Metabolic Phenotyping Study of Mouse Brain Following Microbiome Disruption by C. difficile Colonization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. C. difficile Spore Purification
2.3. Animal Experiment
2.4. Metabolites’ Extraction
2.5. Sample Preparation and Analysis
2.5.1. GC-MS Analysis
2.5.2. GC-MS/MS
2.5.3. HILIC-MS/MS Analysis
2.5.4. RP-LC-HRMS/MS Analysis
2.6. Data Analysis
3. Results
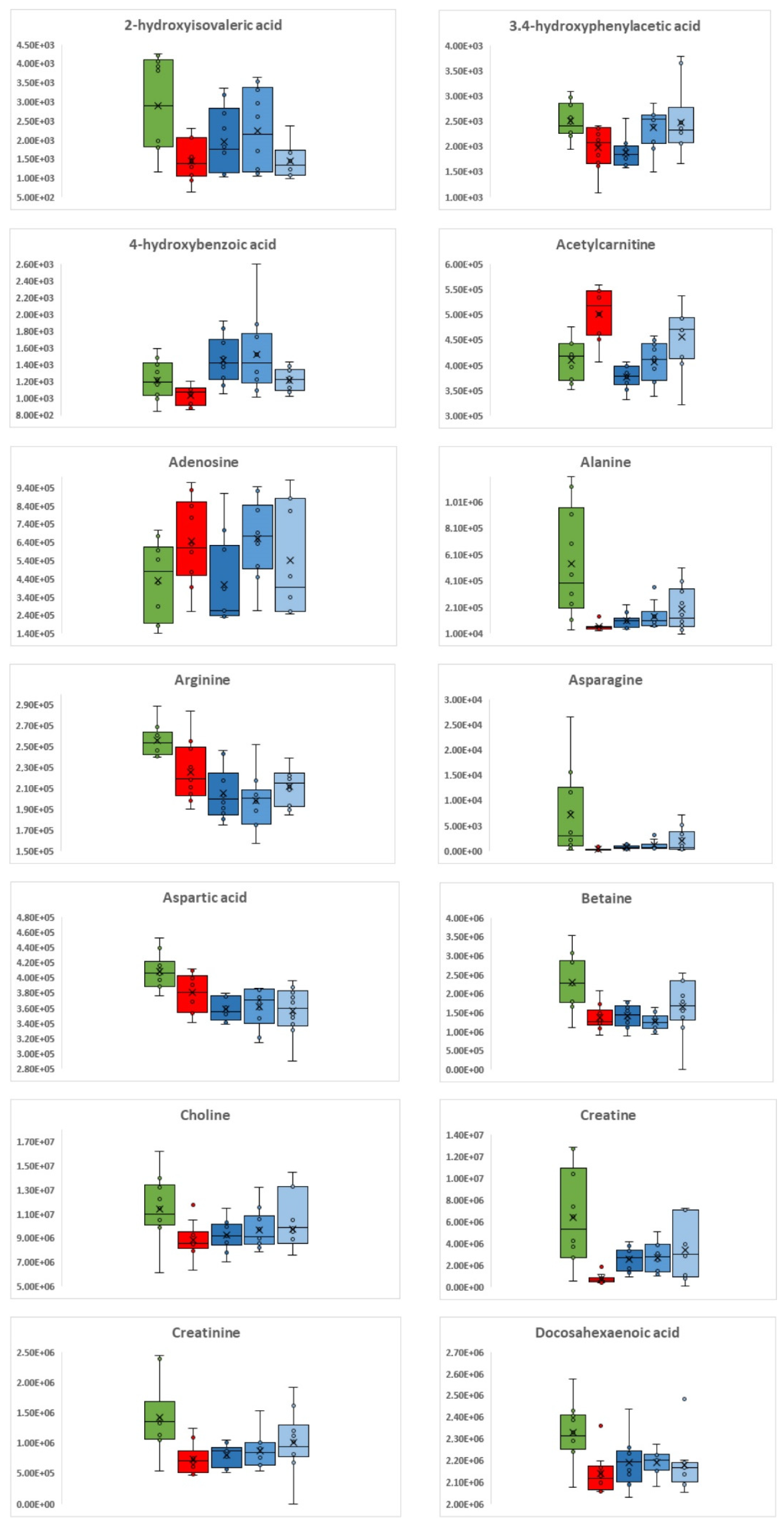
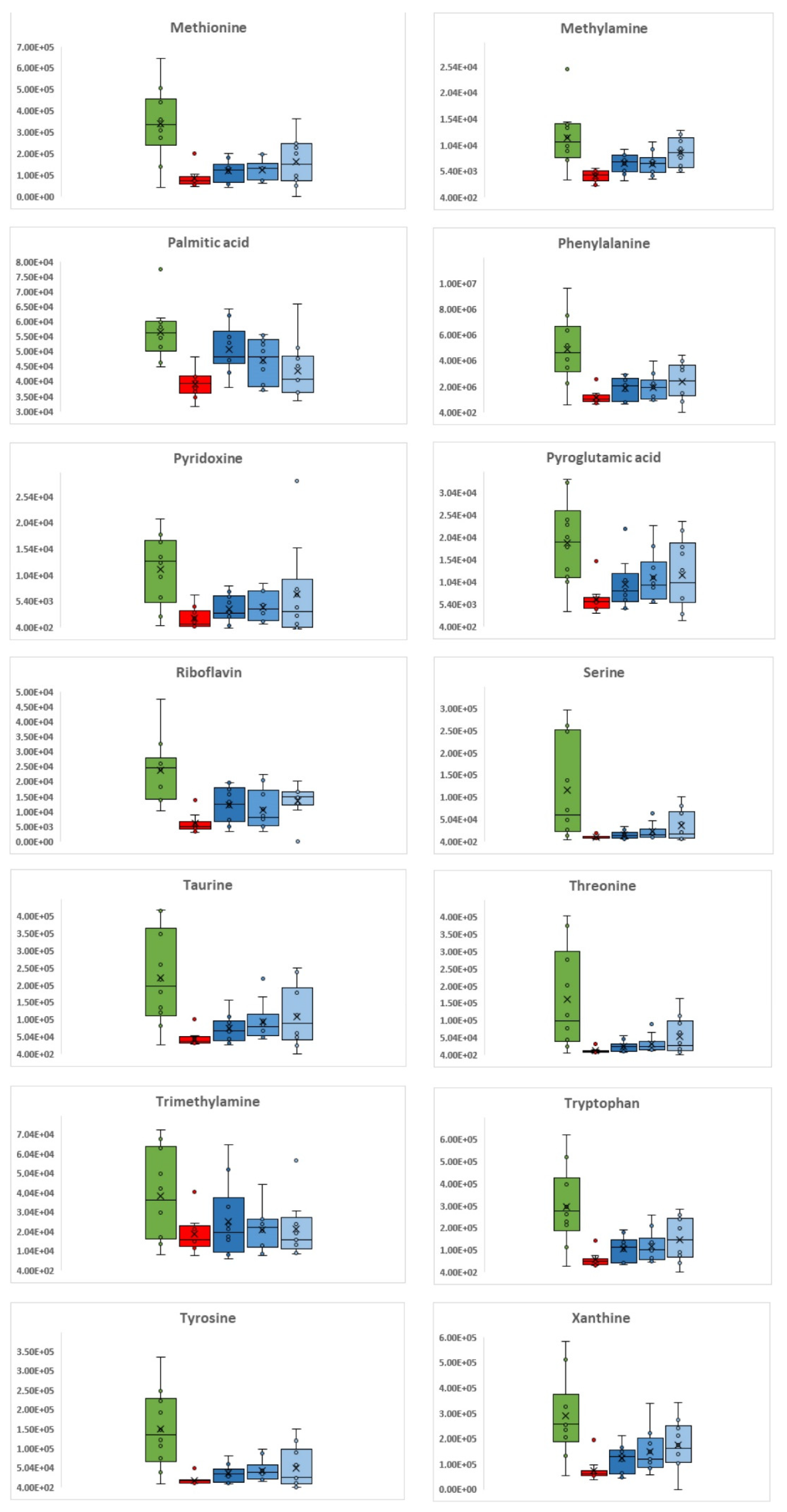
3.1. C. difficile Infection
3.2. C. difficile Therapeutic Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theriot, C.M.; Fletcher, J.R. Human Fecal Metabolomic Profiling Could Inform Clostridioides Difficile Infection Diagnosis and Treatment. J. Clin. Investig. 2019, 129, 3539–3541. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; von Bergen, M.; et al. Gut Microbiota Disturbance during Antibiotic Therapy: A Multi-Omic Approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Impact of Microbial Derived Secondary Bile Acids on Colonization Resistance against Clostridium Difficile in the Gastrointestinal Tract. Anaerobe 2016, 41, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Meng, L.; Yang, H. Therapeutic Effects of Bifidobacterium Breve YH68 in Combination with Vancomycin and Metronidazole in a Primary Clostridioides Difficile-Infected Mouse Model. Microbiol. Spectr. 2022, 10, e0067222. [Google Scholar] [CrossRef]
- Zhou, P.; Zhou, N.; Shao, L.; Li, J.; Liu, S.; Meng, X.; Duan, J.; Xiong, X.; Huang, X.; Chen, Y.; et al. Diagnosis of Clostridium Difficile Infection Using an UPLC–MS Based Metabolomics Method. Metabolomics 2018, 14, 102. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Chen, C.; Bobr, A.; Yao, D.; Lu, Y.; Nelson, V.M.; Sadowsky, M.J.; Khoruts, A. Microbiota Transplantation Restores Normal Fecal Bile Acid Composition in Recurrent Clostridium Difficile Infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G310–G319. [Google Scholar] [CrossRef] [Green Version]
