The Succinated Proteome of FH-Mutant Tumours
Abstract
:1. Introduction
2. Results and Discussion
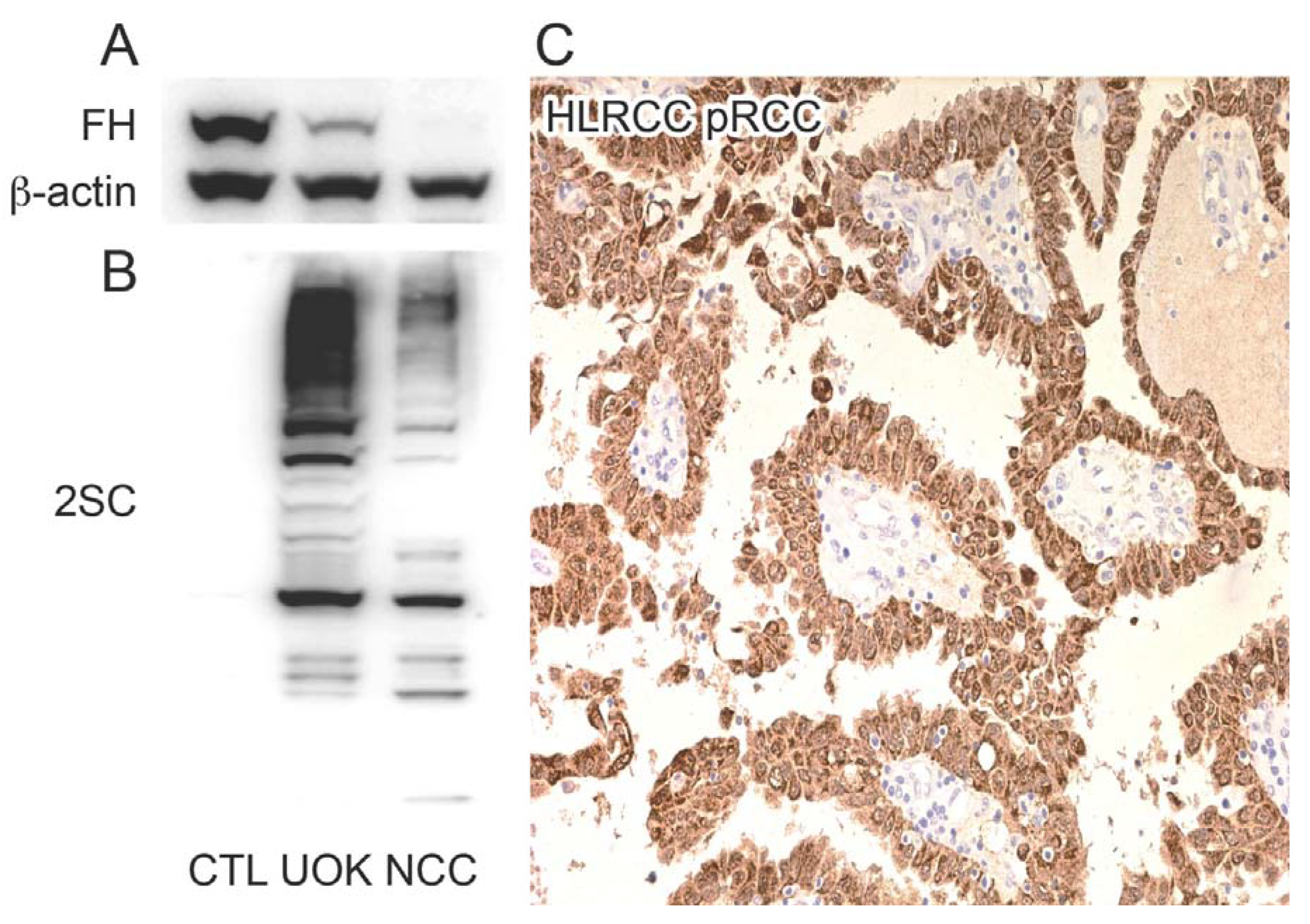
2.1. HLRCC Tumours and Derived Cell Lines Exhibit High Levels of 2SC
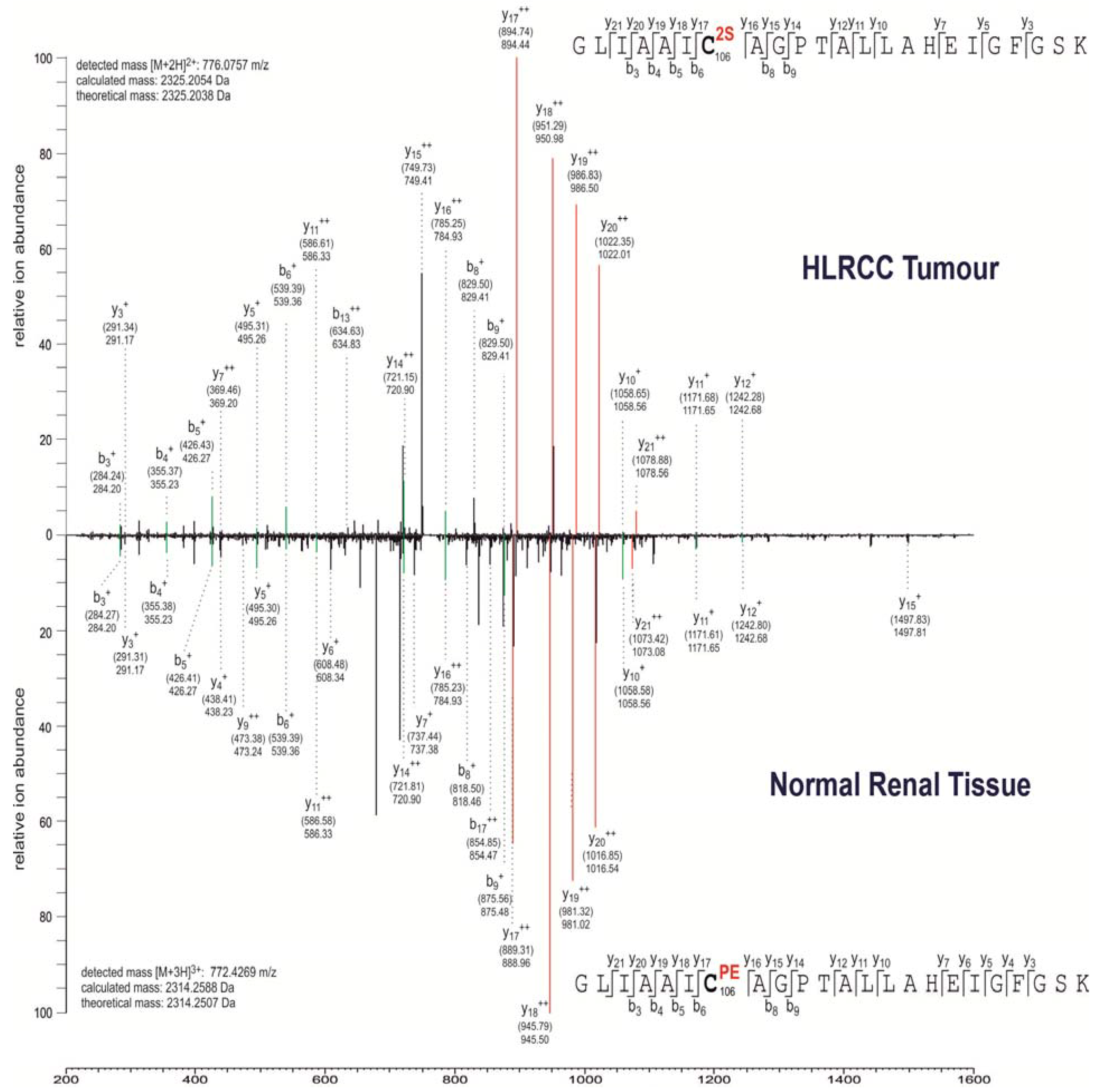
2.2. Multiple Proteins Are Succinated in FH-Mutant Cells and Tumours
| Uniprot ID | Protein Symbol | Protein Name | Succination Site | Source |
|---|---|---|---|---|
| P53396 | ACLY | ATP-citrate synthase | C20 | T, N |
| Q09666 | AHNAK | Neuroblast differentiation-associated protein AHNAK | C1833 | T, N |
| P15121 | AKR1B1 | Aldose reductase | C299 | T |
| P54886-1 | ALDH18A1 | Isoform Long of Delta-1-pyrroline-5-carboxylate synthase | C612 | T, U, N |
| Q5TYW2 | ANKRD20A1 | Ankyrin repeat domain-containing protein 20A1 | C789 | N |
| P04083 | ANXA1 | Annexin A1 | C324 | T, U |
| P16615-1 | ATP2A2 | Isoform 1 of Sarcoplasmicendoplasmic reticulum calcium ATPase 2 | C997 | N |
| Q86VP6-1 | CAND1 | Isoform 1 of Cullin-associated NEDD8-dissociated protein 1 | C942 | U |
| P20810-2 | CAST | Isoform 2 of Calpastatin | C408, C413 | N |
| P23528 | CFL1 | Cofilin-1 | C139 | T, U |
| Q9NX63 | CHCHD3 | Coiled-coil-helix-coiled-coil-helix domain-containing protein 3, mitochondrial | C112 | T |
| Q8N5K1 | CISD2 | CDGSH iron-sulfur domain-containing protein 2 | C92 | T |
| Q00610-1 | CLTC | Isoform 1 of Clathrin heavy chain 1 | C870 | U, N |
| P55060-1 | CSE1L | Isoform 1 of Exportin-2 | C272 | U, N |
| Q6NSH3 | CT45A5 | Cancertestis antigen family 45 member A5 | C22 | U |
| Q9UBR2 | CTSZ | Cathepsin Z | C92 | U |
| Q9H773 | DCTPP1 | dCTP pyrophosphatase 1 | C162 | T |
| P17844 | DDX5 | Probable ATP-dependent RNA helicase DDX5 | C200 | U |
| P33316-2 | DUT | Isoform 2 of Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial | C3 | N |
| P07814 | EPRS | Bifunctional aminoacyl-tRNA synthetase | C105 | T, U |
| O95571 | ETHE1 | Protein ETHE1, mitochondrial | C170 | T, U, N |
| P21333-2 | FLNA | Isoform 2 of Filamin-A | C717, C2543 | T, U, N |
| O75369-1 | FLNB | Isoform 1 of Filamin-B | C2501 | N |
| Q9HA64 | FN3KRP | Ketosamine-3-kinase | C24 | T |
| P02794 | FTH1 | Ferritin heavy chain | C91 | T |
| P04406 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | C152 | T, U, N |
| P07203 | GPX1 | Glutathione peroxidase 1 | C202 | T |
| P53701 | HCCS | Cytochrome c-type heme lyase | C39 | U, N |
