Isolation and Expression of a cDNA Encoding Methylmalonic Aciduria Type A Protein from Euglena gracilis Z
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of a cDNA Encoding MMAA
2.2. Expression of MMAA by E. gracilis

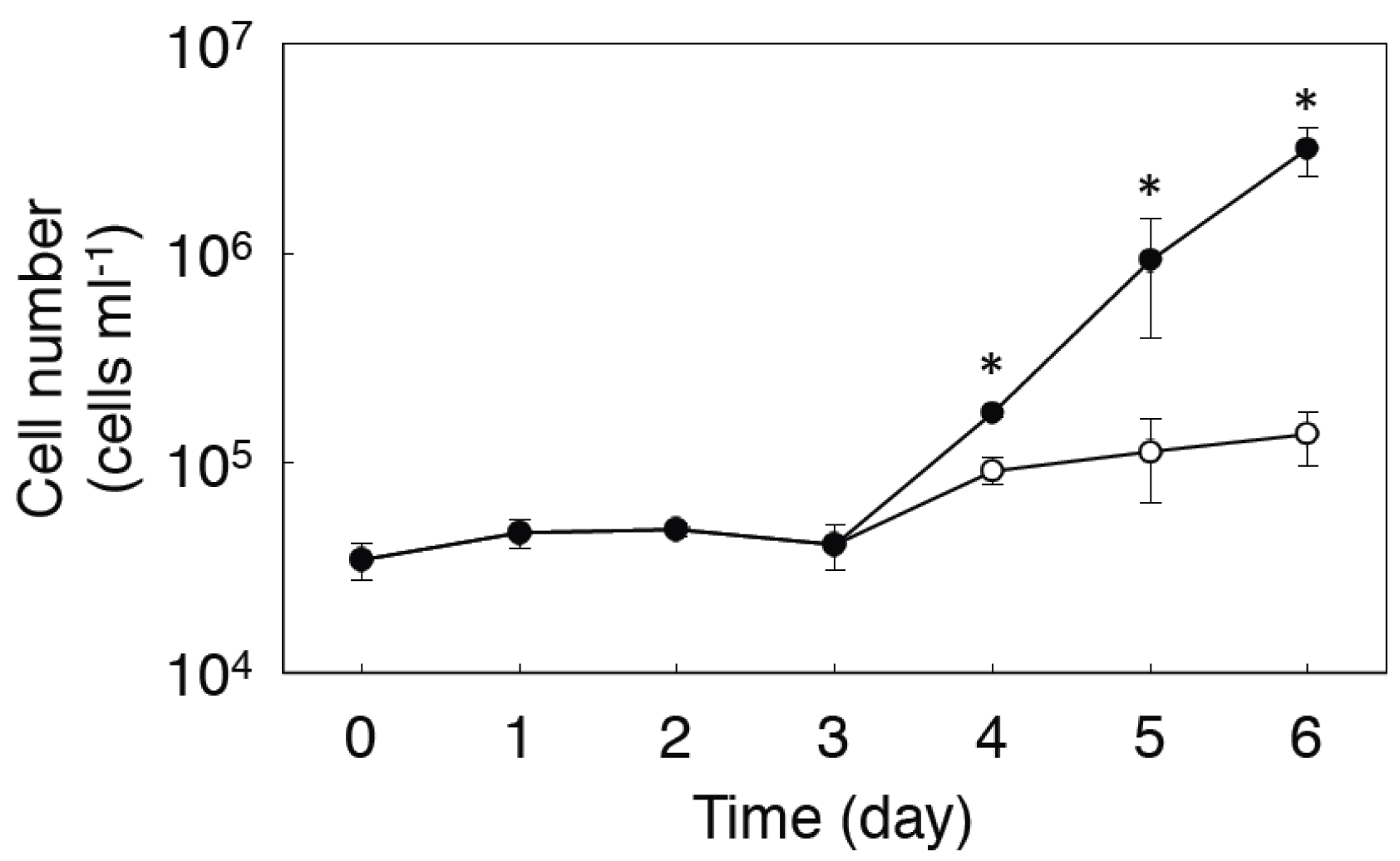
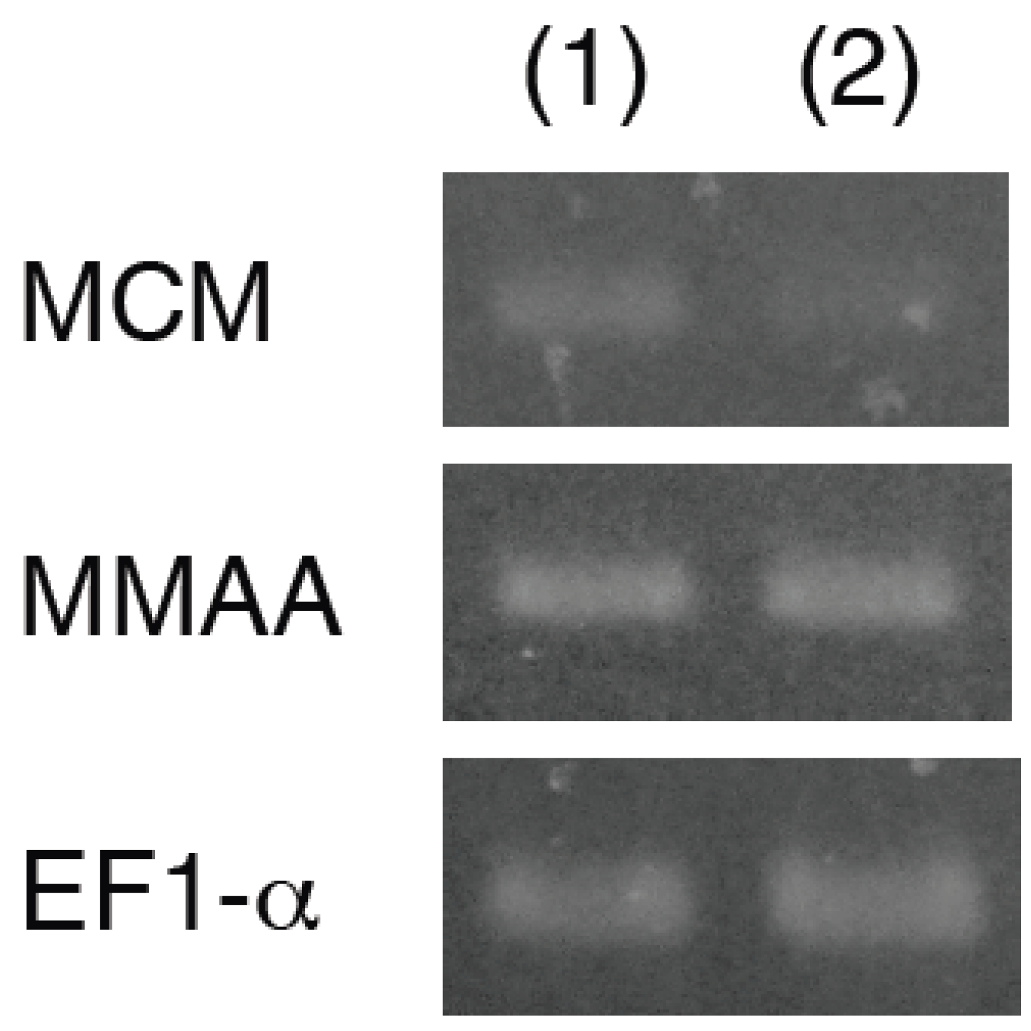
2.3. Effect of Coexpression of MMAA on MCM Activity in E. coli
| Vector pairs | MCM activity (nmol min−1 mg−1 protein) |
|---|---|
| pET/Eg.MCM - pCDF-1b | 12.1 ± 2.0 |
| pET-16b - pCDF-1b/Eg.MMAA | < 0.005 |
| pET-16b - pCDF-1b | < 0.005 |
| pET/Eg.MCM - pCDF/Eg.MMAA | 11.6 ± 1.8 |
3. Experimental Section
3.1. E. gracilis and Culture Conditions
3.2. RT-PCR-Amplification and Sequence Analysis
3.3. Semi-Quantitative RT-PCR Analysis
3.4. Construction of MMAA and MCM Expression Plasmids
3.5. Expression of Recombinant MMAA or MCM Proteins
3.6. Enzyme Extraction and Assay
4. Conclusion
Acknowledgments
Conflict of Interest
References
- Banerjee, R.; Ragsdale, S.W. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 2003, 72, 209–247. [Google Scholar] [CrossRef]
- Ueta, K.; Ishihara, Y.; Yabuta, Y.; Masuda, S.; Watanabe, F. TLC-analysis of a corrinoid compound from Japanese rock-oyster “Iwa-gaki” (Crassostrea. nippona). J. Liquid Chromatography Related Technol. 2011, 34, 928–935. [Google Scholar] [CrossRef]
- Dobson, C.M.; Wai, T.; Leclerc, D.; Wilson, A.; Wu, X.; Doré, C.; Hudson, T.; Rosenblatt, D.S.; Gravel, R.A. Identification of the gene responsible for the cblA complementation group of vitamin B12-responsive methylmalonic acidemia based on analysis of prokaryotic gene arrangements. Proc. Natl. Acad. Sci. USA 2002, 99, 15554–15559. [Google Scholar]
- Lerner-Ellis, J.P.; Dobson, C.M.; Wai, T.; Watkins, D.; Tirone, J.C.; Leclerc, D.; Doré, C.; Lepage, P.; Gravel, R.A.; Rosenblatt, D.S. Mutations in the MMAA gene in patients with the cblA disorder of vitamin B12 metabolism. Hum. Mutat. 2004, 6, 509–516. [Google Scholar]
