Abstract
Organizations recommend evaluating individual ingredients when assessing the safety of personal care or cosmetic products. The goal of this study was to present a screening-level safety assessment methodology to evaluate the safety of a product by identifying individual ingredients, determining their frequency of use in on-market products, and examining published safe-level-of-use information for each ingredient. As a case study, we evaluated WEN by Chaz Dean (WCD) cleansing conditioners since there have been claims of adverse health effects associated with product use. We evaluated 30 ingredients in three on-market WCD cleansing conditioners. We then analyzed the National Library of Medicine’s Household Products Database and the Environmental Working Group’s (EWG) Skin Deep Cosmetic Database, two of the largest publicly available databases, for other on-market personal care and cosmetic products that contained these ingredients. Safe-level-of-use information for each ingredient was obtained by reviewing peer-reviewed literature, the Food and Drug Administration’s (FDA) generally recognized as safe (GRAS) database, available Cosmetic Ingredient Review (CIR) publications, and available product safety publications. The results of this analysis showed that more than 20,000 personal care and cosmetic products contained one or more of the evaluated ingredients used in WCD cleaning conditioners. Published safety information was available for 21 of the 30 evaluated ingredients: seven identified ingredients were designated as GRAS by the FDA and 16 ingredients had safe-level-of-use information available from the CIR. This study presents a screening-level safety assessment methodology that can serve as an initial screening tool to evaluate the safety of an ingredient intended for use in personal care and cosmetic products before a product is launched onto the market. This study provides evidence that the evaluated WCD cleansing conditioner ingredients are commonly used in other personal care and cosmetic products, and ingredients with available safety information are generally considered safe for the intended use. The scope of this analysis is limited to frequency of use information and available toxicological data. Additional testing including in silico, in vitro, and clinical studies may be needed to evaluate the potential toxicity of an ingredient.
1. Introduction
The European Commission (EC)’s Scientific Committee on Consumer Safety (SCCS) reports that the safety of a cosmetic product is based on the safety of its ingredients [1]. Specifically, the SCCS notes that all available data should be reviewed when assessing the safety of an ingredient [1]. In the United States, the Food and Drug Administration (FDA) “does not require cosmetic products and ingredients to have FDA approval before they go on the market” and does not have a list of tests required for any particular cosmetic product or ingredient [2]. The FDA states that manufacturers may use available safety data on individual ingredients or products with similar formulations to assess the safety of the ingredient [2]. Similarly, the Personal Care Products Council (PCPC) stated that the safety evaluation of a product may include comparison to similar marketed formulations with a history of safe use [3].
Individual ingredient safety data are often publicly available and accessible through various sources. For example, safety data can be found in the FDA’s Generally Recognized As Safe (GRAS) database and EC’s Cosmetic Ingredient Database. An ingredient is classified as GRAS by a panel of qualified experts after “having been adequately shown to be safe under the conditions of its intended use” [4]. Although this database primarily evaluates ingredients as food additives, it provides valuable safety information regarding ingredients present in personal care or cosmetic products. Additionally, the Cosmetic Ingredient Review (CIR), an independent entity charged with evaluating the safety of individual ingredients used in cosmetics, routinely publishes safety information [5]. The CIR evaluates ingredients using standardized procedures for review and provides recommendations as to whether an ingredient is (1) safe to use under its intended conditions of use, (2) safe to use under certain limitations and conditions, (3) lacks sufficient data needed to determine its safety, or (4) unsafe under its intended condition of use [6].
The aforementioned available safety data provides valuable information to help ensure consumer safety. Given the complex nature of these data, the goal of this study was to present a screening-level safety assessment methodology to evaluate the safety of a product by identifying individual ingredients and determining their frequency of use in on-market products. Additionally, we examined published safe-level-of-use information for each ingredient. Specifically, we performed a screening-level safety assessment of WEN by Chaz Dean (WCD) cleansing conditioners given recent claims of adverse health effects associated with product use [7].
2. Methods
2.1. Ingredient Identification
We used product formulation sheets to identify 33 individual ingredients present in the 3 most commonly purchased WCD cleansing conditioner products (Sweet Almond Mint, Lavender, and Pomegranate). Chemical Abstract Service (CAS) numbers were collected for each ingredient. Fragrance mixtures were not included in this analysis, as the exact composition of each fragrance mixture is not publicly available. Lavender extract and lavender oil were combined in this analysis. Additionally, we excluded water from this analysis. The resulting 30 identified ingredients that were evaluated are listed in Table 1.

Table 1.
Ingredients identified from on-market hair cleansing conditioners.
