A Comparative Pharmacokinetics Study of the Anti-Parkinsonian Drug Pramipexole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Products
2.3. Study Design and Treatment
2.4. Bioanalytical Method and Pharmacokinetic Analysis
2.4.1. Materials
2.4.2. Validated Bioanalytical Method for Quantification of the Drug Concentration
2.4.3. Sample Extraction
2.4.4. Pharmacokinetic Data Analysis
2.4.5. Incurred Sample Reanalysis
3. Results
3.1. Study Population
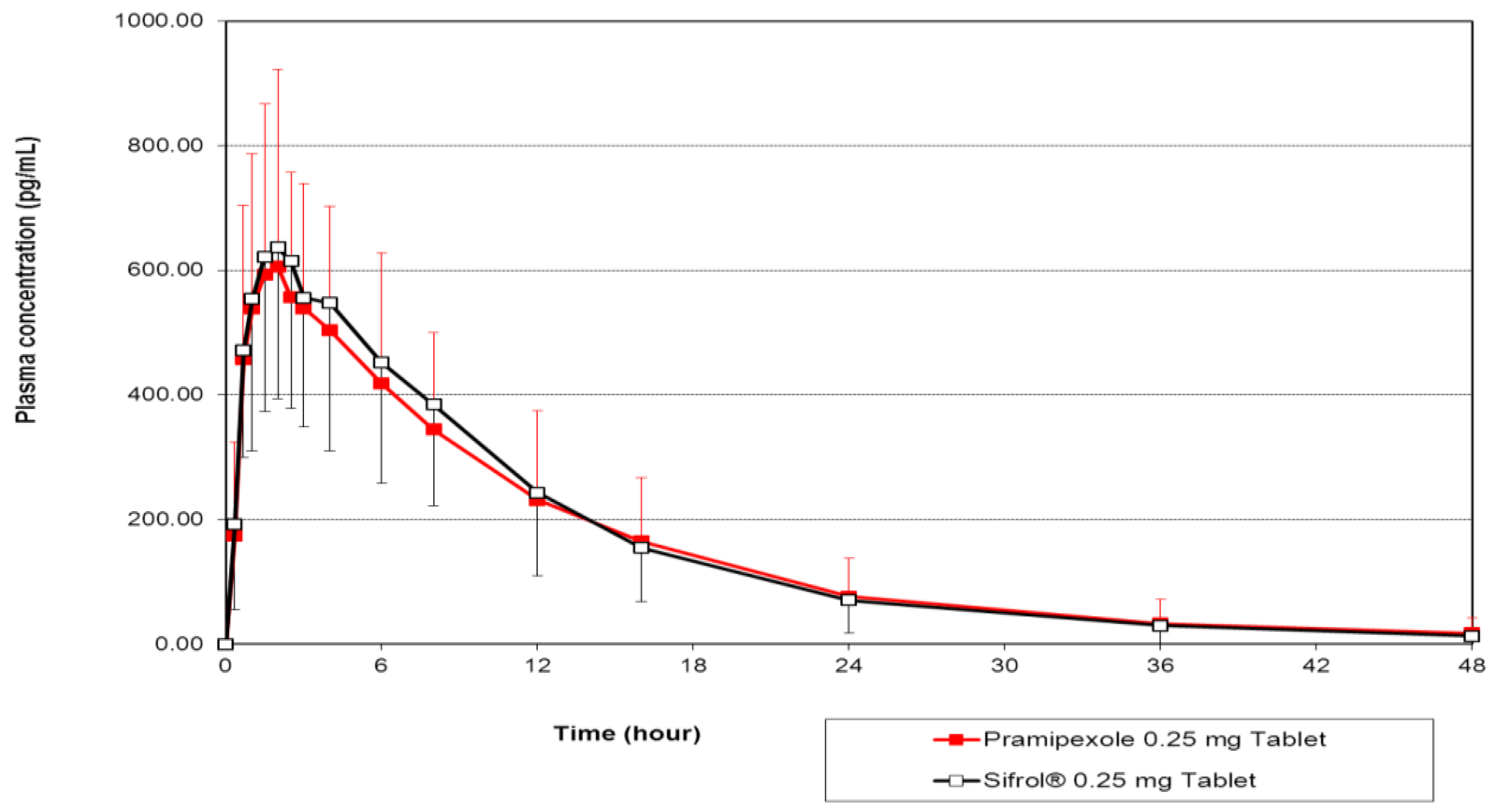
3.2. Pharmacokinetic and Statistical Analysis
3.3. Safety and Tolerability
3.4. Incurred Sample Reanalysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tan, L.C. Epidemiology of Parkinson’s disease. Neurol. Asia 2013, 18, 231–238. [Google Scholar]
- De Rijk, M.C.; Tzourio, C.; Breteler, M.M.; Dartigues, J.F.; Amaducci, L.; Lopez-Pousa, S.; Manubens-Bertran, J.M.; Alpérovitch, A.; Roccam, W.A. Prevalence of parkinsonism and Parkinson’s disease in Europe: The EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Medical management of Parkinson’s disease. P&T 2008, 33, 590–606. [Google Scholar]
- Clarke, C.E. Medical management of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 72 (Suppl. 1), I22–I27. [Google Scholar] [PubMed]
- Pramipexole dihydrochloride. In AHFS Drug Information 2013; McEvoy, G.K. (Ed.) American Society of Health-System Pharmacists: Bethesda, MD, USA, 2013; pp. 2692–2695.