- Behnke, M.; Hansen, S.; Leistner, R.; Diaz, L.A.P.; Gropmann, A.; Sohr, D.; Gastmeier, P.; Piening, B. Nosocomial Infection and Antibiotic Use. Dtsch. Ärzteblatt Int. 2013, 110, 627–633. [Google Scholar] [CrossRef] [Green Version]
- Halverson, T.; Alagiakrishnan, K. Gut Microbes in Neurocognitive and Mental Health Disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef]
- Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells 2022, 11, 1362. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Greene, M.T.; Young, V.B.; Saint, S.; Langa, K.M.; Kao, J.Y.; Aronoff, D.M. Depression, Antidepressant Medications, and Risk of Clostridium Difficile Infection. BMC Med. 2013, 11, 121. [Google Scholar] [CrossRef]
- Koppenol, E.; Terveer, E.M.; Vendrik, K.E.W.; van Lingen, E.; Verspaget, H.W.; Keller, J.J.; Kuijper, E.J.; Giltay, E.J. Fecal Microbiota Transplantation Is Associated with Improved Aspects of Mental Health of Patients with Recurrent Clostridioides Difficile Infections: Effect of FMT on Affect in RCDI Patients. J. Affect. Disord. Rep. 2022, 9, 100355. [Google Scholar] [CrossRef]
- Biazzo, M.; Allegra, M.; Deidda, G. Clostridioides Difficile and Neurological Disorders: New Perspectives. Front. Neurosci. 2022, 16, 946601. [Google Scholar] [CrossRef]
- Arneth, B.M. Gut–Brain Axis Biochemical Signalling from the Gastrointestinal Tract to the Central Nervous System: Gut Dysbiosis and Altered Brain Function. Postgrad. Med. J. 2018, 94, 446–452. [Google Scholar] [CrossRef]
- Sharaby, A.A.; Abugoukh, T.M.; Ahmed, W.; Ahmed, S.; Elshaikh, A.O. Do Probiotics Prevent Clostridium Difficile-Associated Diarrhea? Cureus 2022, 14, e27624. [Google Scholar] [CrossRef]