| P00492 | HPRT1 | Hypoxanthine-guanine phosphoribosyltransferase | C106 | T |
| Q14197 | ICT1 | Peptidyl-tRNA hydrolase ICT1, mitochondrial | C82 | N |
| Q9NWZ3 | IRAK4 | Isoform 1 of Interleukin-1 receptor-associated kinase 4 | C13 | U, N |
| O14880 | MGST3 | Microsomal glutathione S-transferase 3 | C150, C151 | T, N |
| P46013-1 | MKI67 | Isoform Long of Antigen KI-67 | C1285 | U |
| P35579-1 | MYH9 | Isoform 1 of Myosin-9 | C988 | T, U, N |
| Q9NX24 | NHP2 | HACA ribonucleoprotein complex subunit 2 | C18 | T |
| P53384-1 | NUBP1 | Isoform 1 of Cytosolic Fe-S cluster assembly factor NUBP1 | C22, C25 | T |
| Q9H6K4-1 | OPA3 | Isoform 1 of Optic atrophy 3 protein | C164 | N |
| Q99497 | PARK7 | Protein DJ-1 | C106 | T, N |
| Q9Y570-1 | PPME1 | Isoform 1 of Protein phosphatase methylesterase 1 | C381 | N |
| Q06830 | PRDX1 | Peroxiredoxin-1 | C173 | T |
| P30048 | PRDX3 | Thioredoxin-dependent peroxide reductase, mitochondrial | C108 | T |
| P30041 | PRDX6 | Peroxiredoxin-6 | C91 | T, U, N |
| Q15185 | PTGES3 | Prostaglandin E synthase 3 | C58 | T, U, N |
| P49023-2 | PXN | Isoform Alpha of Paxillin | C535, C538 | U |
| P63000-1 | RAC1 | Isoform A of Ras-related C3 botulinum toxin substrate 1 | C178 | T |
| P54727 | RAD23B | UV excision repair protein RAD23 homolog B | C390 | U |
| P50914 | RPL14 | Ribosomal protein L14 variant | C42 | T |
| P05386 | RPLP1 | 60S acidic ribosomal protein P1 | C61 | T |
| P31947-1 | SFN | Isoform 1 of 14-3-3 protein sigma | C38 | T, U, N |
| Q15005 | SPCS2 | Signal peptidase complex subunit 2 | C17, C26 | T, U, N |
| P42224-1 | STAT1 | Isoform Alpha of Signal transducer and activator of transcription 1-alphabeta | C492 | N |
| Q00059 | TFAM | Transcription factor A, mitochondrial | C246 | T, N |
| Q12931 | TRAP1 | Heat shock protein 75 kDa, mitochondrial | C573 | N |
| Q9H4B7 | TUBB1 | Tubulin beta-1 chain | C12 | T, U, N |
| P10599 | TXN | Thioredoxin | C73 | T |
| P09936 | UCHL1 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | C90 (T), C152 (T, U) | T, U |
| P45880-2 | VDAC2 | Isoform 2 of Voltage-dependent anion-selective channel protein 2 | C76 | T, U, N |
| Q9Y277-1 | VDAC3 | Isoform 1 of Voltage-dependent anion-selective channel protein 3 | C65 | T, U, N |
| P08670 | VIM | Vimentin | C328 | T |
| P54577 | YARS | Tyrosyl-tRNA synthetase, cytoplasmic | C424 | U |
