Adverse Effects of Direct Acting Antivirals in HIV/HCV Coinfected Patients: A 4-Year Experience in Miami, Florida
Abstract
:1. Introduction
2. Material and Methods
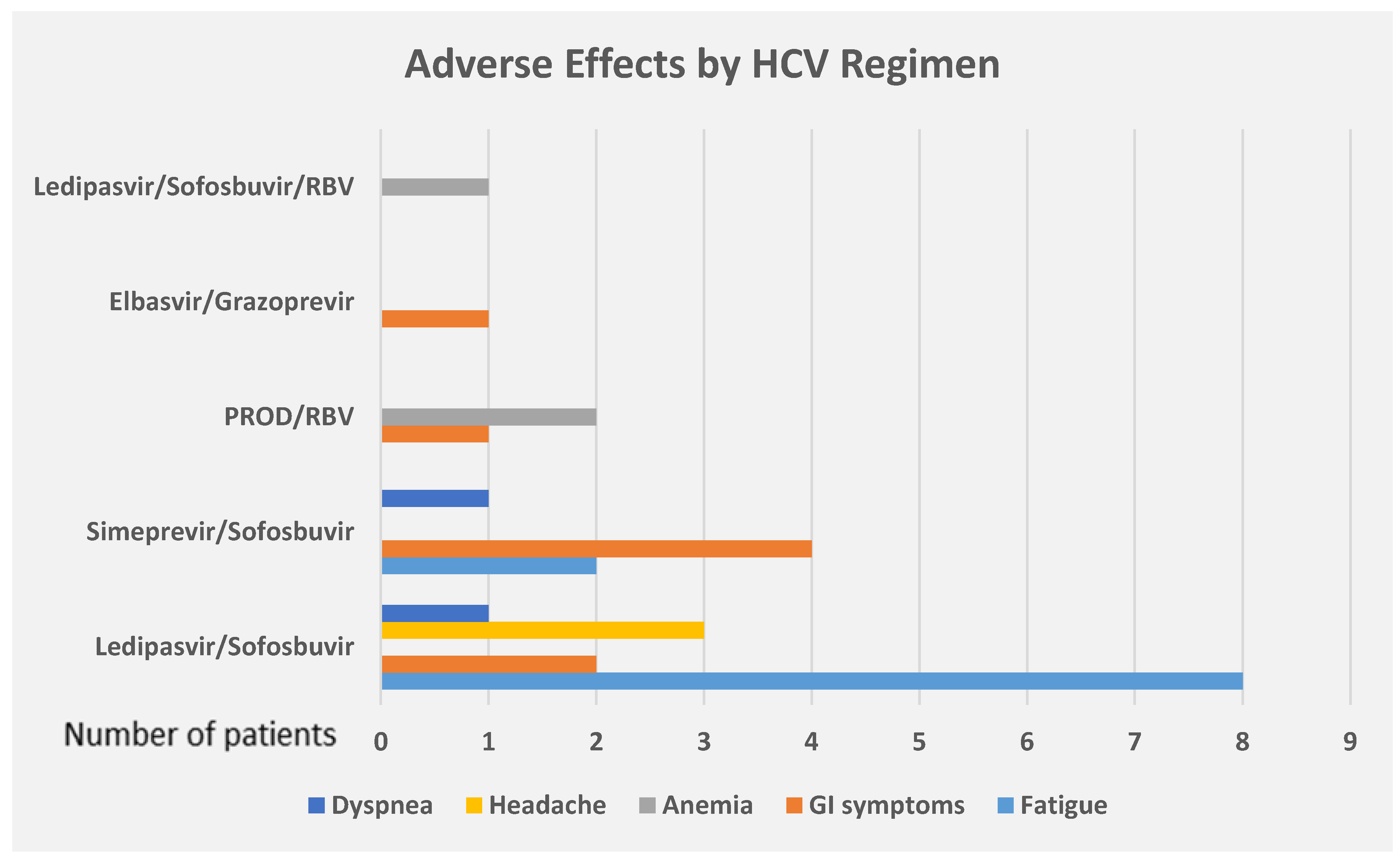
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Assoumou, S.A.; Huang, W.; Young, K.; Horsburgh, C.R.; Linas, B.P. Real-world Outcomes of Hepatitis C Treatment during the Interferon-free Era at an Urban Safety-net Hospital. J. Health Care Poor Underserved 2017, 28, 1333–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renard, S.; Borentain, P.; Salaun, E.; Benhaourech, S.; Maille, B.; Darque, A.; Bregigeon, S.; Colson, P.; Laugier, D.; Gaubert, M.R.; et al. Severe Pulmonary Arterial Hypertension in Patients Treated for Hepatitis C with Sofosbuvir. Chest 2016, 149, e69–e73. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.W.; Luhne, S.; Lange, C.M.; Vermehren, J.; Farnik, H.; Herrmann, E.; Welzel, T.; Zeuzem, S.; Sarrazin, C. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/sofosbuvir treatment. J. Hepatol. 2016, 64, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm (accessed on 23 May 2018).