- Wolfe, T.J.D.; Kates, A.E.; Barko, L.; Darien, B.J.; Safdar, N. Modified Mouse Model of Clostridioides Difficile Infection as a Platform for Probiotic Efficacy Studies. Antimicrob. Agents Chemother. 2019, 63, e00111-19. [Google Scholar] [CrossRef] [Green Version]
- Shelby, R.D.; Janzow, G.E.; Mashburn-Warren, L.; Galley, J.; Tengberg, N.; Navarro, J.; Conces, M.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. A Novel Probiotic Therapeutic in a Murine Model of Clostridioides Difficile Colitis. Gut Microbes 2020, 12, 1814119. [Google Scholar] [CrossRef]
- Mills, J.P.; Rao, K.; Young, V.B. Probiotics for Prevention of Clostridium Difficile Infection. Curr. Opin. Gastroenterol. 2018, 34, 3–10. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kelly, C.R.; Grinspan, A.; Mullish, B.H.; Hurtado, J.; Carrellas, M.; Marcus, J.; Marchesi, J.R.; McDonald, J.A.K.; Gerardin, Y.; et al. Inflammatory Bowel Disease Outcomes Following Fecal Microbiota Transplantation for Recurrent C. Difficile Infection. Inflamm. Bowel Dis. 2021, 27, 1371–1378. [Google Scholar] [CrossRef]
- Martinez-Gili, L.; McDonald, J.a.K.; Liu, Z.; Kao, D.; Allegretti, J.R.; Monaghan, T.M.; Barker, G.F.; Miguéns Blanco, J.; Williams, H.R.T.; Holmes, E.; et al. Understanding the Mechanisms of Efficacy of Fecal Microbiota Transplant in Treating Recurrent Clostridioides Difficile Infection and beyond: The Contribution of Gut Microbial-Derived Metabolites. Gut Microbes 2020, 12, 1810531. [Google Scholar] [CrossRef]
- Antharam, V.C.; McEwen, D.C.; Garrett, T.J.; Dossey, A.T.; Li, E.C.; Kozlov, A.N.; Mesbah, Z.; Wang, G.P. An Integrated Metabolomic and Microbiome Analysis Identified Specific Gut Microbiota Associated with Fecal Cholesterol and Coprostanol in Clostridium Difficile Infection. PLoS ONE 2016, 11, e0148824. [Google Scholar] [CrossRef]