2.3. Succination Preferentially Targets Proteins Involved in Redox Regulation
3. Experimental Section
3.1. Cell Lines and Human Tissue Samples
| Category | Term | Count | % | p Value |
|---|---|---|---|---|
| GOTERM_BP | GO:0000302~response to reactive oxygen species | 7 | 11.67 | 3.50 × 10−7 |
| GOTERM_BP | GO:0042542~response to hydrogen peroxide | 6 | 10.00 | 2.06 × 10−6 |
| GOTERM_BP | GO:0042743~hydrogen peroxide metabolic process | 5 | 8.33 | 2.14 × 10−6 |
| GOTERM_MF | GO:0051920~peroxiredoxin activity | 4 | 6.67 | 3.35 × 10−6 |
| GOTERM_BP | GO:0034614~cellular response to reactive oxygen species | 5 | 8.33 | 4.57 × 10−6 |
| GOTERM_MF | GO:0016684~oxidoreductase activity, acting on peroxide as acceptor | 5 | 8.33 | 7.57 × 10−6 |
| GOTERM_MF | GO:0004601~peroxidase activity | 5 | 8.33 | 7.57 × 10−6 |
| GOTERM_BP | GO:0034599~cellular response to oxidative stress | 5 | 8.33 | 1.98 × 10−5 |
| GOTERM_BP | GO:0042744~hydrogen peroxide catabolic process | 4 | 6.67 | 3.31 × 10−5 |
| GOTERM_BP | GO:0006979~response to oxidative stress | 7 | 11.67 | 3.32 × 10−5 |
| GOTERM_MF | GO:0016209~antioxidant activity | 5 | 8.33 | 3.59 × 10−5 |
| GOTERM_BP | GO:0070301~cellular response to hydrogen peroxide | 4 | 6.67 | 3.96 × 10−5 |
| GOTERM_BP | GO:0045454~cell redox homeostasis | 5 | 8.33 | 9.06 × 10−5 |
| GOTERM_BP | GO:0010035~response to inorganic substance | 7 | 11.67 | 1.15 × 10−4 |
| GOTERM_BP | GO:0006800~oxygen and reactive oxygen species metabolic process | 5 | 8.33 | 1.15 × 10−4 |
| GOTERM_BP | GO:0019725~cellular homeostasis | 9 | 15.00 | 3.23 × 10−4 |
| GOTERM_BP | GO:0042592~homeostatic process | 11 | 18.33 | 4.20 × 10−4 |
| GOTERM_BP | GO:0055114~oxidation reduction | 10 | 16.67 | 5.67 × 10−4 |
3.2. Proteomics and Mass Spectrometry
3.3. Immunohistochemistry (IHC) and Immunoblotting (IB)
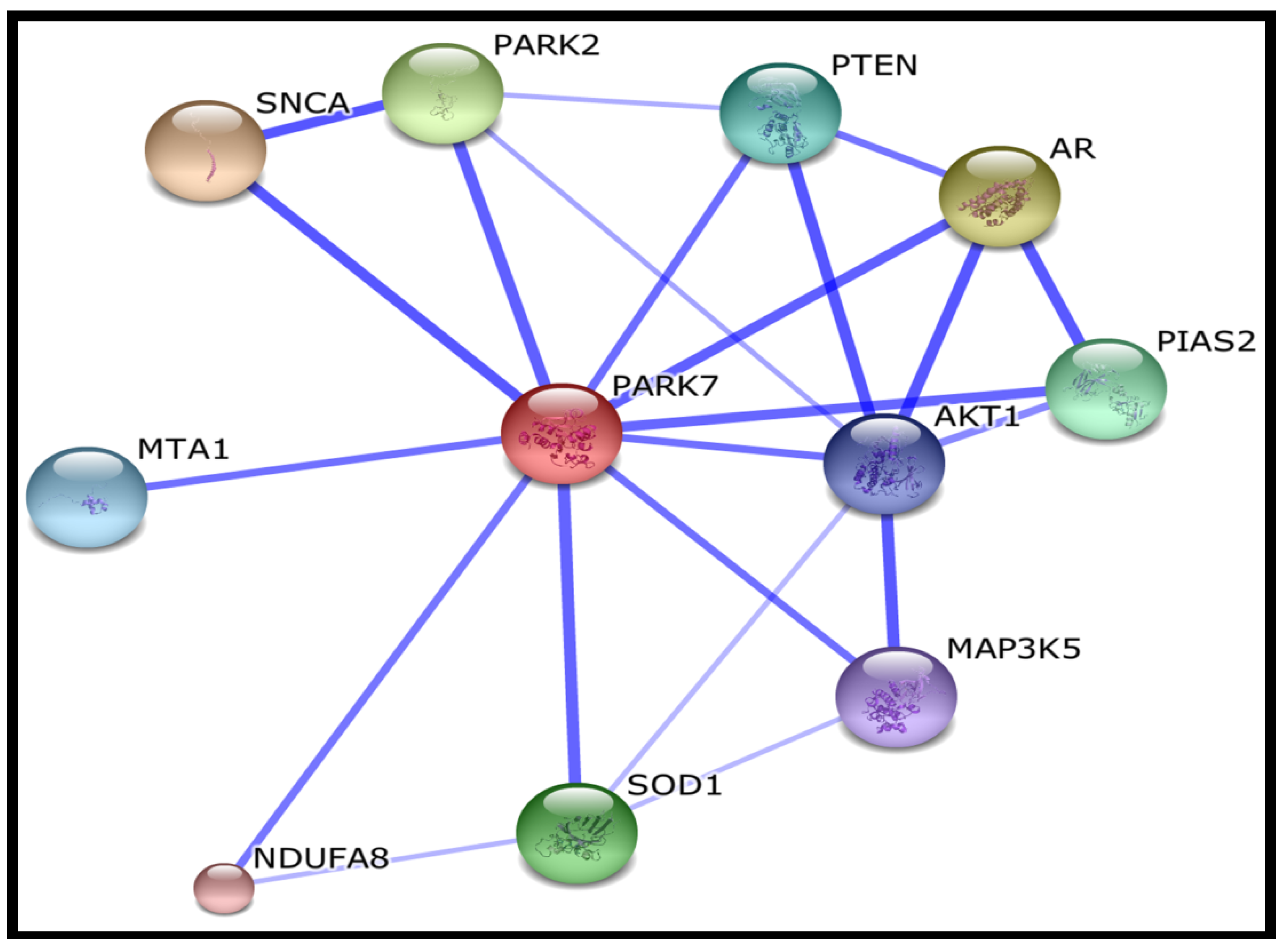
 | SNCA | synuclein, alpha (non A4 component of amyloid precursor) |
 | AR | androgen receptor |
 | PARK2 | Parkinson disease (autosomal recessive, juvenile) 2, parkin |
 | SOD1 | superoxide dismutase 1, soluble |
 | PIAS2 | protein inhibitor of activated STAT, 2 |
 | PTEN | phosphatase and tensin homolog; Tumour suppressor |
 | MTA1 | metastasis associated 1 |
 | AKT1 | v-akt murine thymoma viral oncogene homolog 1 |
 | MAP3K5 | mitogen-activated protein kinase kinase kinase 5 |