- Pramipexole hydrochloride. In Martindale—The Complete Drug Reference, 36th ed.; Sweetman, S.C. (Ed.) The Pharmaceutical Press: London, UK, 2009; p. 814.
- Silber, M.H.; Girish, M.; Izurieta, R. Pramipexole in the Management of Restless Legs Syndrome: An Extended Study. Sleep 2003, 26, 819–821. [Google Scholar] [PubMed]
- World Medical Association. WMA Declaration of Helsinki—Ethical principles for medical research involving human subjects. In Proceedings of the 64th WMA General Assembly, Fortaleza, Brazil, October 2013; World Medical Association, Inc.: Fortaleza, Brazil, 2013. [Google Scholar]
- International Conference on Harmonisation Expert Working Group. ICH Harmonised Tripartite—Guideline for Good Clinical Practice E6(R1). Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (accessed on 16 November 2016).
- Organisation for Economic Cooperation and Development. OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring; OECD Environmental Health and Safety Publications: Paris, France, 1998; pp. 1–41. [Google Scholar]
- Abib, E., Jr.; Duarte, L.F.; Pereira, R. Comparative Bioavailability: Two Pramipexole Formulations in Healthy Volunteers after a Single Dose Administration under Fasting Conditions. J. Bioequiv. Availab. 2012, 4, 56–59. [Google Scholar] [CrossRef]
- US Department of Health and Human Service Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations; Center for Drug Evaluation and Research FDA: Rockville, MD, USA, 2003; pp. 1–23.
