Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
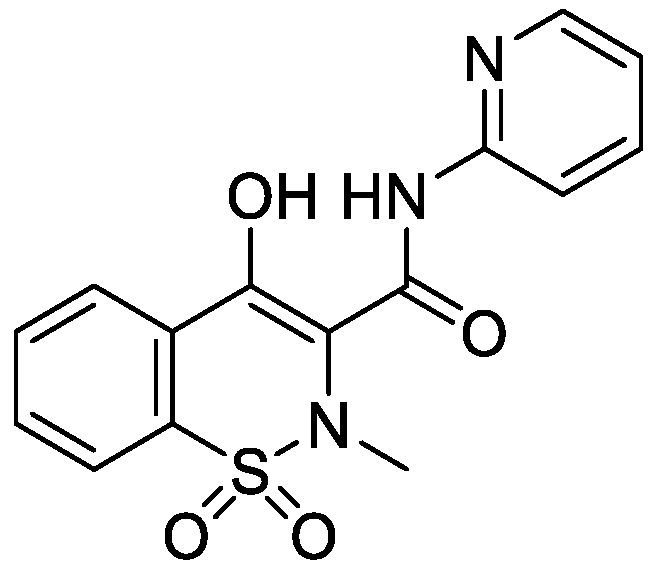
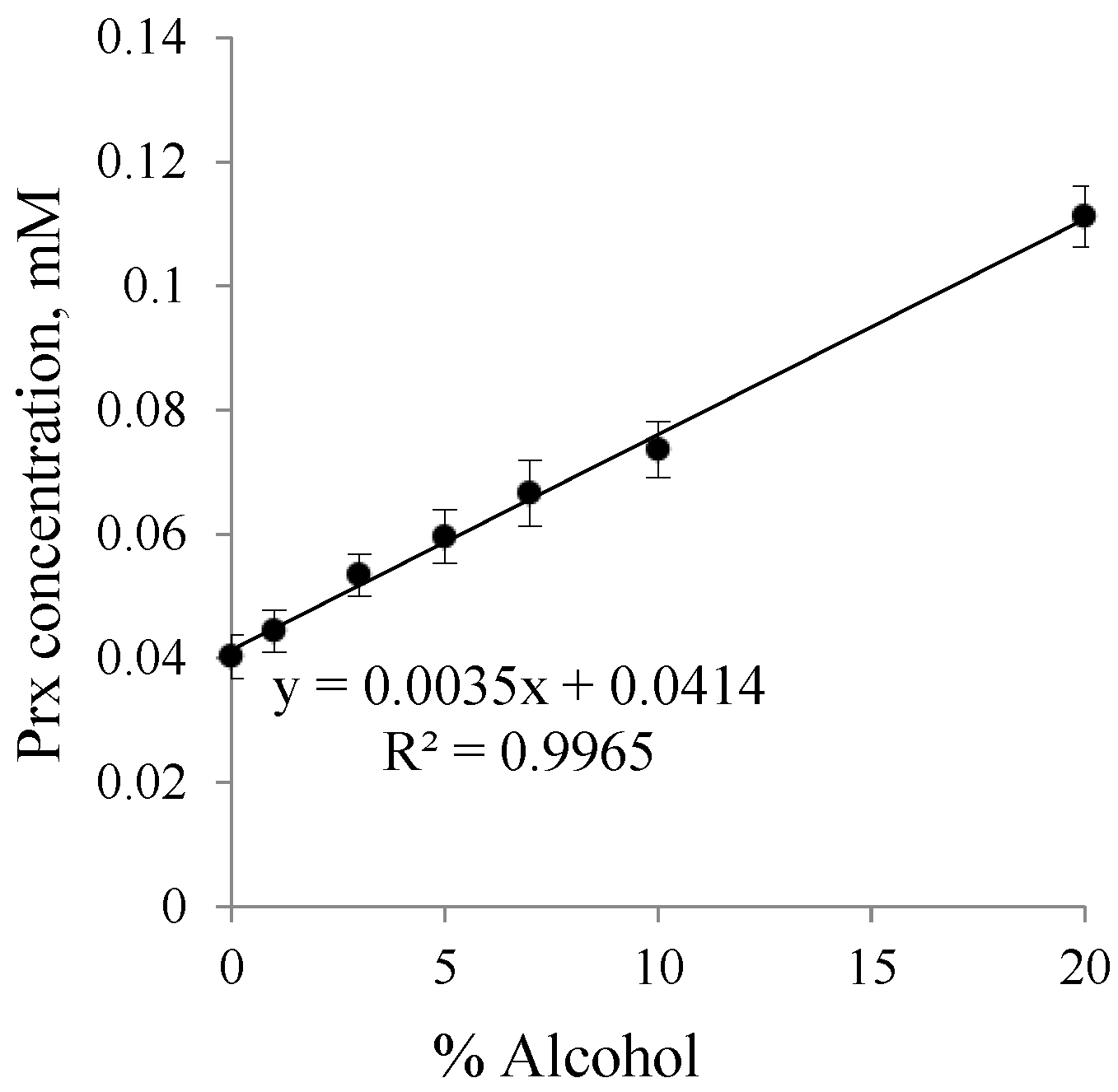