- Hawkins, C.; Grant, J.; Ammerman, L.R.; Palella, F.; Mclaughlin, M.; Green, R.; Mcgregor, D.; Stosor, V. High rates of hepatitis C virus (HCV) cure using direct-acting antivirals in HIV/HCV-coinfected patients: A real-world perspective. J. Antimicrob. Chemother. 2016, 71, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Saracino, A.; Scudeller, L.; Fabrizio, C.; Dell’Acqua, R.; Milano, E.; Milella, M.; Ladisa, N.; Monno, L.; Angarano, G. HCV mono-infected and HIV/HCV co-infected individuals treated with direct-acting antivirals: To what extent do they differ? Int. J. Infect. Dis. 2017, 62, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, L.; Lai, A.; Calvi, E.; Ronzi, P.; Micheli, V.; Binda, F.; Ridolfo, A.L.; Gervasoni, C.; Galli, M.; Antinori, S.; et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: Real-life safety and efficacy. HIV Med. 2017, 18, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Harvoni (Ledipasvir/Sofosbuvir) [Product Monograph]. Gilead Sciences Inc.: Mississauga, ON, Canada, March 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205834s001lbl.pdf (accessed on 9 June 2018).
- Lawitz, E.; Matusow, G.; DeJesus, E.; Yoshida, E.M.; Felizarta, F.; Ghalib, R.; Godofsky, E.; Herring, R.W.; Poleynard, G.; Sheikh, A.; et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2). Hepatology 2016, 64, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutala, B.K.; Mouri, F.; Castelnau, C.; Bouton, V.; Giuily, N.; Boyer, N.; Asselah, T.; Marcellin, P. Efficacy and safety of sofosbuvir-based therapies in patients with advanced liver disease in a real-life cohort. Hepat. Med. 2017, 18, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mariño, Z.; Pascasio-Acevedo, J.M.; Gallego, A.; Diago, M.; Baliellas, C.; Morillas, R.; Prieto, M.; Moreno, J.M.; Sánchez-Antolín, G.; Vergara, M.; et al. High efficacy of Sofosbuvir plus Simeprevir in a large cohort of Spanish cirrhotic patients infected with genotypes 1 and 4. Liver Int. 2017, 37, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.; Dodd, Z.; Guyton, M.; Tookey, P.; Lettner, B.; Matelski, J.; Sockalingam, S.; Altenberg, J.; Powis, J. Understanding real-world adherence in the directly acting antiviral era: A prospective evaluation of adherence among people with a history of drug use at a community-based program in Toronto, Canada. Int. J. Drug Policy 2017, 47, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Saracino, A.; Fabrizio, C.; Scudeller, L.; Milano, E.; Dell’Acqua, R.; Ladisa, N.; Fasano, M.; Minniti, S.; Buccoliero, G.; et al. Safety and effectiveness of a 12-week course of sofosbuvir and simeprevir ± ribavirin in HCV-infected patients with or without HIV infection: A multicentre observational study. Int. J. Antimicrob. Agents 2017, 49, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Copegus (Ribavirin) [Prescribing Information]. Roche Laboratories: Nutley, NJ, USA, January 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021511s023lbl.pdf (accessed on 15 June 2018).
- Lawitz, E.; Sulkowski, M.S.; Ghalib, R.; Rodriguez-Torres, M.; Younossi, Z.M.; Corregidor, A.; DeJesus, E.; Pearlman, B.; Rabinovitz, M.; Gitlin, N.; et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: The COSMOS randomised study. Lancet 2014, 384, 1756–1765. [Google Scholar] [CrossRef]
- Infectious Disease Society of America (IDSA); American Association for the Study of Liver Diseases (AASLD). HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. 2017. Available online: https://www.hcvguidelines.org/ (accessed on 23 May 2018).