- Celis, R.T.; Leadlay, P.F.; Roy, I.; Hansen, A. Phosphorylation of the periplasmic binding protein in two transport systems for arginine incorporation in Escherichia coli K-12 is unrelated to the function of the transport system. J. Bacteriol. 1998, 180, 4828–4833. [Google Scholar]
- Korotkova, N.; Chistoserdova, L.; Kuksa, V.; Lidstrom, M.E. Glyoxylate regeneration pathway in the methylotroph Methylobacterium. extorquens AM1. J. Bacteriol. 2002, 184, 1750–1758. [Google Scholar] [CrossRef]
- Korotkova, N.; Lidstrom, M.E. MeaB is a component of the methylmalonyl-CoA mutase complex required for protection of the enzyme from inactivation. J. Biol. Chem. 2004, 279, 13652–13658. [Google Scholar] [CrossRef]
- Toraya, T. Radical catalysis of B12 enzymes: structure, mechanism, inactivation, and reactivation of diol and glycerol dehydratases. Cell. Mol. Life Sci. 2000, 57, 106–127. [Google Scholar] [CrossRef]
- Mori, K.; Tobimatsu, T.; Toraya, T. A protein factor is essential for in situ reactivation of glycerol-inactivated adenosylcobalamin-dependent diol dehydratase. Biosci. Biotechnol. Biochem. 1997, 61, 1729–1733. [Google Scholar] [CrossRef]
- Mori, K.; Tobimatsu, T.; Hara, T.; Toraya, T. Characterization, sequencing, and expression of the genes encoding a reactivating factor for glycerol-inactivated adenosylcobalamin-dependent diol dehydratase. J. Biol. Chem. 1997, 272, 32034–32041. [Google Scholar]
- Toraya, T.; Mori, K. A reactivating factor for coenzyme B12-dependent diol dehydratase. J. Biol. Chem. 1999, 274, 3372–3377. [Google Scholar] [CrossRef]
- Kajiura, H.; Mori, K.; Tobimatsu, T.; Toraya, T. Characterization and mechanism of action of a reactivating factor for adenosylcobalamin-dependent glycerol dehydratase. J. Biol. Chem. 2001, 276, 36514–36519. [Google Scholar] [CrossRef]
- Padovani, D.; Banerjee, R. A G-protein editor gates coenzyme B12 loading and is corrupted in methylmalonic aciduria. Proc. Natl. Acad. Sci. USA 2009, 106, 21567–21572. [Google Scholar] [CrossRef]
- Takahashi-Íñiguez, T.; García-Arellano, H.; Trujillo-Roldán, M.A.; Flores, M.E. Protection and reactivation of human methylmalonyl-CoA mutase by MMAA protein. Biochem. Biophys. Res. Commun. 2011, 404, 443–447. [Google Scholar] [CrossRef]