2.2. The Calibration Curve Construction
2.3. Determination of the Drug Solubility in the Co-Solvent–Water Binary System
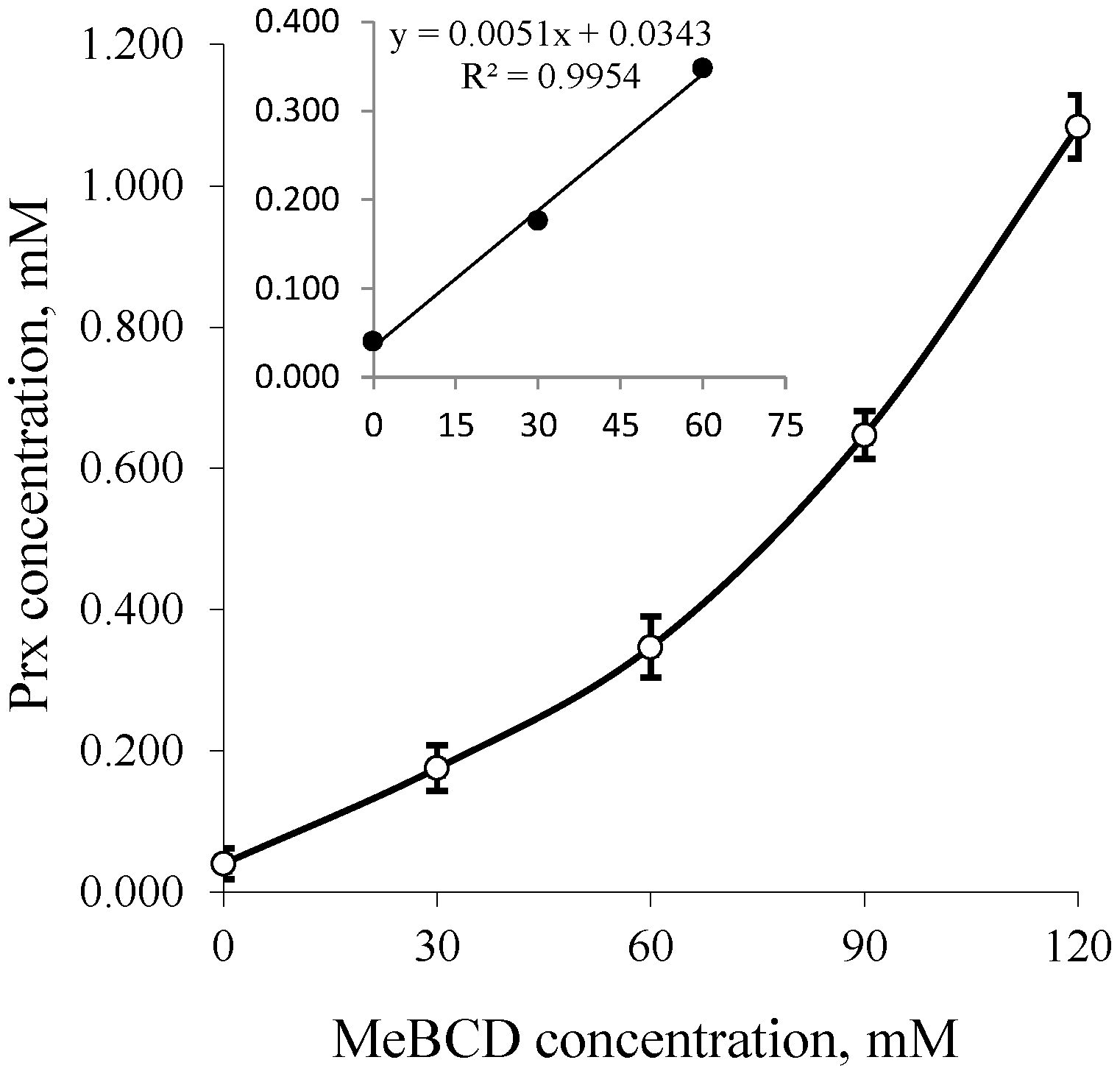