- Griffiths, D.; Fawley, W.; Kachrimanidou, M.; Bowden, R.; Crook, D.W.; Fung, R.; Golubchik, T.; Harding, R.M.; Jeffery, K.J.M.; Jolley, K.A.; et al. Multilocus Sequence Typing of Clostridium Difficile. J. Clin. Microbiol. 2010, 48, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-Induced Shifts in the Mouse Gut Microbiome and Metabolome Increase Susceptibility to Clostridium Difficile Infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef] [Green Version]
- Perez, J.; Springthorpe, V.S.; Sattar, S.A. Clospore: A Liquid Medium for Producing High Titers of Semi-Purified Spores of Clostridium Difficile. J. AOAC Int. 2011, 94, 618–626. [Google Scholar] [CrossRef] [Green Version]
- VanInsberghe, D.; Elsherbini, J.A.; Varian, B.; Poutahidis, T.; Erdman, S.; Polz, M.F. Diarrhoeal Events Can Trigger Long-Term Clostridium Difficile Colonization with Recurrent Blooms. Nat. Microbiol. 2020, 5, 642–650. [Google Scholar] [CrossRef]
- Mormak, D.A.; Casida, L.E. Study of Bacillus Subtilis Endospores in Soil by Use of a Modified Endospore Stain. Appl. Environ. Microbiol. 1985, 49, 1356–1360. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Katchar, K.; Goldsmith, J.D.; Nanthakumar, N.; Cheknis, A.; Gerding, D.N.; Kelly, C.P. A Mouse Model of Clostridium Difficile-Associated Disease. Gastroenterology 2008, 135, 1984–1992. [Google Scholar] [CrossRef]
- Hutton, M.L.; Mackin, K.E.; Chakravorty, A.; Lyras, D. Small Animal Models for the Study of Clostridium Difficile Disease Pathogenesis. FEMS Microbiol. Lett. 2014, 352, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Erikstrup, L.T.; Aarup, M.; Hagemann-Madsen, R.; Dagnaes-Hansen, F.; Kristensen, B.; Olsen, K.E.P.; Fuursted, K. Treatment of Clostridium Difficile Infection in Mice with Vancomycin Alone Is as Effective as Treatment with Vancomycin and Metronidazole in Combination. BMJ Open Gastroenterol. 2015, 2, e000038. [Google Scholar] [CrossRef] [Green Version]
