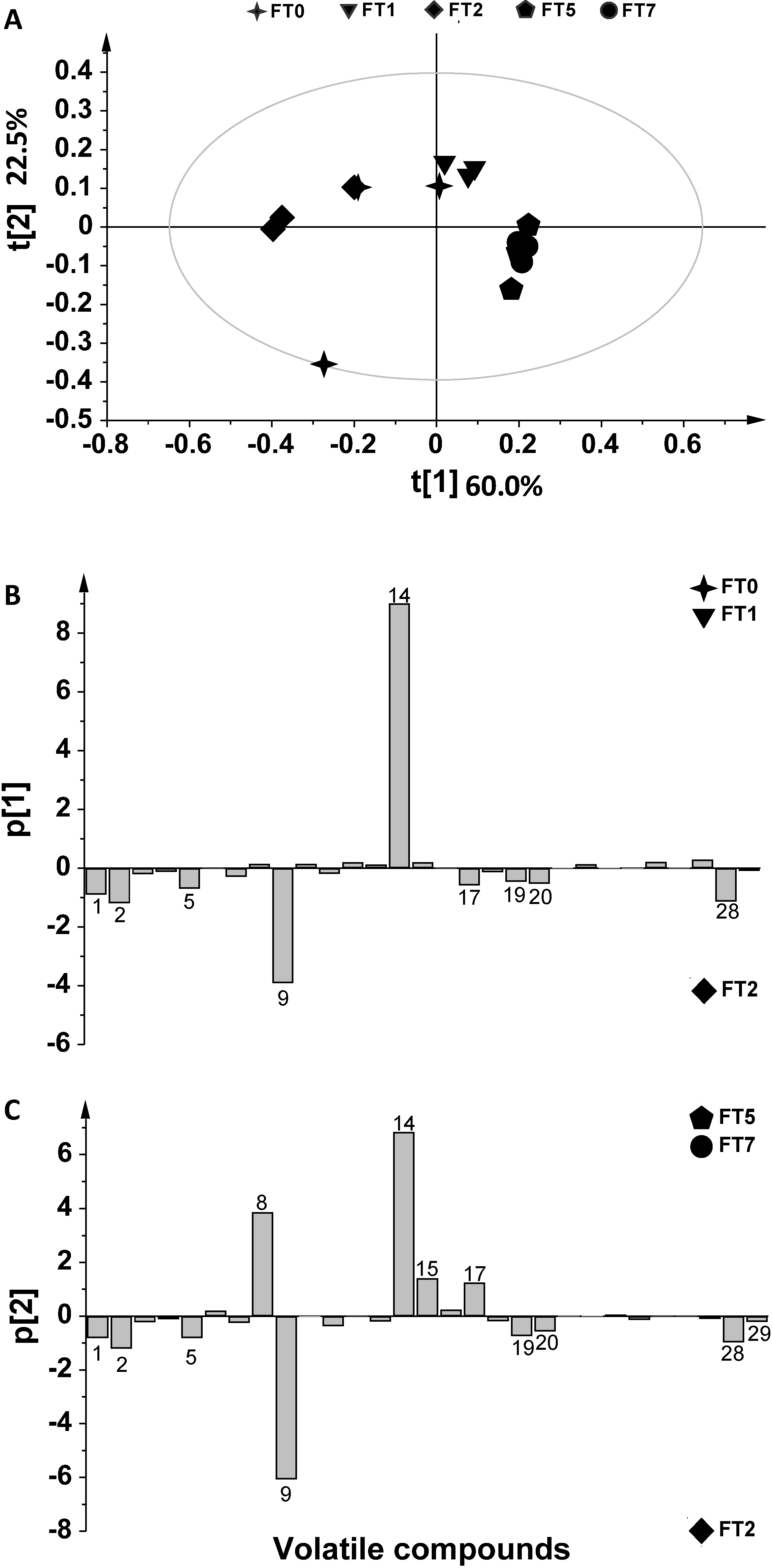
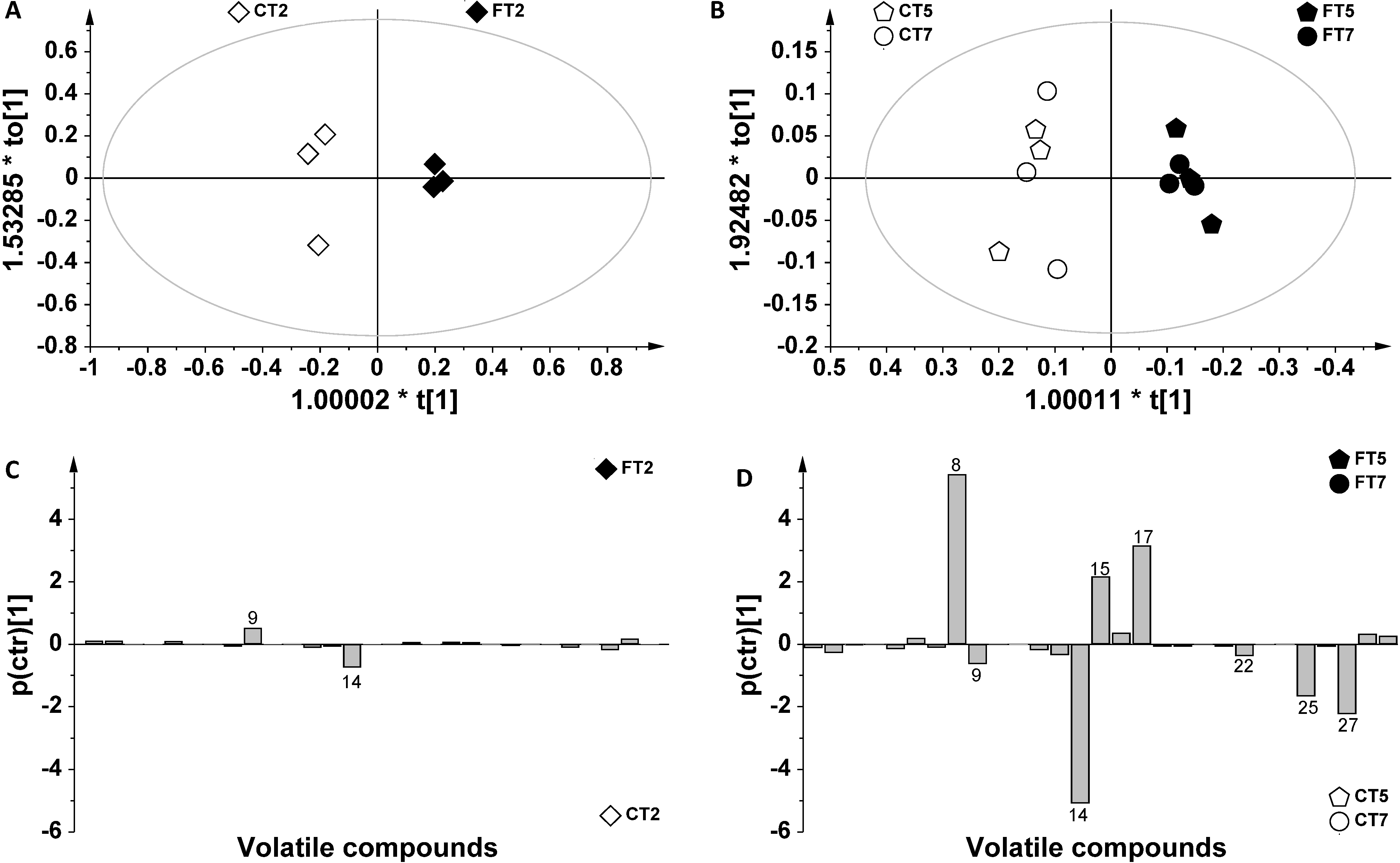
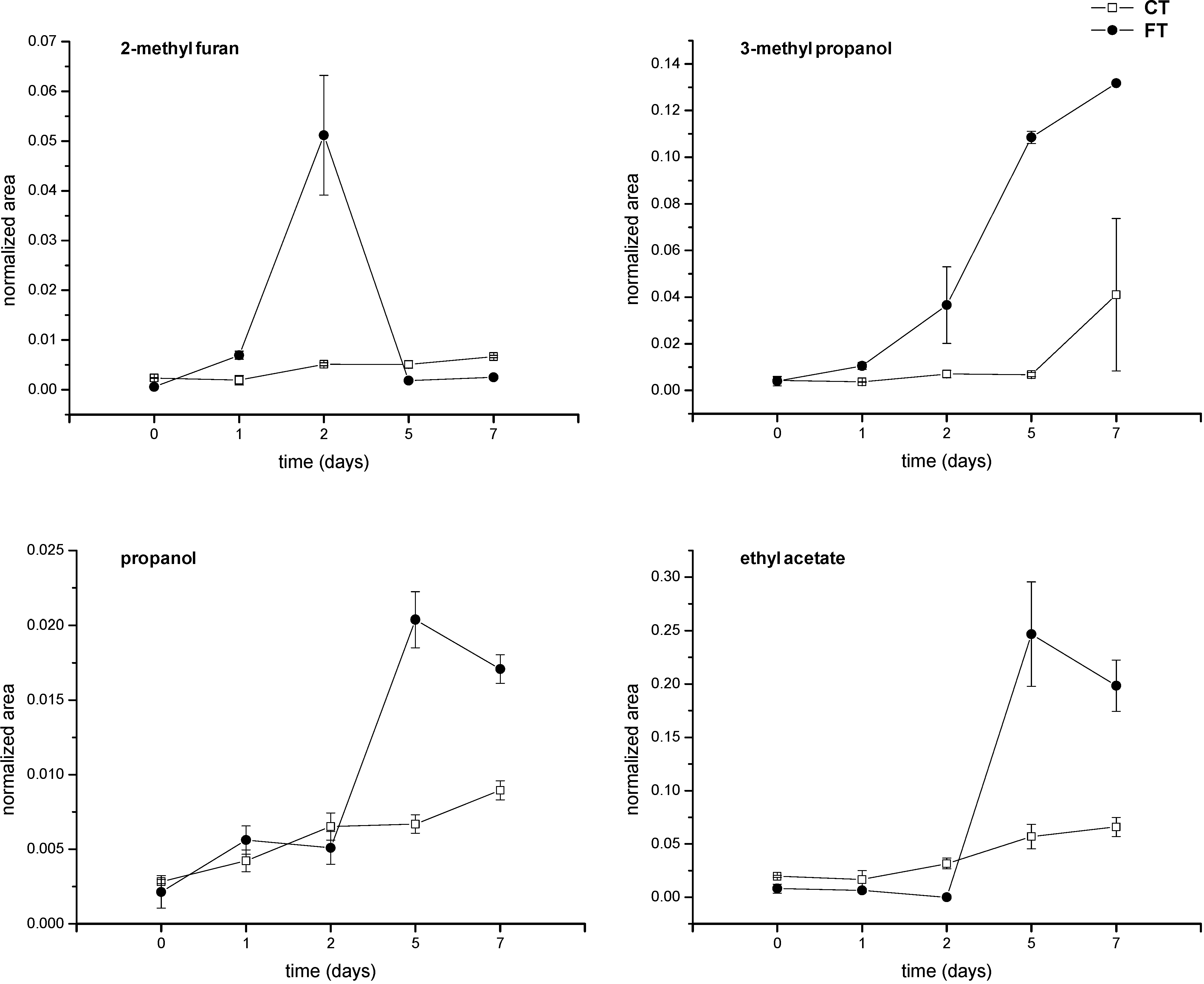
2. Results and Discussion
| Identification Number (n.) | Compound | Class |
|---|---|---|
| 1 | 2-Methylfuran | Aromatics |
| 2 | 2-Ethyl furan | Aromatics |
| 3 | 2-Pentylfuran | Aromatics |
| 4 | 2-Ethyl benzenamine | Aromatics |
| 5 | d-Limonene | Terpene |
| 6 | 4-Ethyl-2-methoxyphenol | Aromatics |
| 7 | p-Xylene | Aromatics |
| 8 | Ethyl acetate | Esters |
| 9 | Ethyl decanoate | Esters |
| 10 | Ethyl 9-decenoate | Esters |
| 11 | 2-Phenylethyl acetate | Esters |
| 12 | 3-Methylbutanal | Aldehydes |
| 13 | Hexanal | Aldehydes |
| 14 | Ethanol | Alcohols |
| 15 | 3-Methyl butanol | Alcohols |
| 16 | Propanol | Alcohols |
| 17 | 2-Methylpropanol | Alcohols |
| 18 | 2-Pentanol | Alcohols |
| 19 | Phenylethyl alcohol | Alcohols |
| 20 | 1-Octen-3-ol | Alcohols |
| 21 | Butanol | Alcohols |
| 22 | 1-Pentanol | Alcohols |
| 23 | Hexanol | Alcohols |
| 24 | 2-Pentanone | Ketones |
| 25 | 2-Butanone | Ketones |
| 26 | 2-Heptanone | Ketones |
| 27 | 3-Hydroxy-2-butanone | Ketones |
| 28 | 2,4-Dimethyl-1-hepten | Hydrocarbons |
| 29 | Acetic acid | Organic acids |

3. Experimental Section
3.1. Fungal Cultures
3.2. Preparation of Wheat Grains
3.3. Gas Chromatography and Mass Spectrometry
3.4. Multivariate Statistical Analysis (MVA) of SPME-GC/MS Data
Author Contributions
Conflicts of interest
References
- Langseth, W.; Bernhost, A.; Rundberget, T.; Kosiak, B.; Manfred, G. Mycotoxin production and cytotoxicity of Fusarium strains isolated from Norwegian cereals. Mycopathologia 1999, 144, 103–113. [Google Scholar] [CrossRef]