- Froese, D.S.; Dobson, C.M.; White, A.P.; Wu, X.; Padovani, D.; Banerjee, R.; Haller, T.; Gerlt, J.A.; Surette, M.G.; Gravel, R.A. Sleeping beauty mutase (sbm) is expressed and interacts with ygfd in Escherichia coli. Microbiol. Res. 2009, 164, 1–8. [Google Scholar] [CrossRef]
- Kitaoka, S.; Nakano, Y.; Miyatake, K.; Yokota, A. In The Biology of Euglena; Buetow, D.E., Ed.; Academic Press: San Diego, CA, USA, 1989; Volume 4, pp. 1–135. [Google Scholar]
- Isegawa, Y.; Nakano, Y.; Kitaoka, S. Conversion and distribution of cobalamin in Euglena gracilis Z, with special reference to its location and probable function within chloroplasts. Plant Physiol. 1984, 76, 814–818. [Google Scholar] [CrossRef]
- Watanabe, F.; Nakano, Y.; Stupperich, E. Different corrinoid specificities for cell growth and the cobalamin uptake system in Euglena gracilis Z. J. Gen. Microbiol. 1992, 138, 1807–1813. [Google Scholar]
- Isegawa, Y.; Watanabe, F.; Kitaoka, S.; Nakano, Y. SubcelluIar distribution of cobalamin-dependent methionine synthase in Euglena gracilis z. Phytochemisty 1994, 35, 59–61. [Google Scholar]
- Torrents, E.; Trevisiol, C.; Rotte, C.; Hellman, U.; Martin, W.; Reichard, P. Euglena gracilis ribonucleotide reductase: the eukaryote class II enzyme and the possible antiquity of eukaryote B12 dependence. J. Biol. Chem. 2006, 281, 5604–5611. [Google Scholar]
- Watanabe, F.; Oki, Y.; Nakano, Y.; Kitaoka, S. Purification and characterization of aquacobalamin reductase (NADPH) from Euglena gracilis. J. Biol. Chem. 1987, 262, 11514–11518. [Google Scholar]
- Miyamoto, E.; Tanioka, Y.; Nishizawa-Yokoi, A.; Yabuta, Y.; Ohnishi, K.; Misono, H.; Shigeoka, S.; Nakano, Y.; Watanabe, F. Characterization of methylmalonyl-CoA mutase involved in the propionate photoassimilation of Euglena gracilis Z. Arch. Microbiol. 2010, 192, 437–446. [Google Scholar] [CrossRef]
- Taxonomically Broad EST Database. Available online: http://amoebidia.bcm.umontreal.ca/pepdb/searches/login.php.
