Synthesis, Spatial Structure and Analgesic Activity of Sodium 3-Benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate Solvates
Abstract
:1. Introduction
2. Materials and Methods
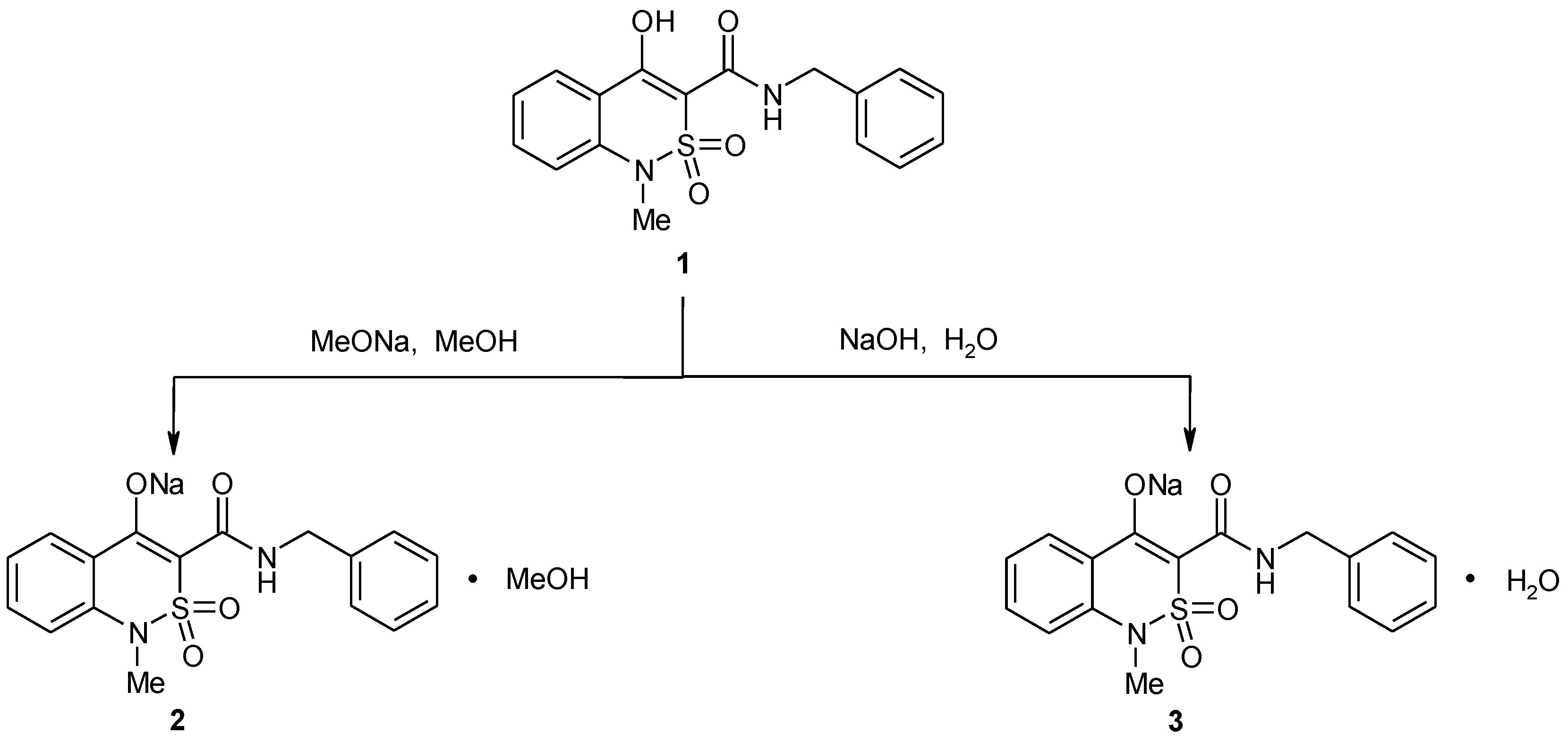
2.1. Chemistry
2.2. Sodium 3-Benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate Methanol Monosolvate (2)
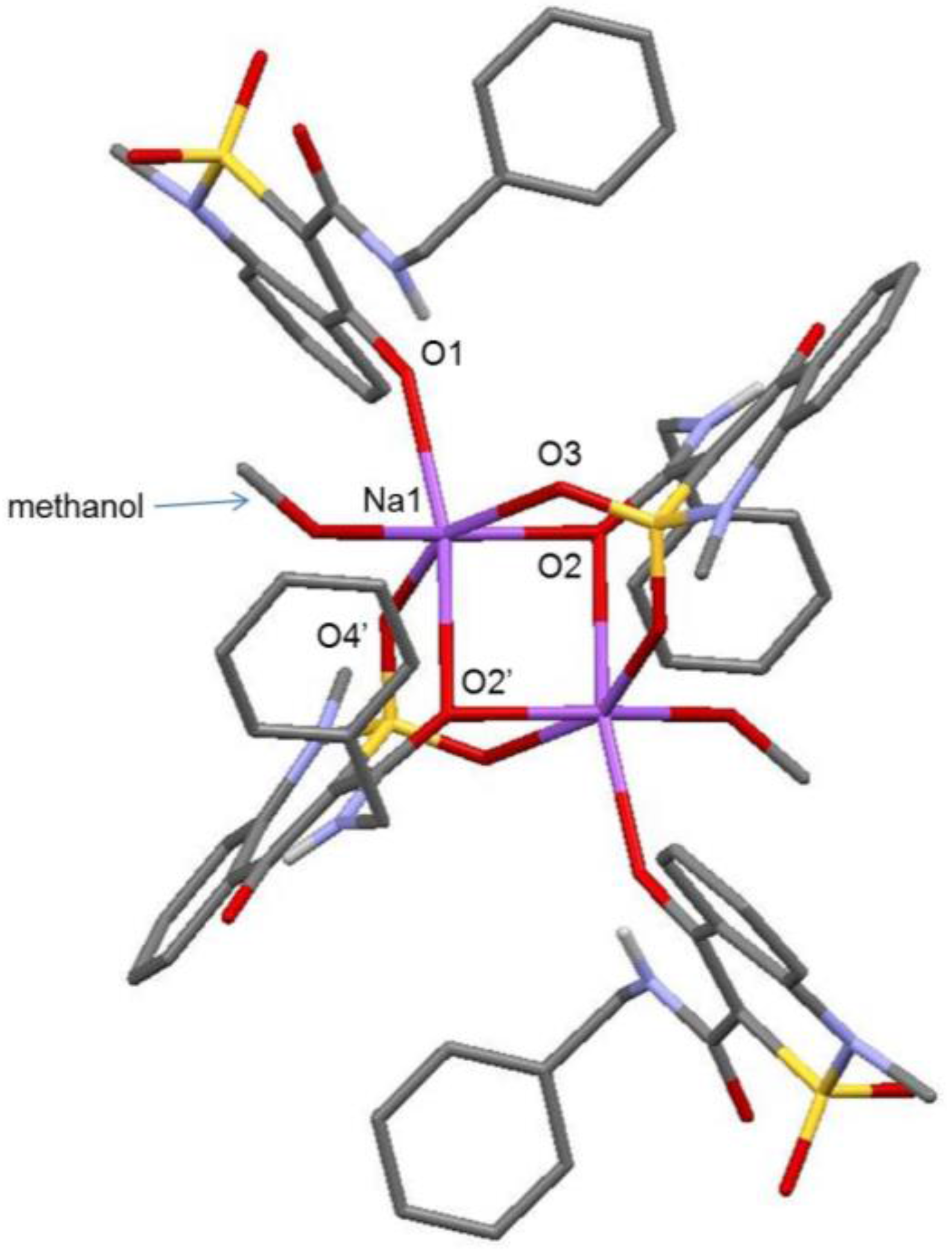
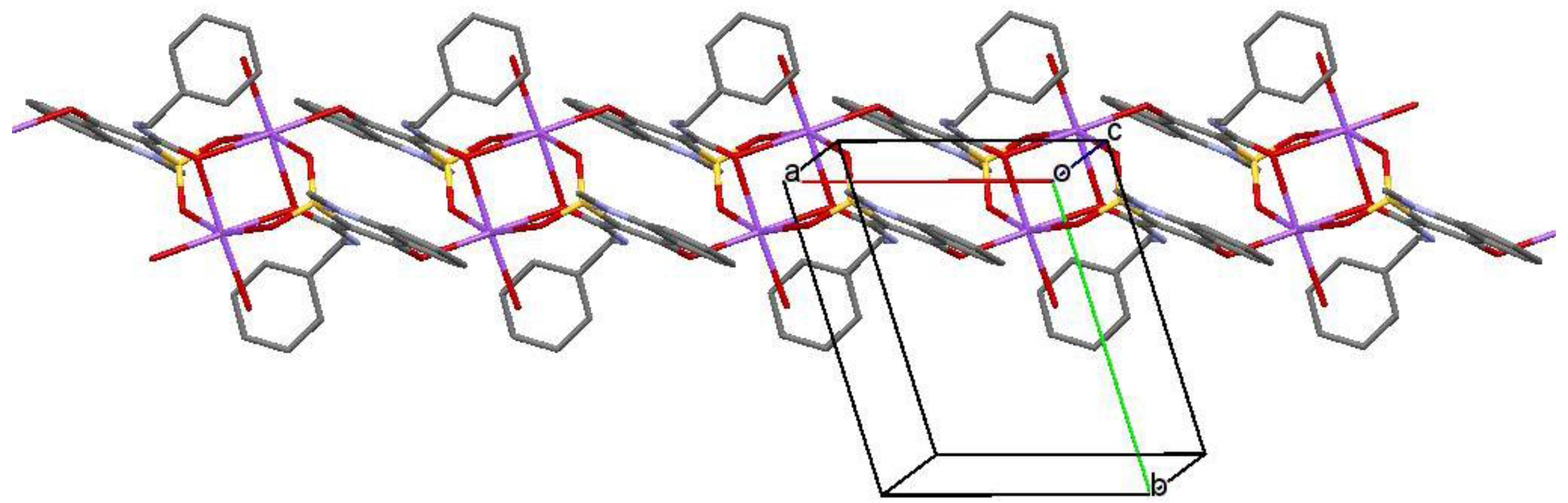
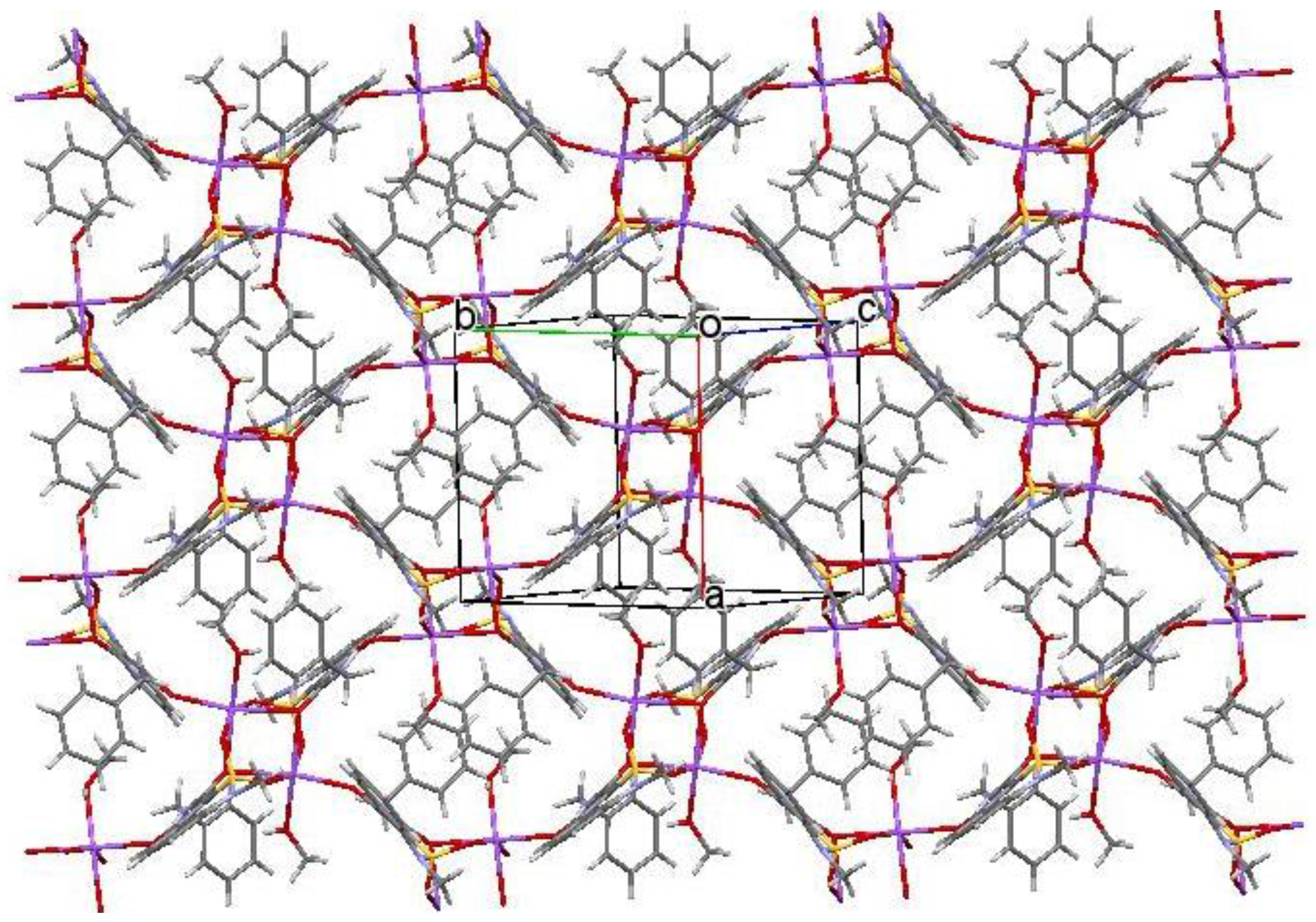
2.3. X-ray Structural Analysis of Sodium Salt Methanol Monosolvate (2)
2.4. Sodium 3-Benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate Monohydrate (3)
2.5. X-ray Structural Analysis of Sodium Salt Monohydrate (3)
2.6. Pharmacology
Analgesic Test
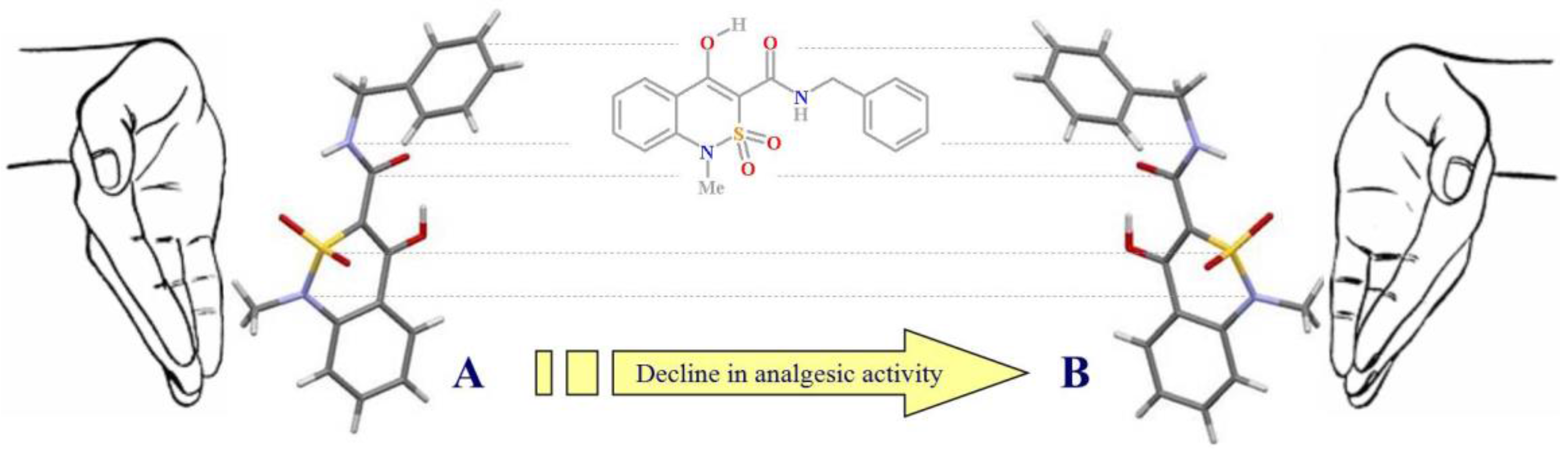
3. Results and Discussion
3.1. Chemistry
3.2. Evaluation of the Analgesic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bock, J.E.; Gavenonis, J.; Kritzer, J.A. Getting in shape: Controlling peptide bioactivity and bioavailability using conformational constraints. ACS Chem Biol. 2013, 8, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational polymorphism. Chem Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: New York, 2007; pp. 150–256. [Google Scholar]
- Morissette, S.L.; Soukasene, S.; Levinson, D.; Cima, M.J.; Almarsson, Ö. Elucidation of crystal form diversity of the HIV protease inhibitor ritonavir by high-throughput crystallization. Proc. Natl. Acad. Sci. USA 2003, 100, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, I.B.; Céolin, R. Rotigotine: Unexpected polymorphism with predictable overall monotropic behavior. J. Pharm. Sci. 2015, 104, 4117–4122. [Google Scholar] [CrossRef] [PubMed]
- Kuczer, M.; Czarniewska, E.; Majewska, A.; Różanowska, M.; Rosiński, G.; Lisowski, M. Novel analogs of alloferon: Synthesis, conformational studies, pro-apoptotic and antiviral activity. Bioorg. Chem. 2016, 66, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cazares, M.; Wu, J.; Houghten, R.A.; Toll, L.; Dooley, C. Potent μ-opioid receptor agonists from cyclic peptides Tyr-c[d-Lys-Xxx-Tyr-Gly]: Synthesis, biological, and structural evaluation. J. Med. Chem. 2016, 59, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Cai, C.; Deng, M.; Wang, Q. Spatial configuration and three-dimensional conformation directed design, synthesis, antiviral activity, and structure-activity relationships of phenanthroindolizidine analogues. J. Agric. Food Chem. 2016, 64, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, A.E.; Głowacka, I.E.; Piotrowska, D.G. 1′-Homonucleosides and their structural analogues: A review. Eur. J. Med. Chem. 2016, 118, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Qu, X.; Swanson, R.; Bohannan, Z.; Bliss, R.; Tsai, J. Relative packing groups in template-based structure prediction: Cooperative effects of true positive constraints. J. Comput. Biol. 2011, 18, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Potemkin, V.A.; Arslambekov, R.M.; Bartashevich, E.V.; Grishina, M.A.; Belik, A.V.; Perspicace, S.; Guccione, S. Multiconformational method for analyzing the biological activity of molecular structures. J. Struct. Chem. 2002, 43, 1045–1049. [Google Scholar] [CrossRef]