- Birzele, B.; Meier, A.; Hindorf, H.; Kramer, J.; Dehne, H.W. Epidemiology of Fusarium infection and deoxynivalenol content in winter wheat in the Rhineland, Germany. Eur. J. Plant Pathol. 2002, 108, 667–673. [Google Scholar] [CrossRef]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.; Doohan, F.; Brennan, J.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Monaghan, S.; Moretti, A.; et al. Within-field variability of Fusarium head blight pathogens and their associated mycotoxins. Eur. J. Plant Pathol. 2008, 120, 21–34. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Mulè, G.; Paciolla, C.; Ritieni, A. Advances on the toxicity of the cereal contamination Fusarium esadepsipeptides. Cereal Res. Commun. 2008, 36, 303–313. [Google Scholar] [CrossRef]
- Petterson, H. Nivalenol production by Fusarium poae. Mycotoxin Res. 1991, 7, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Sundheim, L.; Langseth, W. Trichothecene production and the relationship to vegetative compatibility groups in Fusarium poae. Mycopathologia 1998, 140, 105–114. [Google Scholar] [CrossRef]
- Torp, M.; Langseth, W. Production of T-2 toxin by a Fusarium resembling Fusarium poae. Mycopathologia 1999, 147, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M.N.; Paavanen-Huhtala, S.; Parikka, P.; Yli-Mattila, T. In vitro and in vivo mycotoxin production of Fusarium species isolated from Finnish grains. Arch. Phytopathol. Plant Prot. 2008, 41, 545–558. [Google Scholar] [CrossRef]
- Abramson, D.; Clear, R.M.; Smith, E.M. Trichothecene production by Fusarium spp. isolated from Manitoba grain. Can. J. Plant Pathol. 1994, 15, 147–152. [Google Scholar] [CrossRef]