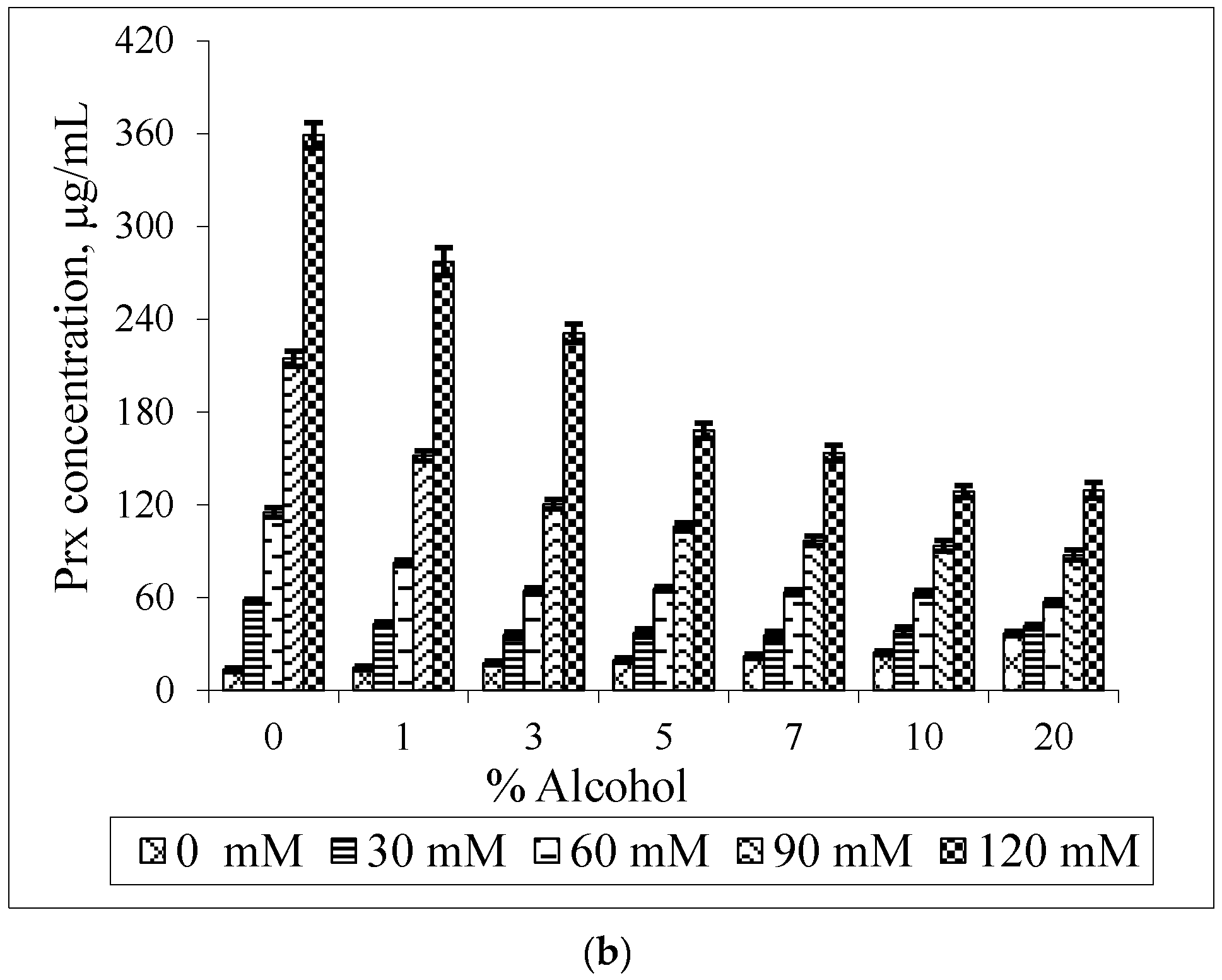
2.4. Phase Solubility Study of the Prx–CDs Binary System
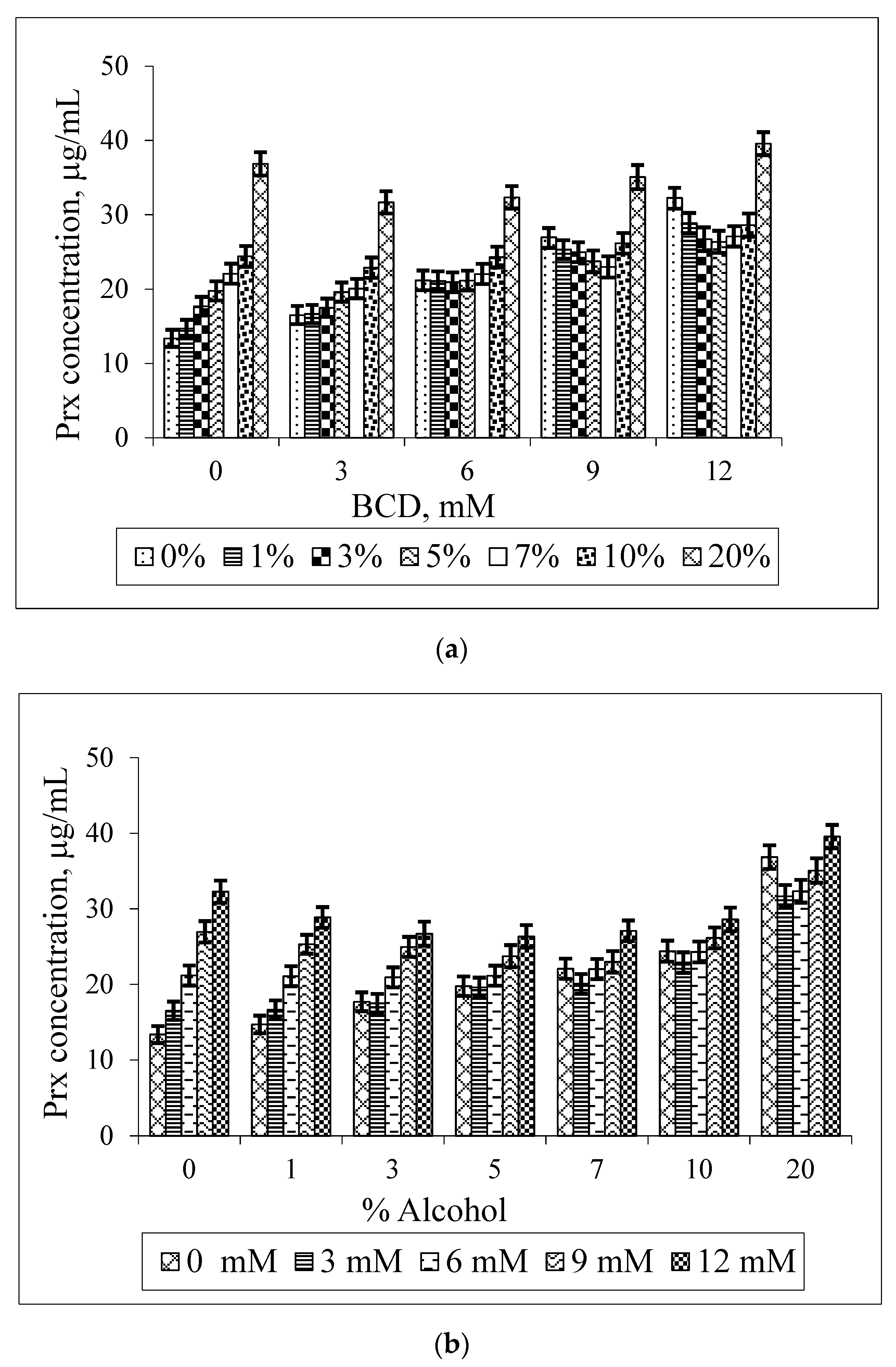