- Macías, M.A.; Acosta, L.M.; Sanabria, C.M.; Palma, A.; Roussel, P.; Gauthier, G.H.; Suescun, L. Crystal structures of five new substituted tetrahydro-1-benzazepines with potential antiparasitic activity. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72 Pt 5, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Shishkina, S.V.; Baumer, V.N.; Gorokhova, O.V.; Petrushova, L.A.; Sim, G. The structure of two pseudo-enantiomeric forms of N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide and their analgesic properties. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72 Pt 5, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Sim, G.; Grinevich, L.A. The effective synthesis of N-(arylalkyl)-1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides as promising analgesics of a new chemical class. Sci. Pharm. 2015, 83, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX Acta Crystallogr, Sect A: Found. Crystallogr 2008, A64, 112–122. [Google Scholar]
- Cambridge Crystallographic Data Center. Available online: www.ccdc.ac.uk/data_request/cifCCDC1486591 (accessed on 21 June 2016).
- Cambridge Crystallographic Data Center. Available online: www.ccdc.ac.uk/data_request/cifCCDC1486592 (accessed on 21 June 2016).
- Vogel, H.G. (Ed.) Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1014–1016.
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994; Volume 2, pp. 741–926. [Google Scholar]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]

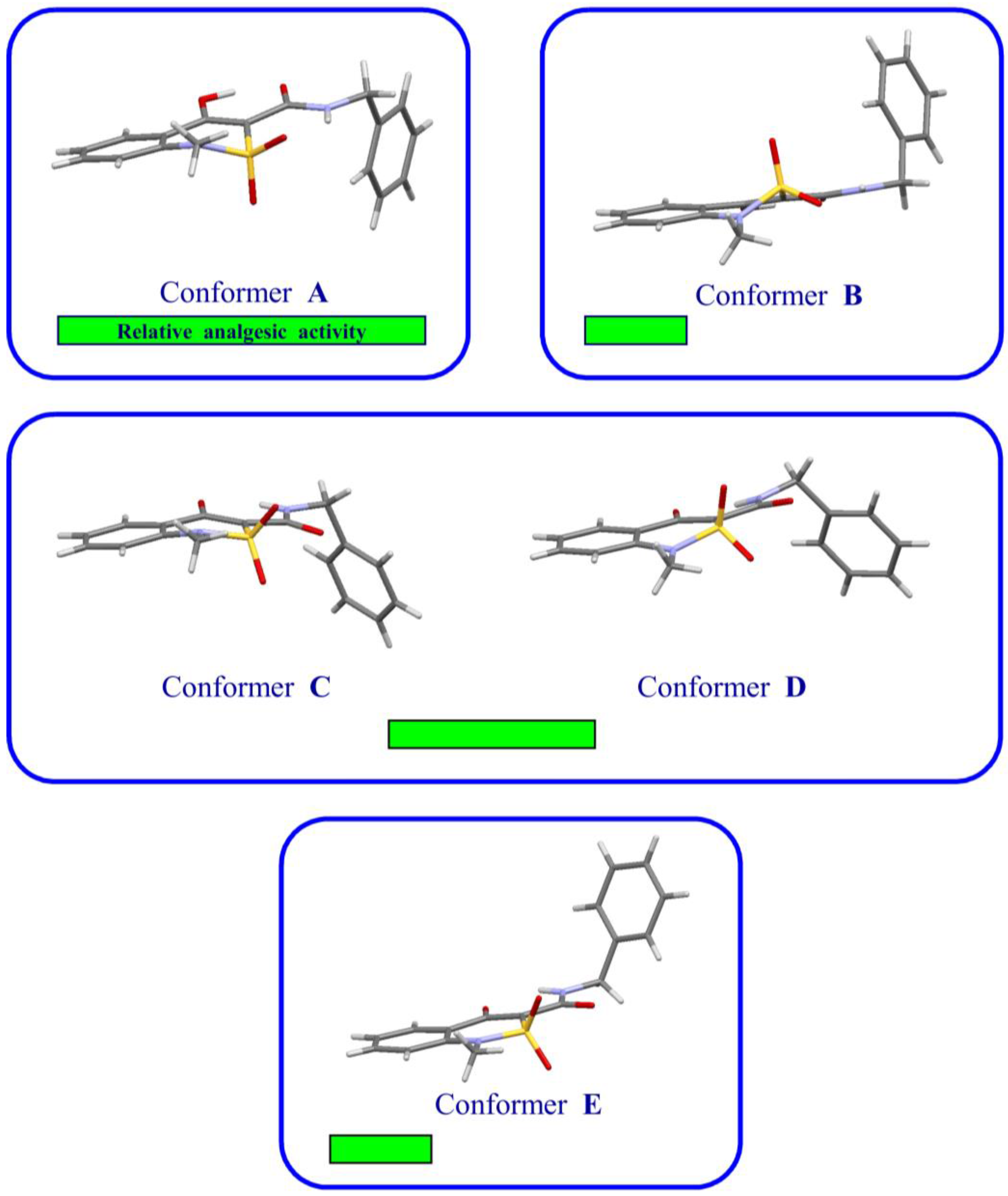
| Entry | Product | Conformer | Latent Period in 1 h after Introduction of the Compounds (s) a | Change of the Latent Period, Compared to Control (%) |
|---|---|---|---|---|
| 1 | 1 | A | 15.98 ± 0.85 | +111.9 |
| 2 | 1 | B | 9.88 ± 0.72 | +31.1 |
| 3 | 2 | C–D (1:1) | 12.27 ± 0.81 | +62.7 |
| 4 | 3 | E | 9.86 ± 0.70 | +30.8 |
| 5 | Meloxicam | - | 11.27 ± 0.83 | +49.5 |
| 6 | Piroxicam | - | 9.45 ± 0.74 | +25.3 |
| 7 | Control | - | 7.54 ± 0.82 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Grinevich, L.A.; Sim, G. Synthesis, Spatial Structure and Analgesic Activity of Sodium 3-Benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate Solvates. Sci. Pharm. 2016, 84, 705-714. https://doi.org/10.3390/scipharm84040705
Ukrainets IV, Petrushova LA, Shishkina SV, Grinevich LA, Sim G. Synthesis, Spatial Structure and Analgesic Activity of Sodium 3-Benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate Solvates. Scientia Pharmaceutica. 2016; 84(4):705-714. https://doi.org/10.3390/scipharm84040705
Chicago/Turabian StyleUkrainets, Igor V., Lidiya A. Petrushova, Svitlana V. Shishkina, Lina A. Grinevich, and Galina Sim. 2016. "Synthesis, Spatial Structure and Analgesic Activity of Sodium 3-Benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate Solvates" Scientia Pharmaceutica 84, no. 4: 705-714. https://doi.org/10.3390/scipharm84040705
APA StyleUkrainets, I. V., Petrushova, L. A., Shishkina, S. V., Grinevich, L. A., & Sim, G. (2016). Synthesis, Spatial Structure and Analgesic Activity of Sodium 3-Benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate Solvates. Scientia Pharmaceutica, 84(4), 705-714. https://doi.org/10.3390/scipharm84040705