- Bokoliya, S.C.; Dorsett, Y.; Panier, H.; Zhou, Y. Procedures for Fecal Microbiota Transplantation in Murine Microbiome Studies. Front. Cell. Infect. Microbiol. 2021, 11, 711055. [Google Scholar] [CrossRef]
- Mouskeftara, T.; Virgiliou, C.; Theodoridis, G.; Gika, H. Analysis of Urinary Organic Acids by Gas Chromatography Tandem Mass Spectrometry Method for Metabolic Profiling Applications. J. Chromatogr. A 2021, 1658, 462590. [Google Scholar] [CrossRef]
- Virgiliou, C.; Sampsonidis, I.; Gika, H.G.; Raikos, N.; Theodoridis, G.A. Development and Validation of a HILIC-MS/MS Multitargeted Method for Metabolomics Applications. Electrophoresis 2015, 36, 2215–2225. [Google Scholar] [CrossRef]
- Deda, O.; Virgiliou, C.; Armitage, E.G.; Orfanidis, A.; Taitzoglou, I.; Wilson, I.D.; Loftus, N.; Gika, H.G. Metabolic Phenotyping Study of Mouse Brains Following Acute or Chronic Exposures to Ethanol. J. Proteome Res. 2020, 19, 4071–4081. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS Spectra Processing, Multi-Omics Integration and Covariate Adjustment of Global Metabolomics Data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Fishbein, S.R.; Robinson, J.I.; Hink, T.; Reske, K.A.; Newcomer, E.P.; Burnham, C.-A.D.; Henderson, J.P.; Dubberke, E.R.; Dantas, G. Multi-Omics Investigation of Clostridioides Difficile-Colonized Patients Reveals Pathogen and Commensal Correlates of C. Difficile Pathogenesis. eLife 2022, 11, e72801. [Google Scholar] [CrossRef]
- Dawkins, J.J.; Allegretti, J.R.; Gibson, T.E.; McClure, E.; Delaney, M.; Bry, L.; Gerber, G.K. Gut Metabolites Predict Clostridioides Difficile Recurrence. Microbiome 2022, 10, 87. [Google Scholar] [CrossRef]
- Jump, R.L.P.; Polinkovsky, A.; Hurless, K.; Sitzlar, B.; Eckart, K.; Tomas, M.; Deshpande, A.; Nerandzic, M.M.; Donskey, C.J. Metabolomics Analysis Identifies Intestinal Microbiota-Derived Biomarkers of Colonization Resistance in Clindamycin-Treated Mice. PLoS ONE 2014, 9, e101267. [Google Scholar] [CrossRef]