- MacBrayne, C.; Fierer, D.S.; Marks, K.M. Ledipasvir/sofosbuvir raises tenofovir diphosphate concentrations in red cells. In Proceedings of the Conference of Retrovirus and Opportunistic Infections, Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Schlabe, S.; Rockstroh, J.K. Advances in the treatment of HIV/HCV coinfection in adults. Expert Opin. Pharmacother. 2018, 19, 49–64. [Google Scholar] [CrossRef] [PubMed]
| Variable | HIV/HCV Patients | No Adverse Effects | Adverse Effects | p | OR (95% CI) |
|---|---|---|---|---|---|
| (n = 78) | (n = 57) | (n = 21) | |||
| Age | 55.64 ± 7.88 | 55.19 ± 8.26 | 56.86 ± 6.77 | 0.41 | - |
| Sex (male) | 53 (67.9%) | 41 (71.9%) | 12 (57.1%) | 0.22 | 0.63 (0.31–1.29) |
| Race | |||||
| (a) Black | 45 (57.7%) | 35 (61.4%) | 10 (47.6%) | 0.27 | 0.67 (0.32–1.38) |
| (b) White | 13 (16.7%) | 6 (10.5%) | 7 (33.3%) | 0.017 | 2.5 (1.26–4.96) |
| (c) Hispanic | 20 (25.6%) | 16 (28.1%) | 4 (19.0%) | 0.42 | 0.68 (0.26–1.79) |
| HAART | 75 (96.2%) | 56 (98.2%) | 19 (90.5%) | 0.11 | 0.38 (0.16–0.93) |
| Hemoglobin | 13.72 ± 1.63 | 13.8 ± 1.56 | 13.54 ± 1.82 | 0.53 | - |
| AST | 74.44 ± 67.471 | 78.12 ± 76.35 | 64.43 ± 32.55) | 0.43 | - |
| ALT | 77.46 ± 82.57 | 83.39 ± 94.77 | 61.38 ± 26.96 | 0.3 | - |
| Albumin | 4.113 ±0.63 | 4.18 ± 0.63 | 3.94 ± 0.60 | 0.14 | - |
| Total bilirubin | 0.96 ± 0.92 | 0.99 ± 0.94 | 0.90 ± 0.89 | 0.72 | - |
| Platelet count | 188.92 ± 70.18 | 188.72 ± 69.60 | 189.48 ± 73.45 | 0.97 | - |
| CD4 count | 637.68 ± 334.35 | 610.54 ± 312.63 | 711.33 ± 386.73 | 0.24 | - |
| CD4/CD8 | 0.92 ± 0.63 | 0.92 ± 0.625 | 0.94 ± 0.65 | 0.87 | - |
| CD4% | 30.14 ± 11.26 | 29.65 ± 11.54 | 31.48 ± 10.59 | 0.53 | - |
| CD4 count < 500 | 29 (37.2%) | 22 (38.6%) | 7 (33.3%) | 0.94 | 0.85 (0.39–1.85) |
| ART regimen | |||||
| (a) TDF/FTC + NNRTI | 15 (19.2%) | 8 (14.0%) | 7 (33.3%) | 0.055 | 2.10 (1.03–4.28) |
| (b) TDF/FTC + PI | 21 (26.9%) | 17 (29.8%) | 4 (19.0%) | 0.34 | 0.64 (0.24–1.68) |
| (c) TDF/FTC + InSTI | 15 (19.2%) | 12 (21.1%) | 3 (14.3%) | 0.5 | 0.70 (0.24–2.07) |
| (d) TAF + InSTI | 4 (5.1%) | 3 (5.3%) | 1 (4.8%) | 0.93 | 0.93 (0.16–5.26) |
| (e) ABC/3TC + InSTI | 7 (9.0%) | 6 (10.5%) | 1 (4.8%) | 0.43 | 0.51 (0.08–3.23) |
| (f) ABC/3TC + PI | 4 (5.1%) | 3 (5.3%) | 1 (4.8%) | 0.93 | 0.93 (0.16–5.26) |
| (g) Other regimens | 9 (11.5%) | 7 (12.3%) | 2 (9.5%) | 0.74 | 0.81 (0.22–2.91) |
| Prior Tx with IFN | 22 (28.2%) | 15 (26.3%) | 7 (33.3%) | 0.54 | 1.27 (0.59–2.73) |