- Torp, M.; Nirenberg, H. Fusarium langsethiae sp. nov. on cereals in Europe. Int. J. Food Microbiol. 2004, 95, 247–256. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Mach, R.; Alekhina, I.A.; Bulat, S.A.; Koskinen, S.; Kullnig-Gradinger, C.M.; Kubicek, C.P.; Klemsdal, S.S. Phylogenetic relationship of Fusarium langsethiae to Fusarium poae and F. sporotrichioides as inferred by IGS, ITS, b-tubulin sequence and UP-PCR hybridization analysis. Int. J. Food Microbiol. 2004, 95, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Gutleb, A.C.; Morrison, E.; Murk, A.J. Cytotoxicity assays for mycotoxins produced by Fusarium strains: A review. Environ. Toxicol. Pharmacol. 2002, 11, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Meca, G.; Soriano, J.M.; Gaspari, A.; Ritieni, A.; Moretti, A.; Mañes, J. Antifungal effects of the bioactive compounds enniatins A, A1, B, B1. Toxicon 2010, 56, 480–485. [Google Scholar] [CrossRef]
- European Communities. Commission Regulation (EC) No 1881/2006. Commission Directive 2006/1881/EC of 19 December 2006, setting maximum levels for certain contaminants in food stuffs. Off. J. Eur. Commun. 2006, L364, 5–24. [Google Scholar]
- Magan, N.; Evans, P. Volatiles as an indicator of fungal activity and differentiation between species, and the potential use of electronic nose technology for early detection of grain spoilage. J. Stored Prod. Res. 2000, 36, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, A.L.; Lappalainen, S.; Pasanen, P. Volatile organic metabolites associated with some toxic fungi and their mycotoxins. Analyst 1996, 121, 1949–1953. [Google Scholar] [CrossRef]
- Börjesson, T.; Stollman, U.; Adamek, P.; Kaspersson, A.K. Analysis of volatile compounds for detection of moulds in stored cereals. Cereal Chem. 1989, 66, 300–304. [Google Scholar]