- Robinson, J.I.; Weir, W.H.; Crowley, J.R.; Hink, T.; Reske, K.A.; Kwon, J.H.; Burnham, C.-A.D.; Dubberke, E.R.; Mucha, P.J.; Henderson, J.P. Metabolomic Networks Connect Host-Microbiome Processes to Human Clostridioides Difficile Infections. J. Clin. Investig. 2019, 129, 3792–3806. [Google Scholar] [CrossRef]
- Gotoh, K.; Sakaguchi, Y.; Kato, H.; Osaki, H.; Jodai, Y.; Wakuda, M.; Také, A.; Hayashi, S.; Morita, E.; Sugie, T.; et al. Fecal Microbiota Transplantation as Therapy for Recurrent Clostridioides Difficile Infection Is Associated with Amelioration of Delirium and Accompanied by Changes in Fecal Microbiota and the Metabolome. Anaerobe 2022, 73, 102502. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Tima, M.; Polinkovsky, A.; Zhang, R.; Emancipator, S.N.; Donskey, C.J. Targeted Metabolomics Analysis Identifies Intestinal Microbiota-Derived Urinary Biomarkers of Colonization Resistance in Antibiotic-Treated Mice. Antimicrob. Agents Chemother. 2017, 61, e00477-17. [Google Scholar] [CrossRef]
- Jenior, M.L.; Leslie, J.L.; Young, V.B.; Schloss, P.D. Clostridium Difficile Alters the Structure and Metabolism of Distinct Cecal Microbiomes during Initial Infection To Promote Sustained Colonization. mSphere 2018, 3, e00261-18. [Google Scholar] [CrossRef] [Green Version]
- Allegretti, J.R.; Kearney, S.; Li, N.; Bogart, E.; Bullock, K.; Gerber, G.K.; Bry, L.; Clish, C.B.; Alm, E.; Korzenik, J.R. Recurrent Clostridium Difficile Infection Associates with Distinct Bile Acid and Microbiome Profiles. Aliment. Pharmacol. Ther. 2016, 43, 1142–1153. [Google Scholar] [CrossRef] [Green Version]