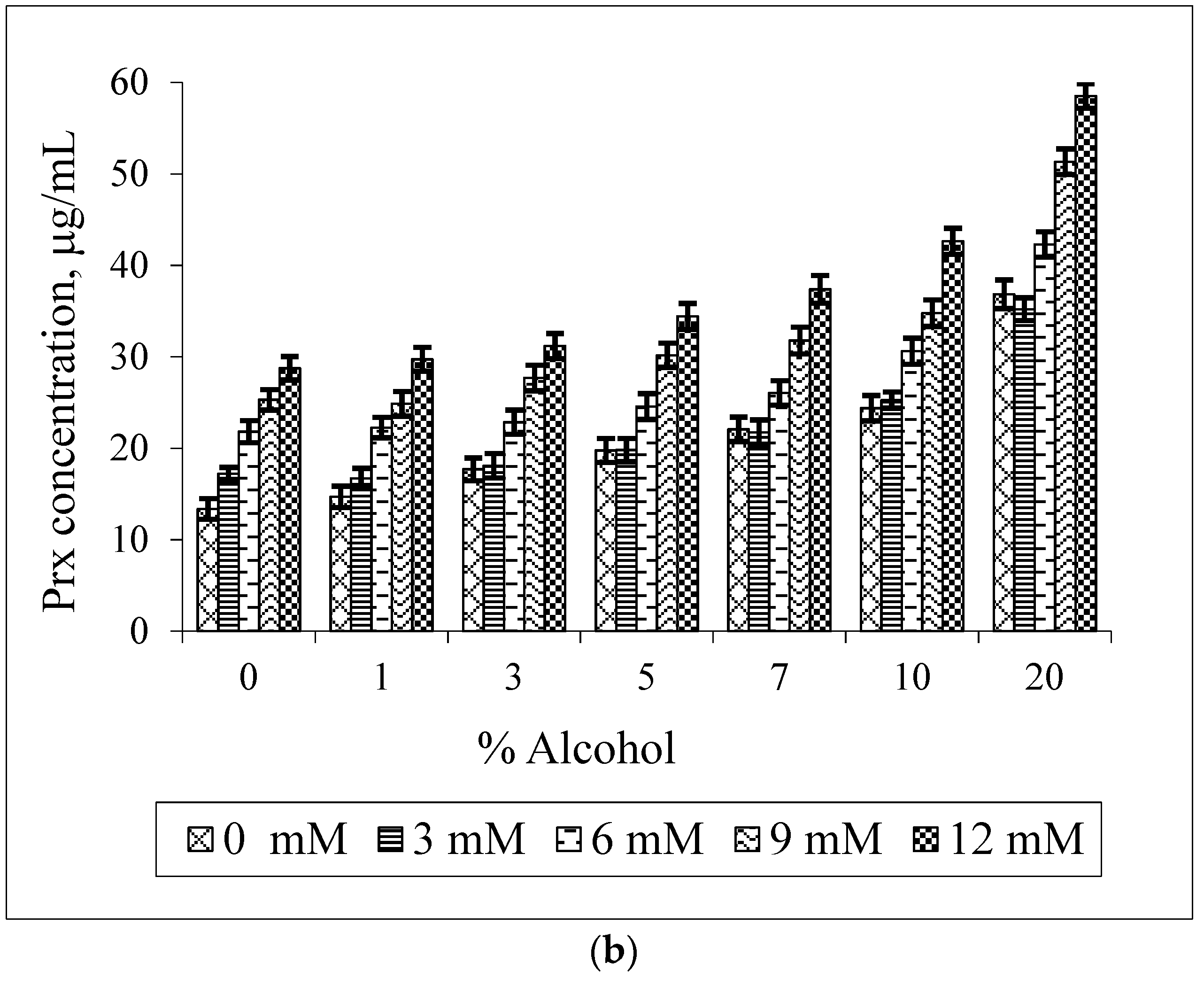
2.5. Phase Solubility Study of the Prx–CDs–Ethanol Ternary System
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lui, R. (Ed.) Water-Insoluble Drug Formulation, 2nd ed.; CRC Press: New York, NY, USA, 2008; Chapter 8–15; pp. 131–435.