- Börjesson, T.; Stollman, U.; Schnürer, J. Volatile metabolites and other indicators of Penicillium aurantiogriseum growth on different substrates. Appl. Environ. Microbiol. 1990, 56, 3705–3710. [Google Scholar] [PubMed]
- Börjesson, T.; Stollman, U.; Schnürer, J. Volatile metabolites produced by six fungal species compared with other indicators of fungal growth on cereal grains. Appl. Environ. Microbiol. 1992, 58, 2599–2605. [Google Scholar] [PubMed]
- Bechtel, D.B.; Kaleikau, L.A.; Gaines, R.L.; Seitz, L.M. The effects of Fusarium graminearum infection on wheat kernels. Cereal Chem. 1985, 62, 191–197. [Google Scholar]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Jelén, H.H. Use of solid phase microextraction (SPME) for profiling fungal volatile metabolites. Lett. Appl. Microbiol. 2003, 36, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Eifler, J.; Martinelli, E.; Santonico, M.; Capuano, R.; Schild, D.; di Natale, C. Differential detection of potentially hazardous Fusarium species in wheat grains by an electronic nose. PLoS One 2011, 6, e21026. [Google Scholar] [CrossRef] [PubMed]
- Lavine, B.K.; Mirjankar, N.; LeBouf, R.; Rossner, A. Prediction of mold contamination from microbial volatile organic compound profiles using head space gas chromatography/mass spectrometry. Microchem. J. 2012, 103, 119–124. [Google Scholar] [CrossRef]
- Börjesson, T.; Stollman, U.; Schnürer, J. Off-odorous compounds produced by molds on oatmeal agar: Identification and relation to other growth characteristics. J. Sci. Food Agric. 1993, 41, 2104–2111. [Google Scholar] [CrossRef]
