Destabilization Mechanism of Ionic Surfactant on Curcumin Nanocrystal against Electrolytes
Abstract
:1. Introduction
2. Results and Discussion
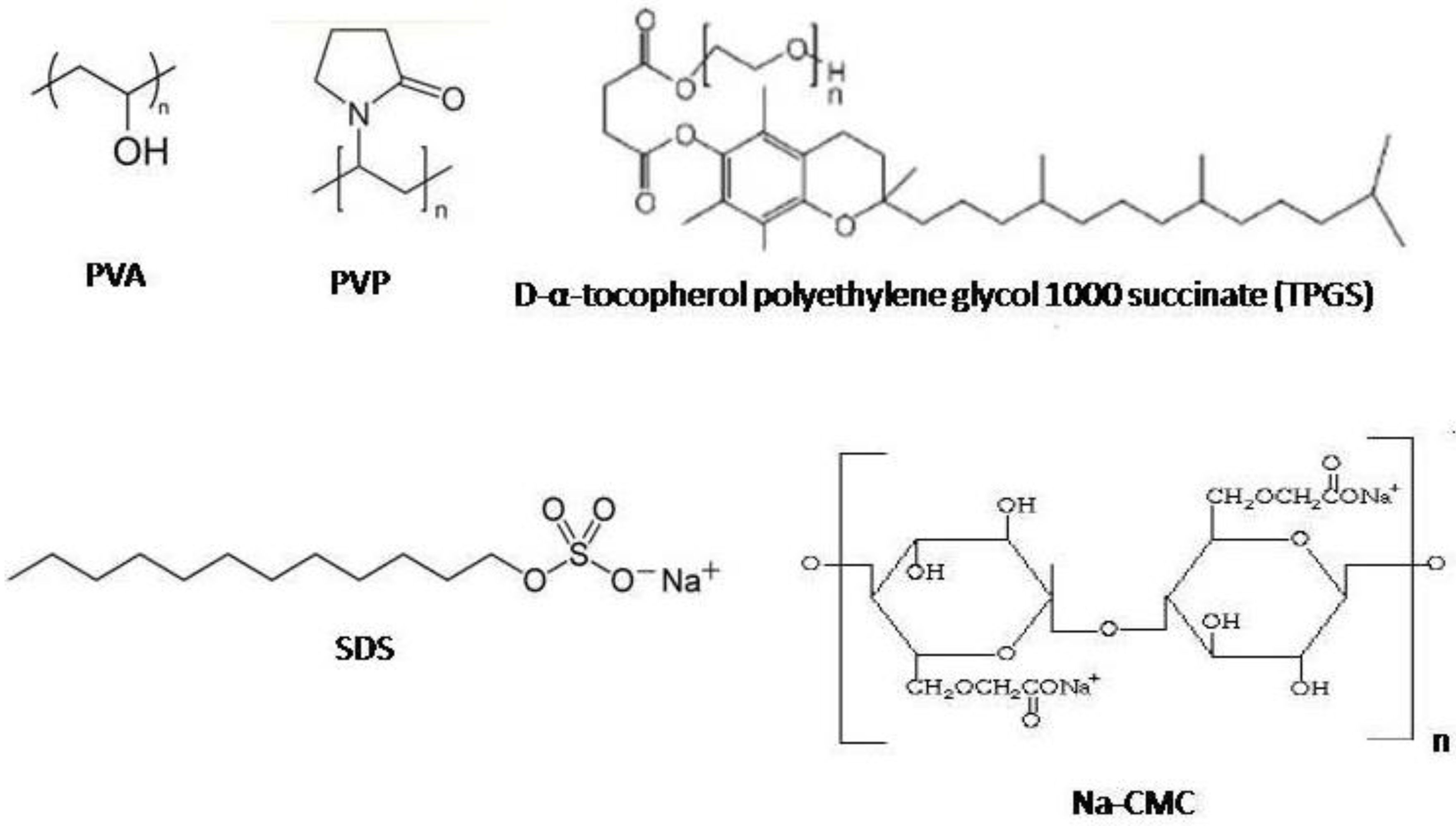
2.1. Effect of Stabilizer Type on Particle Size and Size Distribution
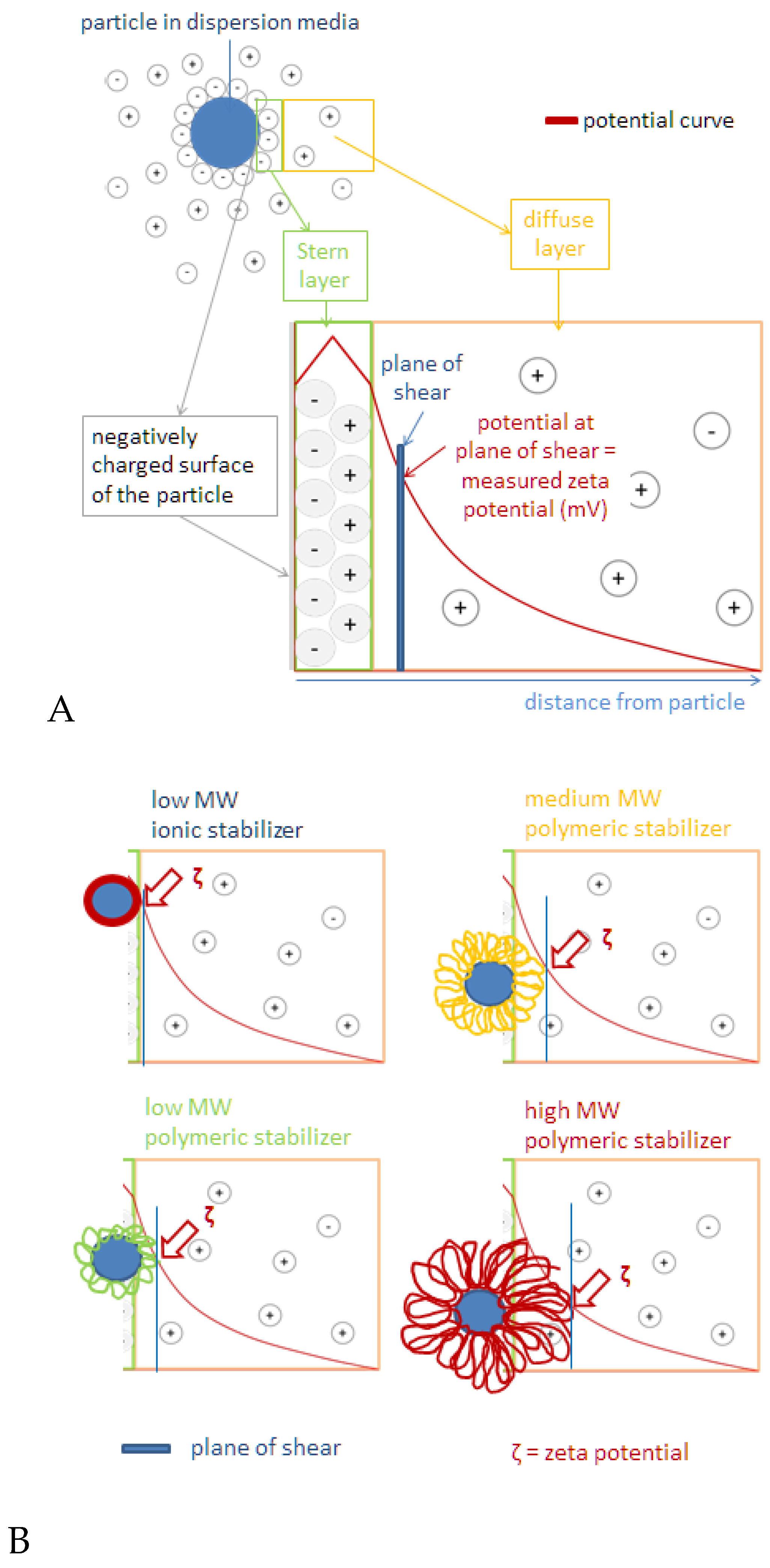
2.2. Surface Charge of Curcumin Nanosuspension
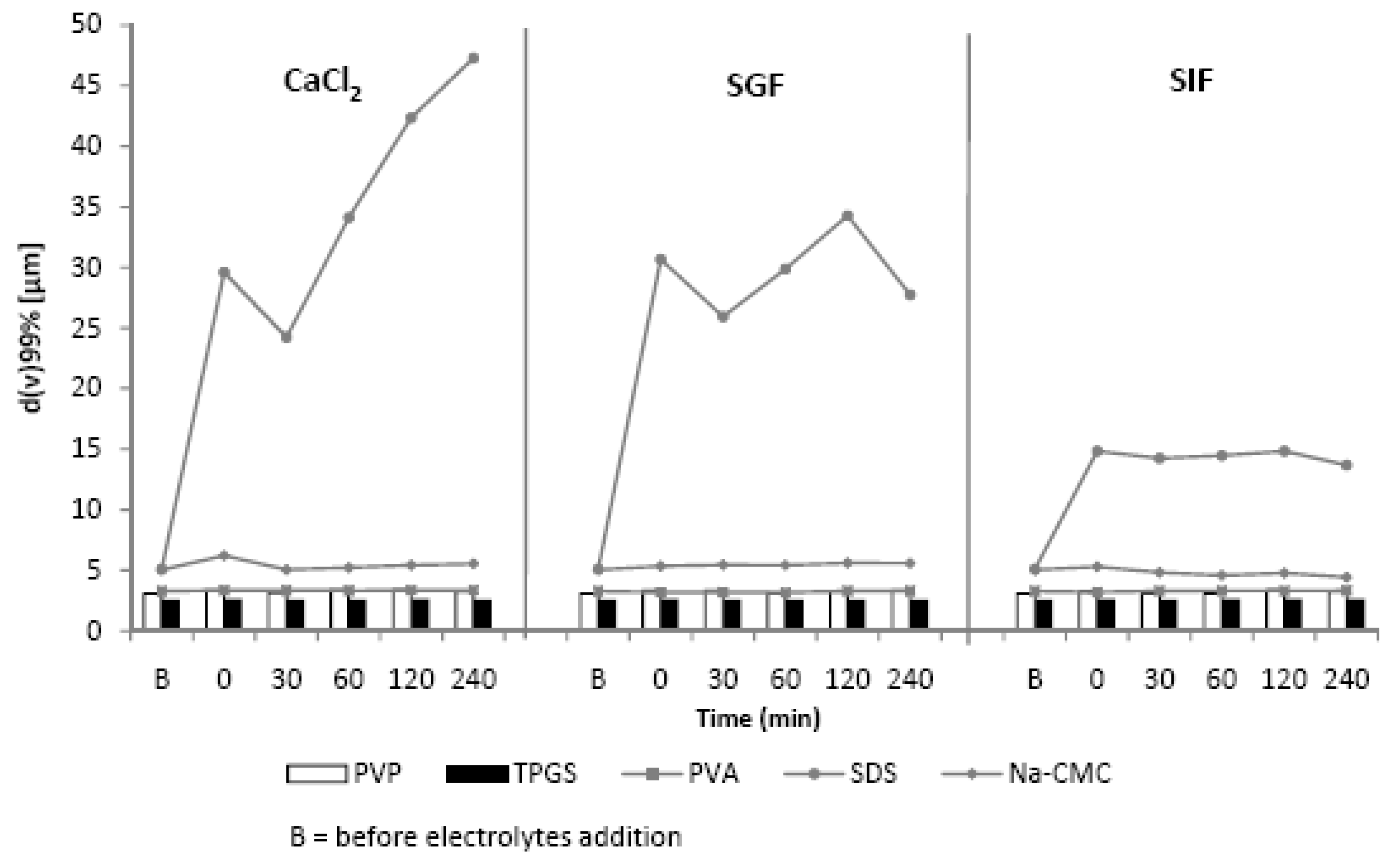
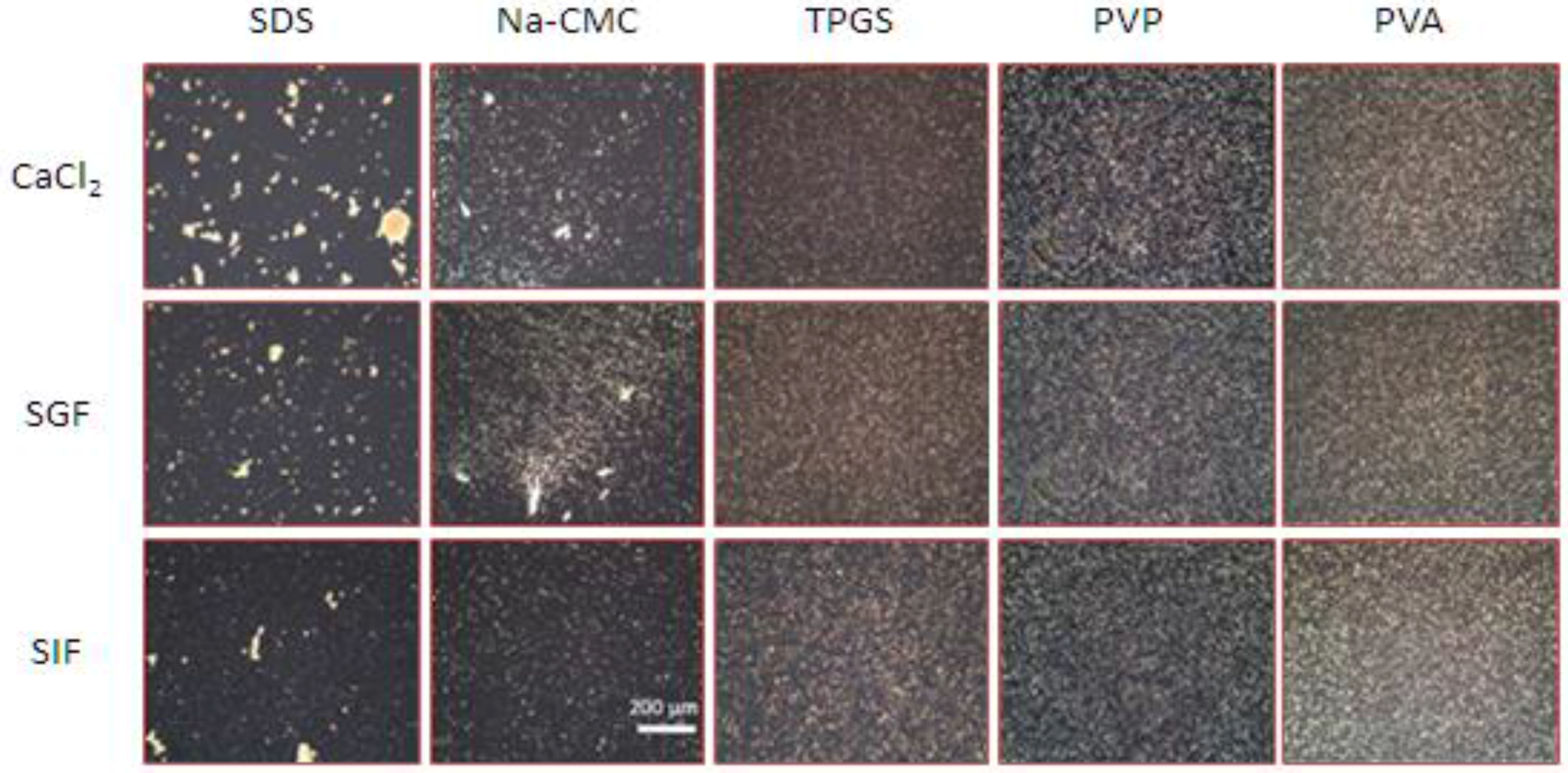
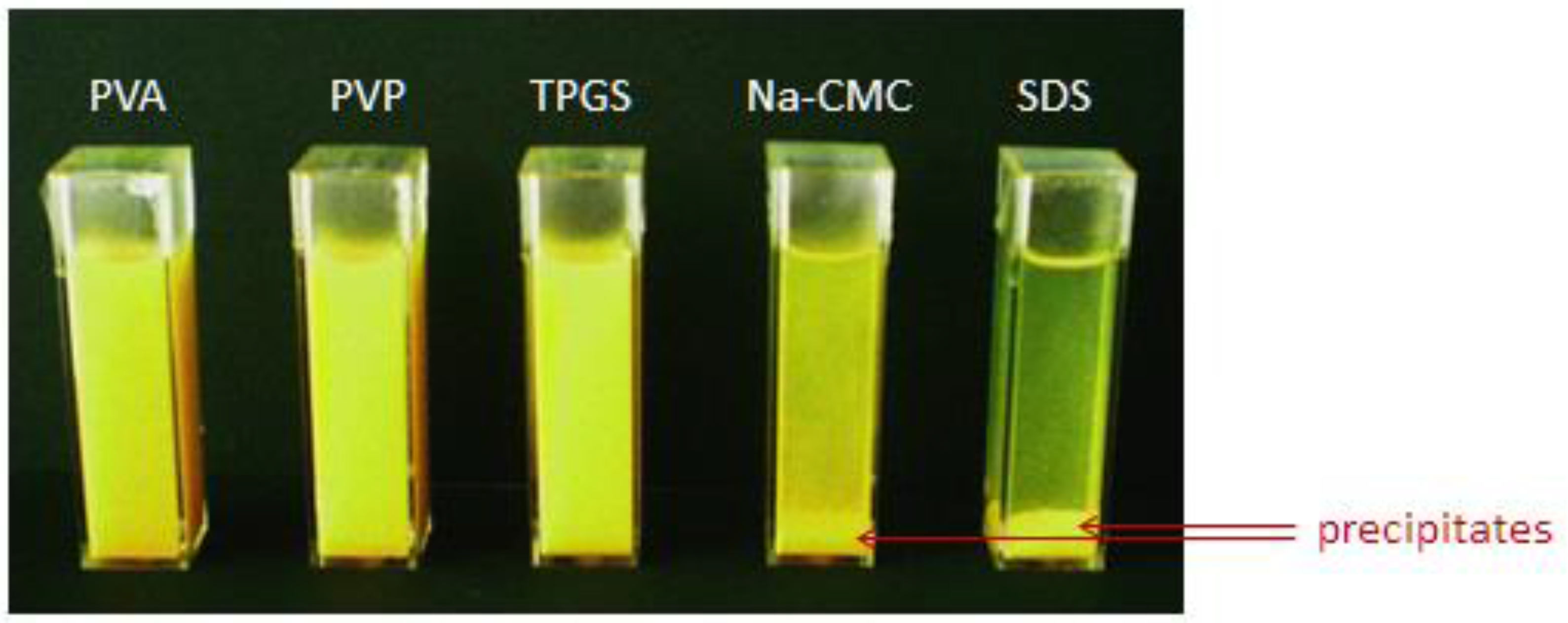
2.3. The Influence of Electrolytes on the Physical Stability of Curcumin Nanosuspension
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Curcumin Nanosuspension
3.2.2. Microscopic Analysis
3.2.3. Zeta Potential (ZP) Determination
3.2.4. Effect of Electrolytes on Physical Stability of Nanosuspensions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zebib, B.; Mouloungui, Z.; Noirot, V. Stabilization of curcumin by complexation with divalent cations in glycerol/water system. Bioinorg. Chem. Appl. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Wan, S.; Sun, Y.; Qi, X.; Tan, F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech 2012, 13, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.; Park, C. Characteristics of polymers enabling nano-comminution of water-insoluble drugs. Int. J. Pharm. 2008, 355, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Keck, C. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Palla, B.J.; Shah, D.O. Stabilization of high ionic strength slurries using surfactant mixtures: Molecular factors that determine optimal stability. J. Colloid Interface Sci. 2002, 256, 143–152. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Ostolska, I.; Szewczuk-Karpisz, K.; Chibowski, S.; Terpiłowski, K.; Gun’ko, V.M.; Zarko, V.I. Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3–SiO2–TiO2). J. Nanopart. Res. 2015, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Peukert, W.; Schwarzer, H.; Stenger, F. Control of aggregation in production and handling of nanoparticles. Chem. Eng. Process. 2005, 44, 245–252. [Google Scholar] [CrossRef]
- Kawakami, K.; Nishihara, Y.; Hirano, K. Effect of hydrophilic polymers on physical stability of liposome dispersions. J. Phys. Chem. 2001, 105, 2374–2385. [Google Scholar] [CrossRef]
- Owen, S.; Chan, D.; Shoichet, M. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Song, S.; Koelsch, P.; Weidner, T.; Wagner, M.; Castner, D. Sodium dodecyl sulfate adsorption onto positively charged surfaces: Monolayer formation with opposing headgroup orientations. Langmuir 2013, 29, 12710–12719. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M. Cyclosporine Nanosuspensions: Optimised Size Characterisation & Oral Formulations. Ph.D. Thesis, Freien Universität Berlin, Berlin, Germany, 2006. [Google Scholar]
- Sjöberg, M.; Bergström, L.; Larsson, A.; Sjöström, E. The effect of polymer and surfactant adsorption on the colloidal stability and rheology of kaolin dispersions. Colloids Surf. A Physicochem. Eng. Asp. 1999, 159, 197–208. [Google Scholar] [CrossRef]