- Strickley, R. Solubilizing Excipients in Oral and Injectable Formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Nagaich, U.; Gulati, N.; Sharma, V.K.; Khosa, R.L. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: A recent review. J. Adv. Pharm. Educ. Res. 2012, 2, 32–67. [Google Scholar]
- Nayak, A.K.; Panigrahi, P.P. Solubility Enhancement of Etoricoxib by Cosolvency Approach. ISRN Phys. Chem. 2012, 2012, 820653. [Google Scholar] [CrossRef]
- Krstic, M.; Popovic, M.; Dobricic, V.; Ibric, S. Influence of Solid Drug Delivery System Formulation on Poorly Water-Soluble Drug Dissolution and Permeability. Molecules 2015, 20, 14684–14698. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Liu, S.; Wang, Q.; Li, X. Nanoemulsions as Novel Oral Carriers of Stiripentol: Insight into the protective effect and Absorption Enhancement. Int. J. Nanomed. 2015, 10, 4937–4946. [Google Scholar]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Kraut, J.A.; Kurtz, I. Toxic Alcohol Ingestions: Clinical Features, Diagnosis, and Management. Clin. J. Am. Soc. Nephrol. 2008, 3, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Becirevic-Lacan, M. Multicomponent Complexes of Piroxicam with Cyclodextrins and Hydroxypropyl Methylcellulose. Drug Dev. Ind. Pharm. 2004, 30, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Pandey, N.K.; Singh, S.K.; Kumar, M. Formulation and characterization of ternary complex of poorly soluble duloxetine hydrochloride. J. Appl. Pharm. Sci. 2015, 5, 88–96. [Google Scholar] [CrossRef]
- Azarbayjani, A.F.; Sajed-Amin, S.; Panahi-Azar, V.; Asadpour-Zeynali, K.; Jouyban, A. Co-solubilization of Lamotrigine by Complexation and Micellization in Binary Solvent Mixtures. Chem. Eng. Res. Des. 2016, 105, 64–70. [Google Scholar] [CrossRef]
- He, Y.; Li, P.; Yalkowsky, S.H. Solubilization of Fluasterone in co-solvent/Cyclodextrin Combinations. Int. J. Pharm. 2003, 264, 25–34. [Google Scholar] [CrossRef]
- Jug, M.; Becirevic-Lacan, M. Influence of hydeoxypropyl-beta-cyclodextrin complexation on Piroxicam Release from Buccaladhesive Tablets. Eur. J. Pharm. Sci. 2004, 21, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Quan, P.; Liu, D.F.; Wei, F.D.; Zhang, Q.; Xu, Q.W. The Influence of Co-solvent on the Complexation of HP-β-cyclodextrins with Oleanolic Acid and Ursolic Acid. AAPS PharmSciTech 2009, 10, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Tirunagari, M.; Mehveen, N.; Qureshi, M.F.; Sultana, J.P.; Tirunagari, V. Solubilization Enhancement of Flurbiprofen using Different Solubilization techniques. Int. J. Pharm. Pharm. Sci. 2012, 4, 97–100. [Google Scholar]
- Ansari, M. Investigations of polyethylene glycol mediated ternary molecular inclusion complexes of silymarin with beta cyclodextrins. J. Appl. Pharm. Sci. 2015, 5, 26–31. [Google Scholar] [CrossRef]
- Viernstein, H.; Weiss-Greiler, P.; Wolschann, P. Solubility enhancement of low soluble biologically active compounds: Temperature and co-solvent dependent inclusion complexation. Int. J. Pharm. 2003, 256, 85–94. [Google Scholar] [CrossRef]
- Rungnim, C.; Phunpee, S.; Kunaseth, M.; Namuangruk, S.; Rungsardthong, K.; Rungrotmongkol, T.; Ruktanonchai, U. Co-solvation effect on the binding mode of the α-mangostin/β-cyclodextrin inclusion complex. Beilstein J. Org. Chem. 2015, 11, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Masson, M.; Jarvinen, T. Cyclodextrins in drug delivery (Review). Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Doliwa, A.; Santoyo, S.; YgartuaInfluence, P. Influence of Piroxicam: Hydroxypropyl-Beta-Cyclodextrin Complexation on the in vitro Permeation and Skin Retention of Piroxicam. Skin Pharmacol. Appl. 2001, 14, 97–107. [Google Scholar] [CrossRef]