- Del Coco, L.; Schena, F.P.; Fanizzi, F.P. 1H nuclear magnetic resonance study of olive oils commercially available as Italian products in the United States of America. Nutrients 2012, 4, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, F.; Ventrella, A.; Bianco, A.; Catucci, L.; Cafagna, I.; Gallo, V.; Mastrorilli, P.; Agostiano, A. Non-targeted 1H NMR fingerprinting and multivariate statistical analyzes for the characterization of the geographical origin of Italian sweet cherries. Food Chem. 2013, 141, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Mannina, L.; Marini, F.; Gobbino, M.; Sobolev, A.P.; Capitani, D. NMR and chemometrics in tracing European olive oils: The case study of Ligurian samples. Talanta 2010, 80, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.O.; Thrane, U. Fusarium taxonomy with relation to trichothecene formation. Toxicol. Lett. 2004, 153, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kinderlerer, J.L. Fungal strategies for detoxification of medium chain fatty acids. Int. Biodeterior. Biodegrad. 1993, 32, 213–224. [Google Scholar] [CrossRef]
- Kaminski, E.; Libbey, L.M.; Stawicki, S.; Wąsowicz, E. Identification of the predominant volatile compounds produced by Aspergillus flavus. Appl. Microbiol. 1972, 24, 721–726. [Google Scholar] [PubMed]
- Kaminski, E.; Stawicki, S.; Wasowicz, E. Volatile flavor compounds produced by molds of Aspergillus, Penicillium and fungi imperfecti. Appl. Microbiol. 1974, 27, 1001–1004. [Google Scholar] [PubMed]
- Wilkins, K.; Scholl, S. Volatile metabolites of some barley storage moulds. Int. J. Food Microbiol. 1989, 8, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.D.; Karahadian, C.; Lindsay, R.C. Musty aroma compounds produced by selected molds and Actinomycetes on agar and whole wheat bread. J. Food Prot. 1986, 49, 964–970. [Google Scholar]