- Monaghan, T.; Mullish, B.H.; Patterson, J.; Wong, G.K.; Marchesi, J.R.; Xu, H.; Jilani, T.; Kao, D. Effective Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection in Humans Is Associated with Increased Signalling in the Bile Acid-Farnesoid X Receptor-Fibroblast Growth Factor Pathway. Gut Microbes 2019, 10, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Seekatz, A.M.; Theriot, C.M.; Rao, K.; Chang, Y.-M.; Freeman, A.E.; Kao, J.Y.; Young, V.B. Restoration of Short Chain Fatty Acid and Bile Acid Metabolism Following Fecal Microbiota Transplantation in Patients with Recurrent Clostridium Difficile Infection. Anaerobe 2018, 53, 64–73. [Google Scholar] [CrossRef]
- Sperringer, J.E.; Addington, A.; Hutson, S.M. Branched-Chain Amino Acids and Brain Metabolism. Neurochem. Res. 2017, 42, 1697–1709. [Google Scholar] [CrossRef]
- Schousboe, A.; Scafidi, S.; Bak, L.K.; Waagepetersen, H.S.; McKenna, M.C. Glutamate Metabolism in the Brain Focusing on Astrocytes. Adv. Neurobiol. 2014, 11, 13–30. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Wu, G. Metabolism of Amino Acids in the Brain and Their Roles in Regulating Food Intake. Adv. Exp. Med. Biol. 2020, 1265, 167–185. [Google Scholar] [CrossRef]
- Pyridoxine—An Overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/neuroscience/pyridoxine (accessed on 8 September 2022).
- Peraza, A.V.; Guzmán, D.C.; Brizuela, N.O.; Herrera, M.O.; Olguín, H.J.; Silva, M.L.; Tapia, B.J.; Mejía, G.B. Riboflavin and Pyridoxine Restore Dopamine Levels and Reduce Oxidative Stress in Brain of Rats. BMC Neurosci. 2018, 19, 71. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.-H.; Uddin, M.S.; Kim, I.-S.; Choi, D.-K. Taurine and Its Analogs in Neurological Disorders: Focus on Therapeutic Potential and Molecular Mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Parksepp, M.; Leppik, L.; Koch, K.; Uppin, K.; Kangro, R.; Haring, L.; Vasar, E.; Zilmer, M. Metabolomics Approach Revealed Robust Changes in Amino Acid and Biogenic Amine Signatures in Patients with Schizophrenia in the Early Course of the Disease. Sci. Rep. 2020, 10, 13983. [Google Scholar] [CrossRef]