- Boonyarattanakalin, K.; Viernstein, H.; Wolschann, P.; Lawtrakul, L. Molecular dynamics of β-CD in water/co-solvent mixtures. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 279–290. [Google Scholar] [CrossRef]
- Boonyarattanakalin, K.; Viernstein, H.; Wolschann, P.; Lawtrakul, L. Influence of Ethanol as a Co-Solvent in Cyclodextrin Inclusion Complexation: A Molecular Dynamics Study. Sci. Pharm. 2015, 83, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.-I.; Tóth, G.; Völgyi, G.; Komjáti, B.; Hancu, G.; Szente, L.; Sohajda, T.; Béni, S.; Muntean, D.-L.; Noszál, B. Chiral separation of asenapine enantiomers by capillaryelectrophoresis and characterization of cyclodextrin complexes byNMR spectroscopy, mass spectrometry and molecular modeling. J. Pharm. Biomed. Anal. 2016, 117, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 210–212.
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 7, 117–122. [Google Scholar]
- Ivanova, D.; Deneva, V.; Nedeltcheva, D.; Kamounah, F.S.; Gergov, G.; Hansen, P.E.; Kawauchi, S.; Antonov, L. Tautomeric transformations of piroxicam in solution: A combined experimental and theoretical study. RSC Adv. 2015, 5, 31852–31860. [Google Scholar] [CrossRef]
- Yazdanian, M.; Jankovsky, B.C.; Hawi, A. The “high solubility” definition of the current FDA guidance on biopharmaceutical classification system may be too strict for acidic drugs. Pharm. Res. 2004, 21, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Demiralay, E.C.; Yilmaz, H. Potentiometric pKa Determination of Piroxicam and Tenoxicam in Acetonitrile-Water Mixtures. SDU J. Sci. 2012, 7, 34–44. [Google Scholar]
- Narazaki, R.; Sanghvi, R.; Yalkowsky, S.H. Estimation of Drug Precipitation upon Dilution of pH-Co-Solvent Solubilized Formulations. Chem. Pharm. Bull. 2007, 55, 1203–1206. [Google Scholar] [CrossRef] [PubMed]




| CDs | S0 (mM) | k1:1 (M−1) | CE × 10−3 (= S0 × k1:1) |
|---|---|---|---|
| BCD | 0.0374 | 132 | 4.9 |
| GBD | 0.0408 | 96 | 3.9 |
| MeBCD | 0.0343 | 149 | 5.1 |
| % Alcohol | k1:1 (M−1) | ||
|---|---|---|---|
| BCD | GCD | MeBCD | |
| 0 | 132 | 96 | 149 |
| 1 | 88 | 90 | 88 |
| 3 | 52 | 76 | 50 |
| 5 | 28 | 75 | 43 |
| 7 | ** | 69 | 35 |
| 10 | ** | 69 | 28 |
| 20 | ** | 61 | 9.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charumanee, S.; Okonogi, S.; Sirithunyalug, J.; Wolschann, P.; Viernstein, H. Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug. Sci. Pharm. 2016, 84, 694-704. https://doi.org/10.3390/scipharm84040694
Charumanee S, Okonogi S, Sirithunyalug J, Wolschann P, Viernstein H. Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug. Scientia Pharmaceutica. 2016; 84(4):694-704. https://doi.org/10.3390/scipharm84040694
Chicago/Turabian StyleCharumanee, Suporn, Siriporn Okonogi, Jakkapan Sirithunyalug, Peter Wolschann, and Helmut Viernstein. 2016. "Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug" Scientia Pharmaceutica 84, no. 4: 694-704. https://doi.org/10.3390/scipharm84040694
APA StyleCharumanee, S., Okonogi, S., Sirithunyalug, J., Wolschann, P., & Viernstein, H. (2016). Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug. Scientia Pharmaceutica, 84(4), 694-704. https://doi.org/10.3390/scipharm84040694