- Nestor, J.; Esquena, J.; Solans, C.; Luckham, P.; Musoke, M.; Levecke, B.; Booten, K.; Tadros, T.F. Interaction forces between particles stabilized by a hydrophobically modified inulin surfactant. J. Colloid Interface Sci. 2007, 311, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachmawati, H.; Shaal, L.; Müller, R.; Keck, C. Development of curcumin nanocrystal: Physical aspects. J. Pharm. Sci. 2013, 102, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhao, D. In Situ Immobilization of Mercury in Water, Soil, and Sediment Using Carboxymethyl Cellulose Stabilized Iron Sulfide Nanoparticles. ACS Symp. Ser. 2013, 1123, 61–77. [Google Scholar] [CrossRef]
- Park, S.; Jung, M.; Lee, J. Nano oxide-dispersed nickel composite plating. Electron. Mater. Lett. 2013, 9, 801–804. [Google Scholar] [CrossRef]
- Doymuş, K. The Effect of Ionic Electrolytes and pH on the Zeta Potential of Fine Coal Particles. Turk. J. Chem. 2007, 31, 589–597. [Google Scholar]
- Jacobasch, H.J.; Simon, F.; Weidenhammer, P. Adsorption of ions onto polymer surfaces and its influence on zeta-potential and adhesion phenomena. Colloid Polym. Sci. 1998, 276, 434–442. [Google Scholar] [CrossRef]
- Illing, A.; Unruh, T. Investigation on the flow behavior of dispersions of solid triglyceride nanoparticles. Int. J. Pharm. 2004, 284, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Müller, R.H. Production and Characterization of a Budesonide Nanosuspension for Pulmonary Administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, C. Measuring Zeta Potential, Methods. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Berlin, Germany, 2008; pp. 1068–1076. [Google Scholar]
- Perrie, Y.; Obrenovic, M.; McCarthy, D.; Gregoriadis, G. Liposome (Lipodine)–mediated DNA vaccination by the oral route. J. Liposome Res. 2002, 12, 185–197. [Google Scholar] [CrossRef] [PubMed]
| # | Stabilizer | CaCl2 (mV) | SGF (mV) | SIF (mV) |
|---|---|---|---|---|