- European Medicines Agency. Guideline on the Investigation of Bioequivalence; Doc. Ref: CPMW/EWP/QWP/1401/98 Rev.1; European Medicines Agency: London, UK, 2010; pp. 1–27. [Google Scholar]
- European Medicines Agency. Guideline on Bioanalytical Method Validation; Doc. Ref: EMEA/CHMP/EWP/192217/2009; European Medicines Agency: London, UK, 2011; pp. 13–14. [Google Scholar]
- Niazi, S.K. Bioequivalence testing rationale and principles. In Handbook of Bioequivalence Testing, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 95–149. [Google Scholar]
- Dixon, W.J.; Massey, F.J. Introduction to Statistical Analysis, 3rd ed.; McGraw-Hill Companies: New York, NY, USA, 1969. [Google Scholar]
- Diletti, E.; Hauschke, D.; Steinijans, V.W. Sample size determination for bioequivalence assessment by means of confidence intervals. Int. J. Clin. Pharmacol. Ther. Toxic. 1991, 29, 1–8. [Google Scholar]
- Pedrazzoli, J., Jr.; Zanin, M.; Coelho, E.C.; Marchioretto, M.; Meurer, E.C.; Barros, F.A.P. Bioequivalence study between two formulations of pramipexole dihydrochloride 0.25 mg tablets in health volunteers after single dose administration. Rev. Bras. Med. 2012, 69, 162–166. [Google Scholar]
- Yadav, M.; Rao, R.; Kurani, H.; Rathod, J.; Patel, R.; Singhal, P.; Shrivastav, P.S. Validated ultra-performance liquid chromatography tandem mass spectrometry method for the determination of pramipexole in human plasma. J. Chromatogr. Sci. 2010, 48, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K. Historical perspective on generic pharmaceuticals. In Handbook of Bioequivalence Testing, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–11. [Google Scholar]
- Setiawati, E.; Yunaidi, D.A.; Handayani, L.R.; Santoso, I.D.; Setiawati, A.; Tjandrawinata, R.R. Bioequivalence study of two clopidogrel film-coated tablet formulations in healthy volunteers. Arzneimittelforschung 2011, 61, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Tjandrawinata, R.R.; Setiawati, E.; Yunaidi, D.A.; Santoso, I.D.; Setiawati, A.; Susanto, L.W. Bioequivalence study of two formulations of bisoprolol fumarate film-coated tablets in healthy subjects. Drug Des. Dev. Ther. 2012, 6, 311–316. [Google Scholar]
- Tjandrawinata, R.R.; Setiawati, E.; Yunaidi, D.A.; Simanjuntak, R.; Santoso, I.D.; Susanto, L.W. Bioequivalence study of two formulations of candesartan cilexetil tablet in healthy subjects under fasting conditions. Drug Des. Dev. Ther. 2013, 7, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Tjandrawinata, R.R.; Setiawati, E.; Putri, R.S.; Gunawan, V.A.; Ong, F.; Susanto, L.W.; Nofiarny, D. Pharmacokinetic equivalence study of two formulations of the anticonvulsant pregabalin. Clin Pharmacol. 2015, 7, 69–75. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Low (60.32 pg/mL) | At concentration of | ||
|---|---|---|---|---|
| Medium (1005.40 pg/mL) | High (1759.45 pg/mL) | |||
| Precision a | intra-assay | 4.55% | 4.57% | 3.74% |
| inter-assay | 7.30% | 5.13% | 5.29% | |
| Accuracy a | intra-assay | 0.65% | −6.74% | −3.81% |
| inter-assay | −0.68% | −7.81% | −6.83% | |
| Stability | at −20°C (stable until 44 days) | −3.81% to +0.49% | – | –3.56% to +7.45% |
| at room temperature (stable until 6 h) | −14.60% to +4.49% | – | −12.41% to −3.51% | |
| freeze and thaw (stable until 4 cycles) | −11.01% to +6.84% | – | −12.87% to +3.83% | |