 | NDUFA8 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 8 |
3.4. Gene Ontology Analysis
| Uniprot ID | Protein Symbol | Succination Site (S) | Mutational Data | References |
|---|---|---|---|---|
| O14880 | GAPDH | C150, C151 | C → S: Abolishes S-acylation; when associated with S-151. C → S: Abolishes S-acylation; when associated with S-150 | [38] |
| Q99497 | PARK7 | C106 | C → A: Abolishes oxidation, association with mitochondria and protease activity. No effect on chaperone activity. Reduced binding to OTUD7B. C → A: Reduced localization in lipid rafts; when associated with A-46. C → D: Abolishes oxidation and association with mitochondria. No effect on chaperone activity. C → S: No effect on mitochondrial translocation. Reduced protease activity. | [49,50,51,52,53,54,55] |
| P10599 | TXN | C73 | C → D: Strongly reduced S-nitrosylation of CASP3. C → S: Loss of nitrosylation, and loss of S-nitrosylating activity towards CASP3. Retains interaction with APEX1 and transcription activation; when associated with S-62 and S-69. C → S: Retains its reducing activity. | [36,56,57] |
| P09936 | UCHL1 | C90 | C → S: Abolishes enzymatic activity. | [58,59,60] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, M.; Soga, T.; Pollard, P.J. Oncometabolites: Linking altered metabolism with cancer. J. Clin. Investig. 2013, 123, 3652–3658. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.R.; DeBerardinis, R.J. Genetically-defined metabolic reprogramming in cancer. Trends Endocrinol. Metab. 2012, 23, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Yamamoto, A.; Wilkins, S.E.; Sokolova, E.; Yates, L.A.; Munzel, M.; Singh, P.; Hopkinson, R.J.; Fischer, R.; Cockman, M.E.; et al. Optimal translational termination requires c4 lysyl hydroxylation of erf1. Mol. Cell 2014, 53, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Acevedo, J.M.; Loenarz, C.; Galagovsky, D.; Liu-Yi, P.; Perez-Pepe, M.; Thalhammer, A.; Sekirnik, R.; Ge, W.; Melani, M.; et al. Sudestada1, a drosophila ribosomal prolyl-hydroxylase required for mrna translation, cell homeostasis, and organ growth. Proc. Natl. Acad. Sci. USA 2014, 111, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Loenarz, C.; Sekirnik, R.; Thalhammer, A.; Ge, W.; Spivakovsky, E.; Mackeen, M.M.; McDonough, M.A.; Cockman, M.E.; Kessler, B.M.; Ratcliffe, P.J.; et