- Tessier, L.H.; Keller, M.; Chan, R.L.; Fournier, R.; Weil, J.H.; Imbault, P. Short leader sequences may be transferred from small RNAs to pre-mature mRNA by trans-splicing in Euglena. EMBO J. 1991, 10, 2621–2625. [Google Scholar]
- Frantz, C.; Ebel, C.; Paulus, F.; Imbaut, P. Characterization of trans-splicing in Euglenoids. Curr. Genet. 2000, 37, 349–355. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tajima, N.; Nishikawa, H.; Gao, Y.; Rapolu, M.; Shibata, H.; Sawa, Y.; Shigeoka, S. Euglena gracilis ascorbate peroxidase forms an intramolecular dimeric structure: its unique molecular characterization. Biochem. J. 2010, 426, 125–134. [Google Scholar] [CrossRef]
- TargetP prediction program. Available online: http://www.cbs.dtu.dk/services/TargetP/.
- Nakao, M.; Hironaka, S.; Harada, N.; Adachi, T.; Bito, T.; Yabuta, Y.; Watanabe, F.; Miura, T.; Yamaji, R.; Inui, H.; Nakano, Y. Cobalamin deficiency results in an abnormal increase in L-methylmalonyl-co-enzyme-A mutase expression in rat liver and COS-7 cells. Br. J. Nutr. 2009, 101, 492–498. [Google Scholar]
- ClustalW Program. Available online: http://clustalw.ddbj.nig.ac.jp/index.php?lang=ja, accessed on 10 February 2013.
- Leipe, D.; Wolf, Y.; Koonin, E.; Aravind, L. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 2002, 317, 41–72. [Google Scholar]
- Haller, T.; Buckel, T.; Rétey, J.; Gerlt, J.A. Discovering new enzymes and metabolic pathways: conversion of succinate to propionate by Escherichia coli. Biochemistry 2000, 39, 4622–4629. [Google Scholar] [CrossRef]
- Chandler, R.J.; Aswani, V.; Tsai, M.S.; Falk, M.; Wehrli, N.; Stabler, S.; Allen, R.; Sedensky, M.; Kazazian, H.H.; Venditti, C.P. Propionyl-CoA and adenosylcobalamin metabolism in Caenorhabditis. elegans: Evidence for a role of methylmalonyl-CoA epimerase in intermediary metabolism. metabolism. Mol. Genet. MeTable 2006, 89, 64–73. [Google Scholar] [CrossRef]
- Koren, L.E.; Hutner, S.H. High-yield media for photosynthesizing Euglena gracilis Z. J. Protozool. 1967, 14, 17. [Google Scholar]
- Image J. Available online: http://rsbweb.nih.gov/ij/, accessed on 10 February 2013.
- Enago. Available online: http://www.enago.jp/, accessed on 10 February 2013.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yabuta, Y.; Takamatsu, R.; Kasagaki, S.; Watanabe, F. Isolation and Expression of a cDNA Encoding Methylmalonic Aciduria Type A Protein from Euglena gracilis Z. Metabolites 2013, 3, 144-154. https://doi.org/10.3390/metabo3010144
Yabuta Y, Takamatsu R, Kasagaki S, Watanabe F. Isolation and Expression of a cDNA Encoding Methylmalonic Aciduria Type A Protein from Euglena gracilis Z. Metabolites. 2013; 3(1):144-154. https://doi.org/10.3390/metabo3010144
Chicago/Turabian StyleYabuta, Yukinori, Ryota Takamatsu, Satoshi Kasagaki, and Fumio Watanabe. 2013. "Isolation and Expression of a cDNA Encoding Methylmalonic Aciduria Type A Protein from Euglena gracilis Z" Metabolites 3, no. 1: 144-154. https://doi.org/10.3390/metabo3010144