2.2. Evaluation of Published Safe Level, FDA GRAS, and EC Cosmetic Ingredient Use Restriction Information
Available information regarding safe level of use (the level at which an ingredient is considered safe for use in cosmetics) was obtained through a series of literature searches performed in PubMed, Medline, CIR ingredient database [5], and Google Scholar. Combinations of the following key words were used: <ingredient name> and safety, toxicity, safe levels, and level of use. Electronic searches were supplemented with additional relevant studies or publications obtained by manual review of the bibliographies of retrieved publications. FDA’s GRAS database [8] was reviewed to identify ingredients that were “generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use” [9]. EC’s Cosmetic Ingredient database [10] was reviewed to identify ingredients with use restrictions.
2.3. Evaluation of Frequency of Use Information
To assess the frequency of use for each ingredient, we utilized two publicly-available databases: the National Library of Medicine’s Household Products Database and the Environmental Working Group (EWG) Skin Deep Cosmetic Database. Within each database, we utilized the identified CAS numbers to retrieve product information for each ingredient. Results were categorized into 28 general product categories (such as hair styling products, nail care products, lip products, etc.). Products that could potentially fit into more than one category (i.e., a 2-in-1 shampoo and conditioner) were included in each applicable category. Duplicate products within each database and among the two databases were removed. It should be noted that the overall total product counts per ingredient is inclusive of duplicates due to product category-crossover.
2.4. National Library of Medicine’s Household Products Database
The National Library of Medicine’s Household Products Database (HPD) was initially compiled in 1995 and is based on the Consumer Product Information Database by DeLima Associates. Information in the HPD is derived from multiple publicly-available sources, including brand-specific labels and Safety Data Sheets from manufacturers [11]. This database was last updated in September 2016. Product lists were available from this database for all 30 evaluated ingredients. Results were filtered to only include personal care products, and products that were identified as “old product” or “discontinued” in the product listing were excluded. Each product was manually categorized into one of the aforementioned 28 categories. In many instances, the product type was evident from the product name; however, for products with ambiguous or unclear names, an additional search was conducted with general search engines (i.e., Google) to identify the product category.
2.5. Environmental Working Group’s Skin Deep Cosmetics Database
The EWG’s Skin Deep Cosmetics Database was initiated in 2004 and utilizes label information provided by companies and manufacturers [12]. To ensure that the database contains the most current products on the market, EWG automatically categorizes any product that has been in the database for longer than three years as an old formulation. If the products have not been verified within the last six years, the products are removed from the database. Product lists for all 30 evaluated ingredients were found in this database. Each product was manually categorized into the aforementioned 28 categories.
3. Results
3.1. FDA GRAS, EC Cosmetic Ingredient Use Restriction, and Published Safe Level Information
Available safety information, including FDA GRAS designation, EC cosmetic ingredient use restriction, and published safe level information for ingredients present in WCD cleansing conditioners are summarized in Table 1. Published safety information was available for 21 of the 30 evaluated ingredients: seven identified ingredients were designated as GRAS by the FDA and 16 ingredients had safe-level-of-use information available from the CIR. Of the 16 ingredients with available safe-level-of-use information, four had specific safe-level-of-use concentration data and 12 were categorized as “safe as presently used” (Table 1). Three ingredients had EC cosmetic ingredient use restrictions (Table 1). Safety information was not available for nine ingredients (Table 1).
3.2. Frequency of Use in Other On-Market Products
The results of our analyses showed that more than 20,000 personal care and cosmetic products contained one or more of the identified ingredients. Importantly, each of the examined ingredients in WCD cleansing conditioners was identified to be used in other cosmetic products. Frequency of use information was summarized by ingredient in Table 1. Data obtained from reviewing the National Library of Medicine’s Household Products Database and the EWG’s Skin Deep Cosmetic Database were sorted by ingredient into 28 pre-determined categories and were summarized in Table 2. The most commonly used ingredients were glycerin (n = 10329), citric acid (n = 6484), and cetearyl alcohol (n = 3941), while the least commonly used ingredients were starch (n = 57), PEG-60 almond glycerides (n = 50), and wild cherry fruit extract (n = 16) (Table 2). A report of each on-market product that contains one of more of the identified ingredients is detailed in Table S1 (Supplementary Materials).

Table 2.
Stratification of Ingredients by Product Types.
4. Discussion
This study presents a screening-level safety assessment methodology that can serve as an initial screening tool to evaluate the safety of personal care and cosmetic product ingredients. This analysis used WCD cleansing conditioners as a case study of the application of this screening level safety assessment framework. The results of this study showed that more than 20,000 personal care and cosmetic products contained one or more of the 30 evaluated ingredients in WCD cleansing conditioners.