al. Hydroxylation of the eukaryotic ribosomal decoding center affects translational accuracy. Proc. Natl. Acad. Sci. USA 2014, 111, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Singleton, R.S.; Liu-Yi, P.; Formenti, F.; Ge, W.; Sekirnik, R.; Fischer, R.; Adam, J.; Pollard, P.J.; Wolf, A.; Thalhammer, A.; et al. Ogfod1 catalyzes prolyl hydroxylation of rps23 and is involved in translation control and stress granule formation. Proc. Natl. Acad. Sci. USA 2014, 111, 4031–4036. [Google Scholar] [CrossRef] [PubMed]
- Loenarz, C.; Schofield, C.J. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat. Chem. Biol. 2008, 4, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Sekirnik, R.; Brissett, N.C.; Krojer, T.; Ho, C.H.; Ng, S.S.; Clifton, I.J.; Ge, W.; Kershaw, N.J.; Fox, G.C.; et al. Ribosomal oxygenases are structurally conserved from prokaryotes to humans. Nature 2014, 510, 422–426. [Google Scholar] [PubMed]
- Thomas, S.A.; Storey, K.B.; Baynes, J.W.; Frizzell, N. Tissue distribution of s-(2-succino) cysteine (2sc), a biomarker of mitochondrial stress in obesity and diabetes. Obesity (Silver Spring) 2012, 20, 263–269. [Google Scholar]
- Frizzell, N.; Lima, M.; Baynes, J.W. Succination of proteins in diabetes. Free Radic. Res. 2011, 45, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bardella, C.; el-Bahrawy, M.; Frizzell, N.; Adam, J.; Ternette, N.; Hatipoglu, E.; Howarth, K.; O’Flaherty, L.; Roberts, I.; Turner, G.; et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and hlrcc patients is a robust biomarker of mutation status. J. Pathol. 2011, 225, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Alderson, N.L.; Wang, Y.; Blatnik, M.; Frizzell, N.; Walla, M.D.; Lyons, T.J.; Alt, N.; Carson, J.A.; Nagai, R.; Thorpe, S.R.; et al. S-(2-succinyl)cysteine: A novel chemical modification of tissue proteins by a krebs cycle intermediate. Arch. Biochem. Biophys. 2006, 450, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Merkley, E.D.; Metz, T.O.; Smith, R.D.; Baynes, J.W.; Frizzell, N. The succinated proteome. Mass Spectrom. Rev. 2014, 33, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Martinez-Garcia, E.; Nguyen, H.; Mullen, A.R.; Dufour, E.; Sudarshan, S.; Licht, J.D.; Deberardinis, R.J.; Chandel, N.S. The proto-oncometabolite fumarate binds glutathione to amplify ros-dependent signaling. Mol. Cell 2013, 51, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Ternette, N.; Yang, M.; Laroyia, M.; Kitagawa, M.; O'Flaherty, L.; Wolhulter, K.; Igarashi, K.; Saito, K.; Kato, K.; Fischer, R.; et al. Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell Rep. 2013, 3, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Blatnik, M.; Frizzell, N.; Thorpe, S.R.; Baynes, J.W. Inactivation of glyceraldehyde-3-phosphate dehydrogenase by fumarate in diabetes: Formation of S-(2-succinyl)cysteine, a novel chemical modification of protein and possible biomarker of mitochondrial stress. Diabetes 2008, 57, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Valera, V.A.; Padilla-Nash, H.M.; Sourbier, C.; Vocke, C.D.; Vira, M.A.; Abu-Asab, M.S.; Bratslavsky, G.; Tsokos, M.; Merino, M.J.; et al. Uok 262 cell line, fumarate hydratase deficient (fh-/fh-) hereditary leiomyomatosis renal cell carcinoma: In vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet. Cytogenet. 2010, 196, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Brock, J.W.; Blatnik, M.; Baatz, J.E.; Bethard, J.; Walla, M.D.; Thorpe, S.R.; Baynes, J.W.; Frizzell, N. Succination of protein thiols during adipocyte maturation: A biomarker of mitochondrial stress. J. Biol. Chem. 2007, 282, 34219–34228. [Google Scholar] [CrossRef] [PubMed]
- Racusen, L.C.; Monteil, C.; Sgrignoli, A.; Lucskay, M.; Marouillat, S.; Rhim, J.G.; Morin, J.P. Cell lines with extended in vitro growth potential from human renal proximal tubule: Characterization, response to inducers, and comparison with established cell lines. J. Lab. Clin. Med. 1997, 129, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.; Newby, L.J.; Sharpe, C.C.; Rossetti, S.; Streets, A.J.; Harris, P.C.; O’Hare, M.J.; Ong, A.C. Hyperproliferation of pkd1 cystic cells is induced by insulin-like growth factor-1 activation of the ras/raf signalling system. Kidney Int. 2007, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Yang, M.; Soga, T.; Pollard, P.J. Rare insights into cancer biology. Oncogene 2014, 33, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Soga, T.; Pollard, P.J.; Adam, J. The emerging role of fumarate as an oncometabolite. Front. Oncol. 2012, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Uniprot. Available online: http://www.uniprot.org (accessed on 21 March 2014).