- Jelén, H.H.; Grabarkiewicz-Szczesna, J. Volatile compounds of Aspergillus strains with different abilities to produce Ochratoxin A. J. Food Agric. Chem. 2005, 53, 1678–1683. [Google Scholar] [CrossRef]
- Agri-Food Toxigenic Fungi Culture Collection ITEM. Available online: http://www.ispa.cnr.it/Collection (accessed on 10 October 2014).
- ChemSpider Search and Share Chemistry. Available online: http://www.chemspider.com (accessed on 10 October 2014).
- Lindon, J.C.; Nicholson, J.K.; Holmes, E. The Handbook of Metabonomics and Metabolomics; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Eastment, H.; Krzanowski, W. Cross-Validatory choice of the number of components from a principal component analysis. Technometrics 1982, 24, 73–77. [Google Scholar]
- De Jong, S. Multivariate Calibration; Martens, H., Naes, T., Eds.; Wiley: New York, NY, USA, 1990. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laddomada, B.; Del Coco, L.; Durante, M.; Presicce, D.S.; Siciliano, P.A.; Fanizzi, F.P.; Logrieco, A.F. Volatile Metabolite Profiling of Durum Wheat Kernels Contaminated by Fusarium poae. Metabolites 2014, 4, 932-945. https://doi.org/10.3390/metabo4040932
Laddomada B, Del Coco L, Durante M, Presicce DS, Siciliano PA, Fanizzi FP, Logrieco AF. Volatile Metabolite Profiling of Durum Wheat Kernels Contaminated by Fusarium poae. Metabolites. 2014; 4(4):932-945. https://doi.org/10.3390/metabo4040932
Chicago/Turabian StyleLaddomada, Barbara, Laura Del Coco, Miriana Durante, Dominique S. Presicce, Pietro A. Siciliano, Francesco P. Fanizzi, and Antonio F. Logrieco. 2014. "Volatile Metabolite Profiling of Durum Wheat Kernels Contaminated by Fusarium poae" Metabolites 4, no. 4: 932-945. https://doi.org/10.3390/metabo4040932