| Linearity: the linearity of the standard calibration curves was obtained (r of 0.9974 on day 1, 0.9975 on day 2, and 0.9998 on day 3). | ||||
| LLOQ: the LLOQ has been established at 20.12 pg/mL. | ||||
| Selectivity: The % diff of the analyte and internal standard interferences ranged from 2.71%–17.92% and 0.01%–0.03%, respectively. From the result, it can be concluded that there were no interferences of the analyte and internal standard compounds. | ||||
| Range: the range of quantification was established as 20.12–2011.60 pg/mL. | ||||
| Subject | Gender | Age (Years) | Weight (kg) | Height (m) | BMI (kg/m2) | Smoking Status (Cigarettes/Day) a |
|---|---|---|---|---|---|---|
| 1 | F | 18 | 51.5 | 1.52 | 22.29 | - |
| 2 | M | 34 | 43 | 1.54 | 18.13 | 3 |
| 3 | F | 49 | 57 | 1.54 | 24.03 | - |
| 4 | F | 46 | 43 | 1.50 | 19.11 | - |
| 5 | F | 46 | 60 | 1.55 | 24.97 | - |
| 6 | M | 18 | 65 | 1.75 | 21.22 | 6 |
| 7 | F | 32 | 54.5 | 1.64 | 20.26 | - |
| 8 | F | 29 | 51 | 1.54 | 21.50 | - |
| 9 | F | 42 | 54 | 1.47 | 24.99 | - |
| 10 | F | 38 | 59 | 1.54 | 24.88 | - |
| 11 | F | 23 | 61 | 1.57 | 24.75 | - |
| 12 | F | 42 | 48 | 1.50 | 21.33 | - |
| 13 | F | 29 | 51 | 1.56 | 20.96 | - |
| 14 | F | 44 | 56 | 1.50 | 24.89 | - |
| 15 | M | 36 | 64 | 1.60 | 25.00 | 8 |
| 16 | F | 50 | 60 | 1.57 | 24.34 | - |
| 17 | M | 23 | 55 | 1.63 | 20.70 | 6 |
| 18 | M | 23 | 52 | 1.65 | 19.10 | 8 |
| 19 | M | 46 | 45 | 1.56 | 18.49 | - |
| 20 | M | 26 | 61 | 1.69 | 21.36 | 3 |
| 21 | M | 25 | 48 | 1.58 | 19.23 | 6 |
| 22 | M | 28 | 49 | 1.63 | 18.44 | 5 |
| 23 | F | 24 | 57 | 1.57 | 23.12 | - |
| Mean | - | 33.52 | 54.13 | 1.57 | 21.87 | - |
| SD | - | 10.37 | 6.35 | 0.07 | 2.46 | - |
| Min | - | 18 | 43 | 1.47 | 18.13 | - |
| Max | - | 50 | 65 | 1.75 | 25.00 | - |
| %CV | - | 30.93% | 11.73% | 4.23% | 11.26% | - |
| Parameter | Test Formulation Mean (SD) | Reference Formulation Mean (SD) | Geometric Mean Ratio of T/R (90% CI) a | p Values | %CV |
|---|---|---|---|---|---|
| AUC0-t (pg·h/mL) b | 7355.76 (3793.14) | 7542.56 (3492.79) | 95.89% (90.73%–101.34%) | 0.2053 b | 10.67% |
| AUC0-∞ (pg·h/mL) b | 7996.24 (3861.00) | 8196.72 (3555.44) | 95.53% (89.75%–101.68%) | 0.2208 b | 12.05% |
| Cmax (pg/mL) b | 684.43 (308.51) | 727.41 (251.07) | 92.11% (84.35%–100.58%) | 0.1228 b | 17.05% |
| t1/2 (h) | 8.82 (4.02) | 8.83 (3.95) | - | NS d | - |
| tmax (h) c | 2.00 (0.67–4.00) | 1.75 (0.67–3.00) | - | NS e | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putri, R.S.I.; Setiawati, E.; Aziswan, S.A.; Ong, F.; Tjandrawinata, R.R.; Susanto, L.W. A Comparative Pharmacokinetics Study of the Anti-Parkinsonian Drug Pramipexole. Sci. Pharm. 2016, 84, 715-723. https://doi.org/10.3390/scipharm84040715
Putri RSI, Setiawati E, Aziswan SA, Ong F, Tjandrawinata RR, Susanto LW. A Comparative Pharmacokinetics Study of the Anti-Parkinsonian Drug Pramipexole. Scientia Pharmaceutica. 2016; 84(4):715-723. https://doi.org/10.3390/scipharm84040715
Chicago/Turabian StylePutri, Ratih S. I., Effi Setiawati, Syifa A. Aziswan, Fenny Ong, Raymond R. Tjandrawinata, and Liana W. Susanto. 2016. "A Comparative Pharmacokinetics Study of the Anti-Parkinsonian Drug Pramipexole" Scientia Pharmaceutica 84, no. 4: 715-723. https://doi.org/10.3390/scipharm84040715