Based on the large number of personal care and cosmetic product(s) that contained the identified ingredients, we conclude that WCD cleansing conditioner ingredients are generally commonly used in other personal care and cosmetic products. Of the ingredients that are more unique, such as wild cherry fruit extract, most of its uses are in hair care products, demonstrating that it is commonly used in the applicable product category. Additionally, published safety information was available for 21 of the 30 evaluated ingredients. However, it is important to note that ingredients such as methylchloroisothiazolinone and methylisothiazolinone have been shown to induce sensitization and have use restriction levels according to the EC; thus, specific concentrations of use must be considered when evaluating the safety of these ingredients. Findings from this analysis suggest that ingredients in WCD cleansing conditioners with available safety information are generally considered safe for the intended uses. While safety information was not available for the remaining nine ingredients, their widespread use in cosmetic products across multiple product categories demonstrate a history of safe use. Where available, concentrations of use for such ingredients lacking safety information should be compared in new formulations versus historical usage.
Based on the information obtained from this screening-level safety assessment methodology, a summary of findings can be prepared for each ingredient with available safety information. An example of an ingredient-specific safety assessment for citric acid is included below. Citric acid is a white solid that is soluble in water and some organic solvents [13,14]. It is widely used as a flavor, fragrance, pH adjuster, chelating agent, skin conditioning agent, and buffering agent in foods, beverages, cosmetics, pharmaceuticals, detergents and cleaning products, and pesticides due to its low toxicity [13,14]. According to our analysis, it was used in approximately 6500 personal care and cosmetic products (Table 2). It was most commonly found in shampoos (1590 products); body wash, face wash, and exfoliant products (1142 products); conditioners (856 products); moisturizer, cream, lotion, and body oil products (650 products); nail polishes (453); hand washes (261 products); hair dyes (218 products); and foundation, powder, beauty balm, and concealer products (89 products) (Table 2). The CIR panel reported that citric acid was used at concentrations from 0.0000005 to 10% in cosmetic products, and concluded that citric acid was considered safe in the present practices of use [14]. Citric acid is present in WCD cleansing conditioners at a concentration up to 0.3%. Additionally, citric acid is generally regarded as safe by the U.S. FDA [9].
It should be noted that safety information was not available for all WCD cleansing conditioner ingredients. Importantly, each of the examined ingredients in WCD cleansing conditioners was identified to be used in other cosmetic products. Additionally, it is possible that both safety information and frequency of use information are not available from peer-reviewed literature, U.S. FDA, or personal care and cosmetic products databases. In such a scenario, concentrations of use for such ingredients should be compared against formulations with a history of safe use. If product comparison information is also lacking, further testing may be necessary. For example, specific ingredients within a fragrance mixture are typically not listed, thus, safety and frequency of use information will be limited. Under these circumstances, additional safety assessments and relevant tests including in silico, in vitro, and clinical studies must be conducted to comprehensively evaluate the safety of the ingredient. Another scenario where additional testing may be necessary is when available safety information indicates a potential safety concern associated with the use of an ingredient. A framework of step-wise decision-making is provided in Figure 1.
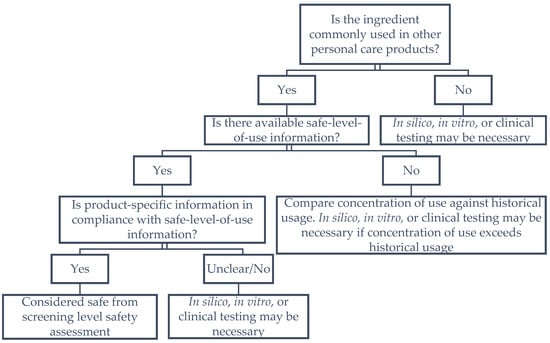
Figure 1.
Screening level safety assessment framework.
Although this screening-level safety assessment methodology provides valuable information regarding the safety and frequency of use of any ingredient, the methodology is not without limitations. As part of this assessment, two publicly available databases were evaluated. Though these databases were extensive and well established, the scope of this analysis was limited to the personal care and cosmetic products included in these databases; it is likely that these two databases do not contain all on-market personal care and cosmetic products. Additionally, new products that were recently introduced onto the market may not be added to these databases immediately. Thus, it is fair to assume that the total number of personal care and cosmetic products containing the listed ingredients will be greater than the number listed in this analysis.
Another limitation is the use of FDA GRAS information. This designation is related to the Federal Food, Drug, and Cosmetic Act (FFDCA) food additive tolerance requirements and is intended for ingredients added to food. Although this safety information is intended for food additives, it is also relevant for personal care and cosmetic products. The primary route of exposure of a food additive is ingestion, where the substance is ingested and readily absorbed into the body. On the other hand, the primary route of exposure for a personal care or cosmetic product is dermal exposure, where the substance must penetrate the skin before it is absorbed into the body, leading to a lower absorption potential compared to when ingested. Although oral and inhalation exposures may be associated with the use of some cosmetic products (e.g., lip/mouth products and aerosols). This difference in absorption (bioavailability) suggests that the margin of safety is larger when a substance is used in personal care or cosmetic products compared to in foods. Noting this difference, safety information pertaining to use as food additives provide valuable information for personal care and cosmetic products.