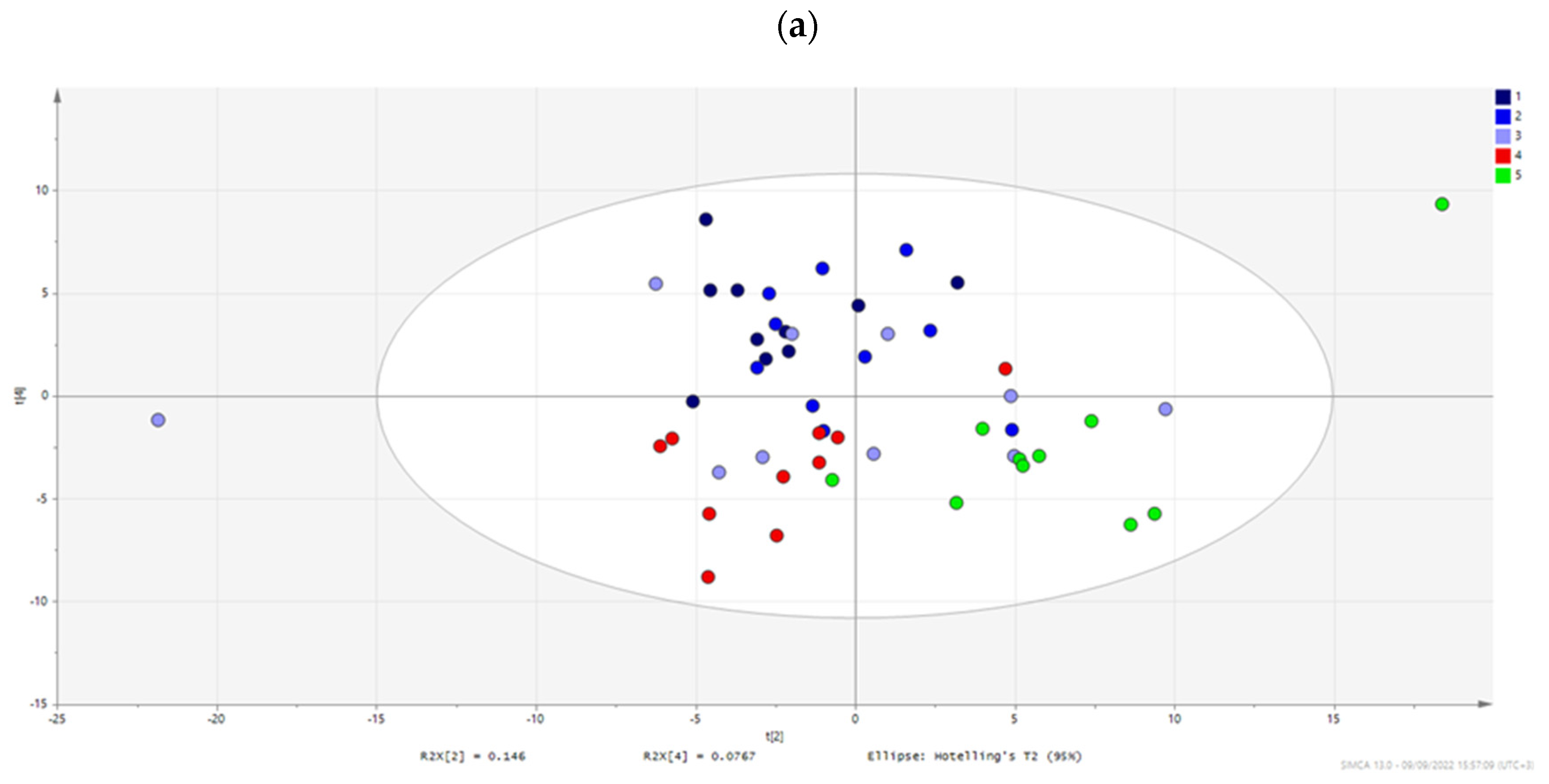
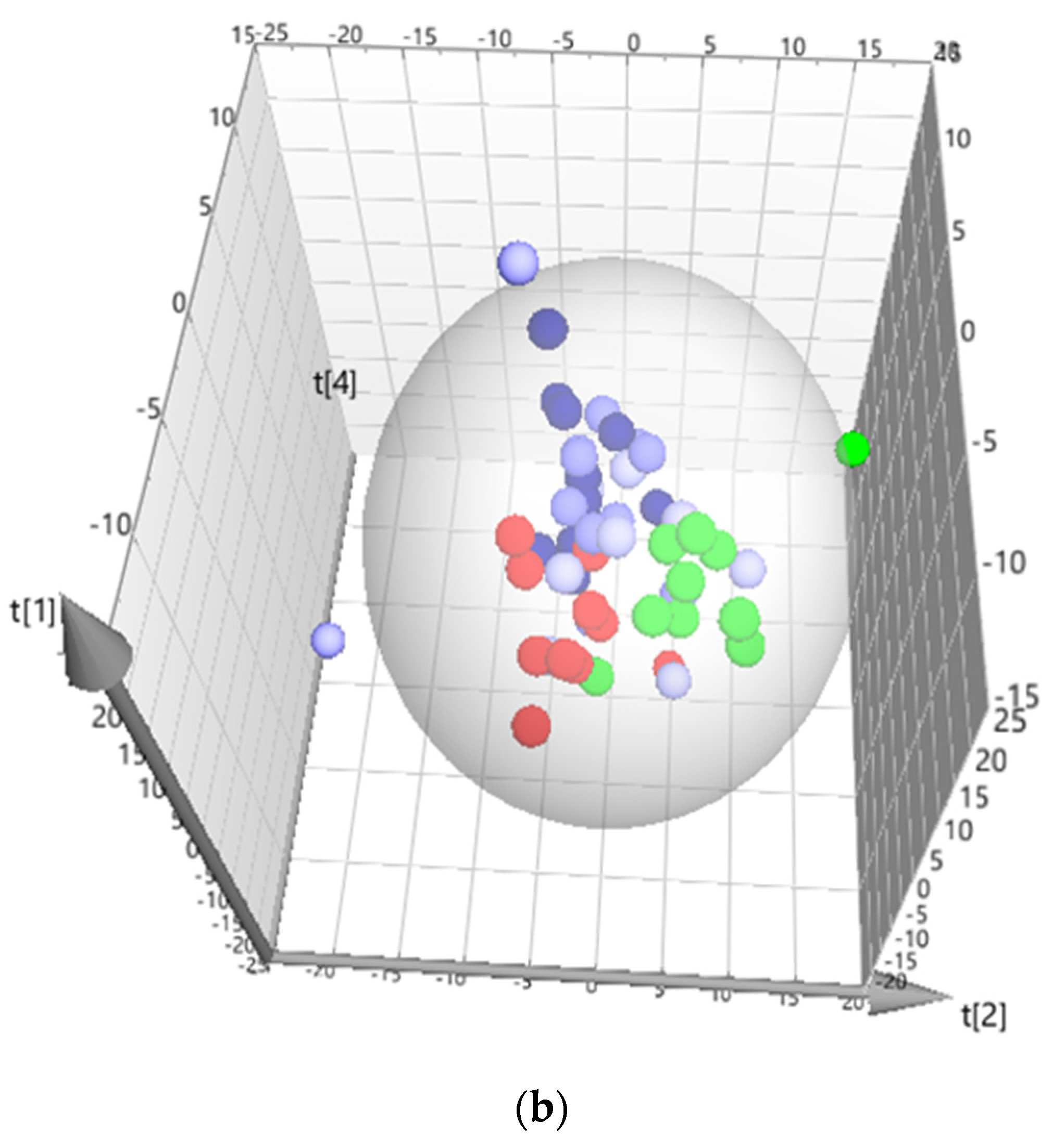
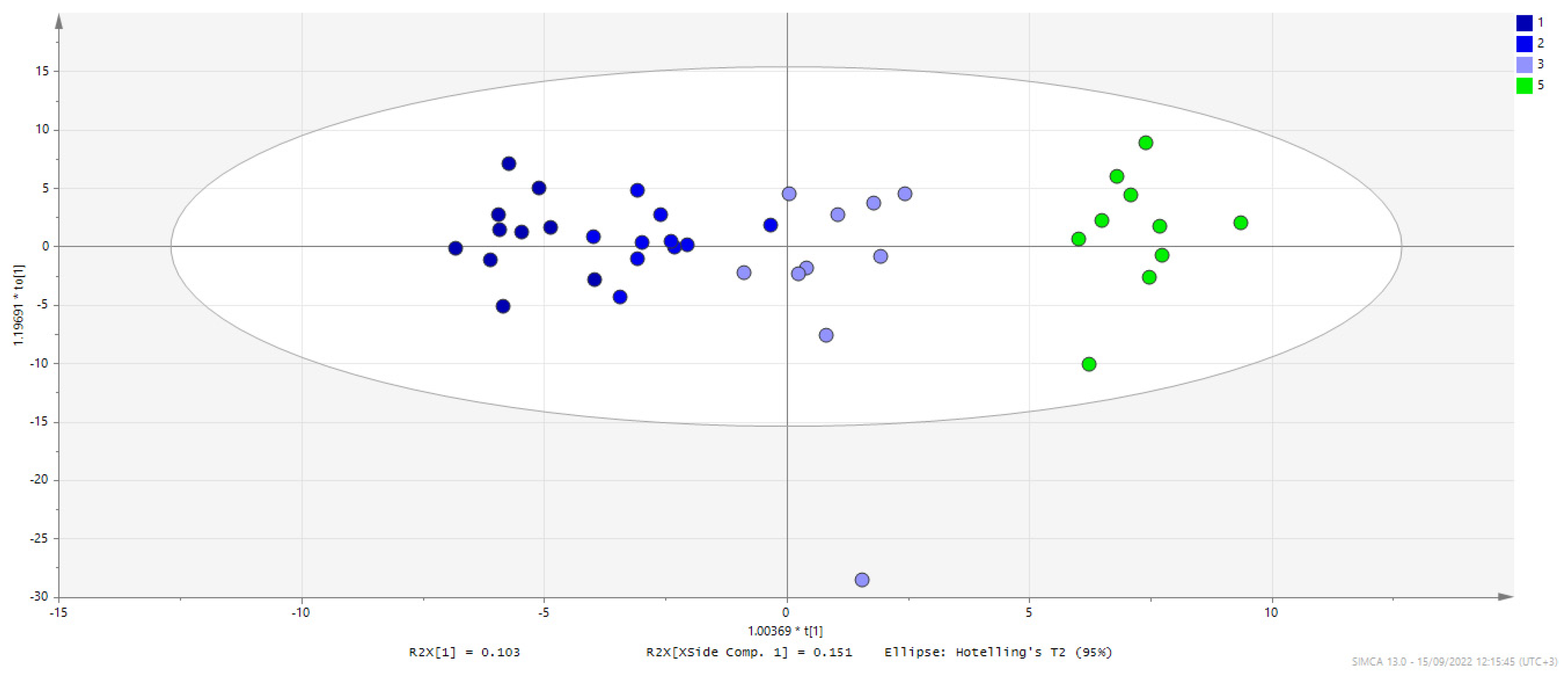
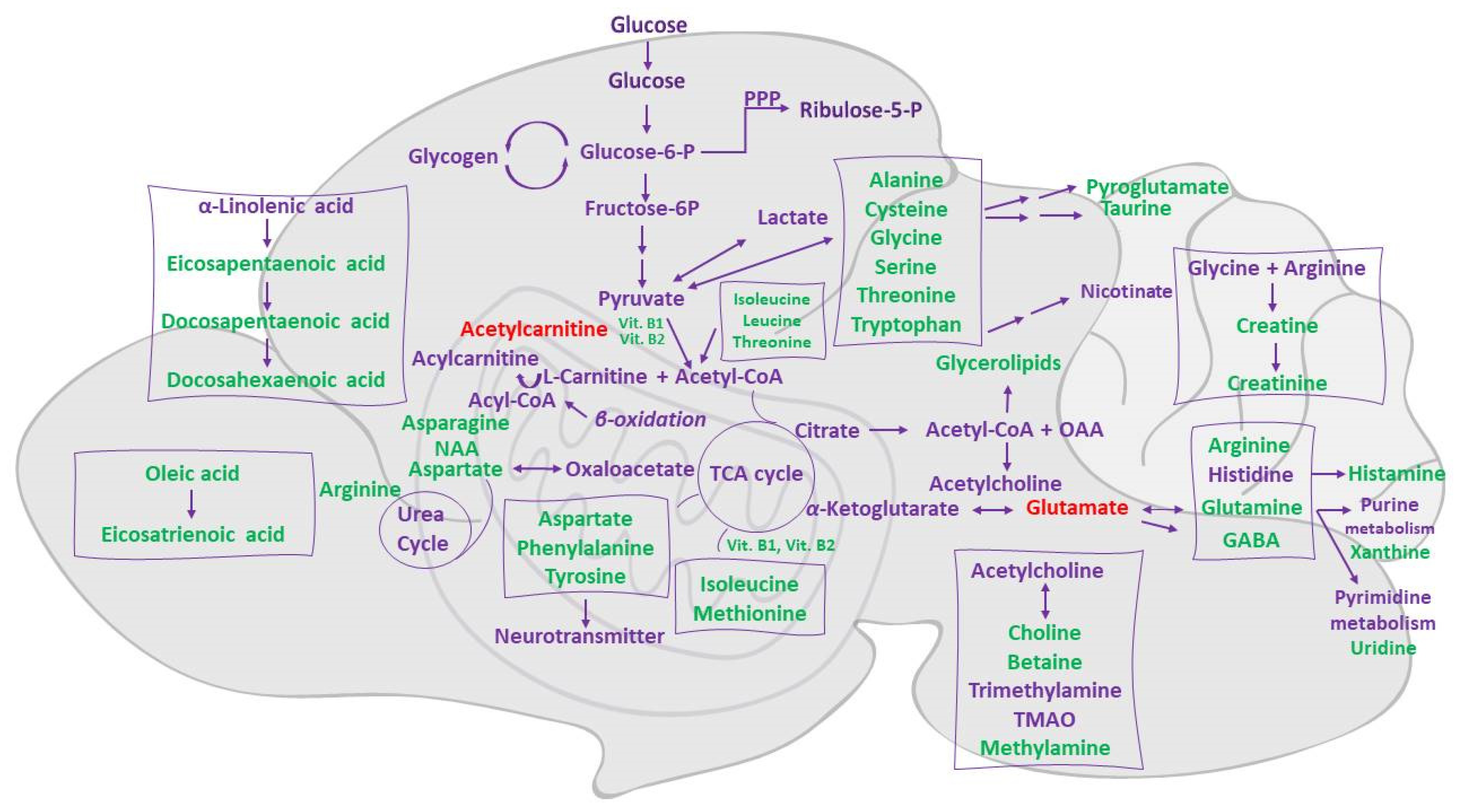
| Model | Type | N | R2X(cum) | R2Y(cum) | Q2(cum) | CV ANOVA |
|---|---|---|---|---|---|---|
| 1,2,3,4,5 | PCA-X | 50 | 0.557 | 0.335 | ||
| 1,2,3,4,5 | OPLS | 50 | 0.481 | 0.946 | 0.834 | 1.139E-08 |
| 1,2,3,5 | OPLS | 40 | 0.518 | 0.966 | 0.898 | 3.246E-08 |
| 1,4 | OPLS-DA | 20 | 0.610 | 0.985 | 0.854 | 3.587E-04 |
| 1,5 | OPLS-DA | 20 | 0.625 | 0.991 | 0.909 | 2.453E-05 |
| 2,4 | OPLS-DA | 20 | 0.543 | 0.987 | 0.789 | 2.702E-03 |
| 2,5 | OPLS-DA | 20 | 0.526 | 0.989 | 0.832 | 7.549E-04 |
| 3,4 | OPLS-DA | 20 | 0.616 | 0.962 | −0.041 | 1.000E+00 |
| 3,5 | OPLS-DA | 20 | 0.577 | 0.972 | 0.824 | 9.762E-04 |
| 4,5 | OPLS-DA | 20 | 0.600 | 0.990 | 0.840 | 5.839E-04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deda, O.; Kachrimanidou, M.; Armitage, E.G.; Mouskeftara, T.; Loftus, N.J.; Zervos, I.; Taitzoglou, I.; Gika, H. Metabolic Phenotyping Study of Mouse Brain Following Microbiome Disruption by C. difficile Colonization. Metabolites 2022, 12, 1039. https://doi.org/10.3390/metabo12111039
Deda O, Kachrimanidou M, Armitage EG, Mouskeftara T, Loftus NJ, Zervos I, Taitzoglou I, Gika H. Metabolic Phenotyping Study of Mouse Brain Following Microbiome Disruption by C. difficile Colonization. Metabolites. 2022; 12(11):1039. https://doi.org/10.3390/metabo12111039
Chicago/Turabian StyleDeda, Olga, Melina Kachrimanidou, Emily G. Armitage, Thomai Mouskeftara, Neil J. Loftus, Ioannis Zervos, Ioannis Taitzoglou, and Helen Gika. 2022. "Metabolic Phenotyping Study of Mouse Brain Following Microbiome Disruption by C. difficile Colonization" Metabolites 12, no. 11: 1039. https://doi.org/10.3390/metabo12111039