- Forman, H.J.; Ursini, F.; Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell Cardiol. 2014, 73, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ooi, A.; Wong, J.C.; Petillo, D.; Roossien, D.; Perrier-Trudova, V.; Whitten, D.; Min, B.W.; Tan, M.H.; Zhang, Z.; Yang, X.J.; et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 2011, 20, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Hatipoglu, E.; O’Flaherty, L.; Ternette, N.; Sahgal, N.; Lockstone, H.; Baban, D.; Nye, E.; Stamp, G.W.; Wolhuter, K.; et al. Renal cyst formation in fh1-deficient mice is independent of the hif/phd pathway: Roles for fumarate in keap1 succination and nrf2 signaling. Cancer Cell 2011, 20, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Abou-Sleiman, P.M.; Muqit, M.M.; Wood, N.W. Expanding insights of mitochondrial dysfunction in parkinson’s disease. Nat. Rev. Neurosci. 2006, 7, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, S.; Afzal, S.; Tardivel-Lacombe, J.; Park, D.S.; Iovanna, J.L.; Mak, T.W. Dj-1/park7 is an important mediator of hypoxia-induced cellular responses. Proc. Natl. Acad. Sci. USA 2009, 106, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Baumunk, D.; Reichelt, U.; Hildebrandt, J.; Krause, H.; Ebbing, J.; Cash, H.; Miller, K.; Schostak, M.; Weikert, S. Expression parameters of the metabolic pathway genes pyruvate dehydrogenase kinase-1 (pdk-1) and dj-1/park7 in renal cell carcinoma (rcc). World J. Urol. 2013, 31, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Ambrosi, G.; Gammoh, O.; Blandini, F.; Cereda, C. Sod1 and dj-1 converge at nrf2 pathway: A clue for antioxidant therapeutic potential in neurodegeneration. Oxid. Med. Cell. Longev. 2013, 2013, 836760. [Google Scholar]
- Merikallio, H.; Paakko, P.; Kinnula, V.L.; Harju, T.; Soini, Y. Nuclear factor erythroid-derived 2-like 2 (nrf2) and dj1 are prognostic factors in lung cancer. Hum. Pathol. 2012, 43, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Drexhage, J.A.; Flor, T.; Gerritsen, W.; van der Valk, P.; de Vries, H.E. Nrf2 and dj1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic. Biol. Med. 2010, 49, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Bhatnagar, A.; Ansari, N.H.; Srivastava, S.K. Identification of the reactive cysteine residue in human placenta aldose reductase. Biochim. Biophys. Acta 1993, 1164, 268–272. [Google Scholar]
- Wood, Z.A.; Schroder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Poynton, R.A.; Hampton, M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta 2014, 1840, 906–912. [Google Scholar]
- Mitchell, D.A.; Marletta, M.A. Thioredoxin catalyzes the s-nitrosation of the caspase-3 active site cysteine. Nat. Chem. Biol. 2005, 1, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A. Biogenesis of iron-sulfur clusters in mammalian cells: New insights and relevance to human disease. Dis. Model. Mech. 2012, 5, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.T.; Hess, D.T.; Thompson, J.W.; Hultman, R.; Moseley, M.A.; Stamler, J.S.; Casey, P.J. Site-specific analysis of protein s-acylation by resin-assisted capture. J. Lipid Res. 2011, 52, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Yang, M.; Bauerschmidt, C.; Kitagawa, M.; O’Flaherty, L.; Maheswaran, P.; Ozkan, G.; Sahgal, N.; Baban, D.; Kato, K.; et al. A role for cytosolic fumarate hydratase in urea cycle metabolism and renal neoplasia. Cell Rep. 2013, 3, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- DAVID Bioinformatics Resources. Available online: http://david.abcc.ncifcrf.gov/ (accessed on 21 March 2014).
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. David bioinformatics resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef] [PubMed]
- International Protein Index. Available online: http://www.ebi.ac.uk/IPI (accessed on 21 March 2014).
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef] [PubMed]
- Trudgian, D.C.; Thomas, B.; McGowan, S.J.; Kessler, B.M.; Salek, M.; Acuto, O. Cpfp: A central proteomics facilities pipeline. Bioinformatics 2010, 26, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, L.; Adam, J.; Heather, L.C.; Zhdanov, A.V.; Chung, Y.L.; Miranda, M.X.; Croft, J.; Olpin, S.; Clarke, K.; Pugh, C.W.; et al. Dysregulation of hypoxia pathways in fumarate hydratase-deficient cells is independent of defective mitochondrial metabolism. Hum. Mol. Genet. 2010, 19, 3844–3851. [Google Scholar] [CrossRef] [PubMed]
- STRINGv9.1. Available online: http://string-db.org/ (accessed on 21 March 2014).