Overall, this study provides evidence that the evaluated WCD cleansing conditioner ingredients are commonly used in other personal care and cosmetic products, and the ingredients with available safety information are generally considered safe in the present practice of use. It is important to note that the scope of this analysis is limited to frequency of use information and available toxicological data; further testing may be required to fully evaluate the potential toxicity of an ingredient. The presented screening-level safety assessment methodology can serve as an initial screening tool to evaluate the safety of an ingredient intended for use in personal care and cosmetic products before a product is launched on to the market.
Supplementary Materials
The following are available online at http://www.mdpi.com/2079-9284/5/2/38/s1, Table S1: Product Listing by Ingredient and Product Category.
Author Contributions
All authors (E.S.F., D.A.D., K.M.T., R.M.N., M.T.H., C.P., D.J.P., and A.D.M.) conceived and designed the study; E.S.F., M.T.H., and C.P. performed the study; E.S.F., D.A.D., K.M.T., and A.D.M. analyzed the data; E.S.F., K.M.T., and A.D.M. wrote the paper.
Conflicts of Interest
Authors E.S.F., D.A.D., K.M.T., R.M.N., M.T.H., C.P., D.J.P., and A.D.M. are employed by Cardno ChemRisk, a consulting firm that provides scientific advice to the government, corporations, law firms, and various scientific/professional organizations. Cardno ChemRisk has been engaged by WEN by Chaz Dean, Inc. (WCD), which produces personal care products, including the product examined in this study. This paper was prepared and written exclusively by the authors without review or comment by any outside entity. It is possible that this work will be relied upon in litigation. Funding for the research and preparation of this article was provided by WCD. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Scientific Committee on Consumer Safety (SCCS). The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 9th revision, 29 September 2015, SCCS/1564/15, revision of 25 April 2016; SCCS: Brussels, Belgium, 2016. [Google Scholar]
- Food and Drug Administration (FDA). FDA Authority Over Cosmetics: How Cosmetics Are Not FDA-Approved, but Are FDA-Regulated; FDA: Silver Spring, MD, USA, 2016. Available online: https://www.fda.gov/Cosmetics/GuidanceRegulation/LawsRegulations/ucm074162.htm (accessed on 18 May 2018).
- Personal Care Products Council (PCPC). Personal Care Products Council Technical Guidelines: Safety Evaluation Guidelines; PCPC: Washington, DC, USA, 2014. [Google Scholar]
- Food and Drug Administration (FDA). Generally Recognized as Safe (GRAS); 1/4/2018; FDA: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ (accessed on 18 May 2018).
- Cosmetic Ingredient Review (CIR). Cosmetic Ingredient Review. 2016. Available online: https://www.cir-safety.org/about (accessed on 18 May 2018).
- Cosmetic Ingredient Review (CIR). Cosmetic Ingredient Review Procedures; CIR: Washington, DC, USA, 2010. [Google Scholar]
- Kwa, M.; Welty, L.J.; Xu, S. Adverse events reported to the US Food and Drug Administration for cosmetics and personal care products. JAMA Intern. Med. 2017, 177, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). GRAS Substances (SCOGS) Database; 3/28/18; FDA: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/default.htm (accessed on 18 May 2018).
- Food and Drug Administration (FDA). CFR—Code of Federal Regulations Title 21; FDA: Silver Spring, MD, USA, 2017.
- European Commission. European Commission Cosmetic Ingredient Database; European Commision: Brussels, Belgium, 2018; Available online: https://ec.europa.eu/growth/sectors/cosmetics/cosing_en (accessed on 5 June 2018).
- U.S. Deparment of Health & Human Services. Household Products Database, Health & Safety Information on Household Products; 9/2017; U.S. Deparment of Health & Human Services: Bethesda, MD, USA, 2017. Available online: https://householdproducts.nlm.nih.gov/about.htm (accessed on 18 May 2018).
- Environmental Working Group (EWG). Environmental Working Group's Skin Deep Cosmetics Database; EWG: Washington, DC, USA, 2018; Available online: https://www.ewg.org/skindeep/site/about.php#.Wv8fdIgvzAI (accessed on 18 May 2018).
- Soccol, C.R.; Vandenberghe, L.P.; Rodrigues, C.; Pandey, A. New perspectives for citric acid production and application. Food Technol. Biotechnol. 2006, 44, 141–149. [Google Scholar]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of citric acid, inorganic citrate salts, and alkyl citrate esters as used in cosmetics. Int. J. Toxicol. 2014, 33 (Suppl. 2), 16S–46S. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).