| 1 | PVA | −1.6 | −1.2 | −4.7 |
| 2 | PVP | −7.4 | −5.2 | −20.3 |
| 3 | TPGS | −8.9 | −4.5 | −19.6 |
| 4 | SDS | −7.3 | −19.1 | −45.9 |
| 5 | Na-CMC | −17.6 | −9.8 | −33.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachmawati, H.; Rahma, A.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Destabilization Mechanism of Ionic Surfactant on Curcumin Nanocrystal against Electrolytes. Sci. Pharm. 2016, 84, 685-693. https://doi.org/10.3390/scipharm84040685
Rachmawati H, Rahma A, Al Shaal L, Müller RH, Keck CM. Destabilization Mechanism of Ionic Surfactant on Curcumin Nanocrystal against Electrolytes. Scientia Pharmaceutica. 2016; 84(4):685-693. https://doi.org/10.3390/scipharm84040685
Chicago/Turabian StyleRachmawati, Heni, Annisa Rahma, Loaye Al Shaal, Rainer H. Müller, and Cornelia M. Keck. 2016. "Destabilization Mechanism of Ionic Surfactant on Curcumin Nanocrystal against Electrolytes" Scientia Pharmaceutica 84, no. 4: 685-693. https://doi.org/10.3390/scipharm84040685