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. String 8—A global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009, 37, D412–D416. [Google Scholar] [CrossRef] [PubMed]
- Shendelman, S.; Jonason, A.; Martinat, C.; Leete, T.; Abeliovich, A. Dj-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004, 2, e362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekito, A.; Koide-Yoshida, S.; Niki, T.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. Dj-1 interacts with hipk1 and affects h2o2-induced cell death. Free Radic. Res. 2006, 40, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Jang, W.H.; Zhao, X.; Jeong, B.S.; Mouradian, M.M. Mitochondrial localization of dj-1 leads to enhanced neuroprotection. J. Neurosci. Res. 2009, 87, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Chin, L.S. Parkinson disease protein dj-1 converts from a zymogen to a protease by carboxyl-terminal cleavage. Hum. Mol. Genet. 2010, 19, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.S.; Davis, B.K.; Clements, C.M.; Accavitti-Loper, M.A.; Mak, T.W.; Ting, J.P. Dj-1 enhances cell survival through the binding of cezanne, a negative regulator of nf-kappab. J. Biol. Chem. 2011, 286, 4098–4106. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, J.S.; Park, J.Y.; Suh, Y.H.; Jou, I.; Joe, E.H.; Park, S.M. Dj-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum. Mol. Genet. 2013, 22, 4805–4817. [Google Scholar] [CrossRef] [PubMed]
- Canet-Aviles, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The parkinson’s disease protein dj-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Matsui, M.; Iwata, S.; Nishiyama, A.; Mori, K.; Yodoi, J. Ap-1 transcriptional activity is regulated by a direct association between thioredoxin and ref-1. Proc. Natl. Acad. Sci. USA 1997, 94, 3633–3638. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Morton, S.U.; Fernhoff, N.B.; Marletta, M.A. Thioredoxin is required for s-nitrosation of procaspase-3 and the inhibition of apoptosis in jurkat cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11609–11614. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.N.; Price, J.S.; Wilkinson, K.D. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: Identification of two active site residues. Biochemistry 1996, 35, 6735–6744. [Google Scholar] [CrossRef] [PubMed]
- Boudreaux, D.A.; Maiti, T.K.; Davies, C.W.; Das, C. Ubiquitin vinyl methyl ester binding orients the misaligned active site of the ubiquitin hydrolase uchl1 into productive conformation. Proc. Natl. Acad. Sci. USA 2010, 107, 9117–9122. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Li, H.; Kawamura, R.; Osaka, H.; Wang, Y.L.; Hara, Y.; Hirokawa, T.; Manago, Y.; Amano, T.; Noda, M.; et al. Alterations of structure and hydrolase activity of parkinsonism-associated human ubiquitin carboxyl-terminal hydrolase l1 variants. Biochem. Biophys. Res. Commun 2003, 304, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ooi, A.; Dykema, K.; Ansari, A.; Petillo, D.; Snider, J.; Kahnoski, R.; Anema, J.; Craig, D.; Carpten, J.; Teh, B.T.; et al. Cul3 and nrf2 mutations confer an nrf2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res. 2013, 73, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Gerlinger, M.; Futreal, P.A.; Pusztai, L.; Swanton, C. Intratumor heterogeneity: Seeing the wood for the trees. Sci. Transl. Med. 2012, 4, 127ps110. [Google Scholar] [CrossRef]
- Fisher, R.; Larkin, J.; Swanton, C. Inter and intratumour heterogeneity: A barrier to individualized medical therapy in renal cell carcinoma? Front. Oncol. 2012, 2, 49. [Google Scholar]
- Frizzell, N.; Thomas, S.A.; Carson, J.A.; Baynes, J.W. Mitochondrial stress causes increased succination of proteins in adipocytes in response to glucotoxicity. Biochem. J. 2012, 445, 247–254. [Google Scholar] [PubMed]
- Piroli, G.G.; Manuel, A.M.; Walla, M.D.; Jepson, M.J.; Brock, J.W.; Rajesh, M.P.; Tanis, R.M.; Cotham, W.E.; Frizzell, N. Identification of protein succination as a novel modification of tubulin. Biochem. J. 2014. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, M.; Ternette, N.; Su, H.; Dabiri, R.; Kessler, B.M.; Adam, J.; Teh, B.T.; Pollard, P.J. The Succinated Proteome of FH-Mutant Tumours. Metabolites 2014, 4, 640-654. https://doi.org/10.3390/metabo4030640
Yang M, Ternette N, Su H, Dabiri R, Kessler BM, Adam J, Teh BT, Pollard PJ. The Succinated Proteome of FH-Mutant Tumours. Metabolites. 2014; 4(3):640-654. https://doi.org/10.3390/metabo4030640
Chicago/Turabian StyleYang, Ming, Nicola Ternette, Huizhong Su, Raliat Dabiri, Benedikt M. Kessler, Julie Adam, Bin Tean Teh, and Patrick J. Pollard. 2014. "The Succinated Proteome of FH-Mutant Tumours" Metabolites 4, no. 3: 640-654. https://doi.org/10.3390/metabo4030640
APA StyleYang, M., Ternette, N., Su, H., Dabiri, R., Kessler, B. M., Adam, J., Teh, B. T., & Pollard, P. J. (2014). The Succinated Proteome of FH-Mutant Tumours. Metabolites, 4(3), 640-654. https://doi.org/10.3390/metabo4030640