| Liver biopsy | 33 (42.3%) | 22 (38.6%) | 11 (52.4%) | 0.27 | 1.50 (0.72–3.11) |
| Elastography | 15 (19.2%) | 13(22.8%) | 2 (9.5%) | 0.19 | 0.44 (0.12–1.70) |
| Genotype | |||||
| (a) 1a | 47 (61.0%) | 34 (59.6%) | 13 (61.9%) | 0.86 | 1.07 (0.50–2.23) |
| (b) 1b | 25 (32.5%) | 18 (31.6%) | 7 (33.3%) | 0.88 | 1.06 (0.49–2.30) |
| (c) Others | 6 (7.7%) | 5(8.8%) | 1(4.8%) | 0.56 | 0.60 (0.09–3.73) |
| HCV10log | 6.18 ± 0.76 | 6.137 ± 0.82 | 6.30 ± 0.56 | 0.4 | - |
| Creatinine | 1.05 ± 0.38 | 1.09 ± 0.41 | 0.95 ± 0.24 | 0.14 | - |
| Advanced liver disease (F3, F4) | 28 (35.9%) | 20 (35.1%) | 8 (38.1%) | 0.81 | 1.10 (0.52–2.33) |
| Cirrhosis | 12 (15.4%) | 7 (12.3%) | 5 (23.8%) | 0.21 | 1.72 (0.78–3.80) |
| HCV treatment | |||||
| (a) Ledipasvir/Sofosbuvir | 56 (71.8%) | 44 (77.2%) | 12 (57.1%) | 0.08 | 0.52 (0.26–1.07) |
| (b) Simeprevir/Sofosbuvir | 12 (15.4%) | 7 (12.3%) | 5 (23.8%) | 0.21 | 1.72 (0.78–3.80) |
| (c) PROD/RBV | 2 (2.6%) | 0 (0%) | 2 (9.5%) | 0.018 | 4.00 (2.71–5.90) |
| (d) Elbasvir/Grazoprevir | 2 (2.6%) | 1 (1.8%) | 1 (4.8%) | 0.46 | 1.90 (0.45–7.99) |
| (e) Ledipasvir/Sofosbuvir + RBV | 2 (2.6%) | 1 (1.8%) | 1 (4.8%) | 0.46 | 1.90 (0.45–7.99) |
| (f) Sofosbuvir + RBV | 2 (2.6%) | 2 (3.5%) | 0 (0%) | 0.39 | - |
| (g) PROD | 1 (1.3%) | 1 (1.8%) | 0 (0%) | 0.54 | - |
| (h) Sofosbuvir/Velpatasvir | 1 (1.3%) | 1 (1.8%) | 0 (0%) | 0.54 | - |
| Tx duration (12 weeks) | 71 (91.0%) | 53 (98.1%) | 18 (90.0%) | 0.11 | 0.38 (0.16–0.93) |
| SVR12 (ITT) | 64 (82.1%) | 44 (77.2%) | 20 (95.2%) | 0.07 | 4.38 (0.64–29.94) |
| Completed HCV Tx | 73 (93.6%) | 54 (94.7%) | 19 (90.5%) | 0.5 | 0.65 (0.21–2.03) |
| Lost to follow-up | 4 (5.1%) | 3 (5.3%) | 1 (4.8%) | 0.93 | 0.93 (0.16–5.26) |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzales Zamora, J.A. Adverse Effects of Direct Acting Antivirals in HIV/HCV Coinfected Patients: A 4-Year Experience in Miami, Florida. Diseases 2018, 6, 51. https://doi.org/10.3390/diseases6020051
Gonzales Zamora JA. Adverse Effects of Direct Acting Antivirals in HIV/HCV Coinfected Patients: A 4-Year Experience in Miami, Florida. Diseases. 2018; 6(2):51. https://doi.org/10.3390/diseases6020051
Chicago/Turabian StyleGonzales Zamora, Jose Armando. 2018. "Adverse Effects of Direct Acting Antivirals in HIV/HCV Coinfected Patients: A 4-Year Experience in Miami, Florida" Diseases 6, no. 2: 51. https://doi.org/10.3390/diseases6020051





