Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation Development
2.2. Preliminary Stability Evaluation (PSE)
2.2.1. Centrifuging Test
2.2.2. Thermal Stress Test
2.3. Normal Stability Test
- −10.0 ± 0.5 °C (freezer CRC30: Consul®, São Paulo, Brazil);
- 5.0 ± 0.5 °C (refrigerator Whirlpool BR562: Brastemp®, São Paulo, Brazil);
- 50.0 ± 0.5 °C (incubator 031–7M–12: Quimis®, Diadema, Brazil);
- 25.0 ± 2 °C (room temperature—Indirect exposure to sunlight).
2.4. pH Assessment
2.5. Polarized Light Microscopy
2.6. Parameters and Criteria Used on Stability Tests
2.7. Organoleptic Parameters
- (M) modified;
- (SM) slightly modified;
- (NM) no modifications regarding visual aspect, color, and scent.
3. Results and Discussion
3.1. Preliminary Stability Testing
3.2. Normal Stability Tests
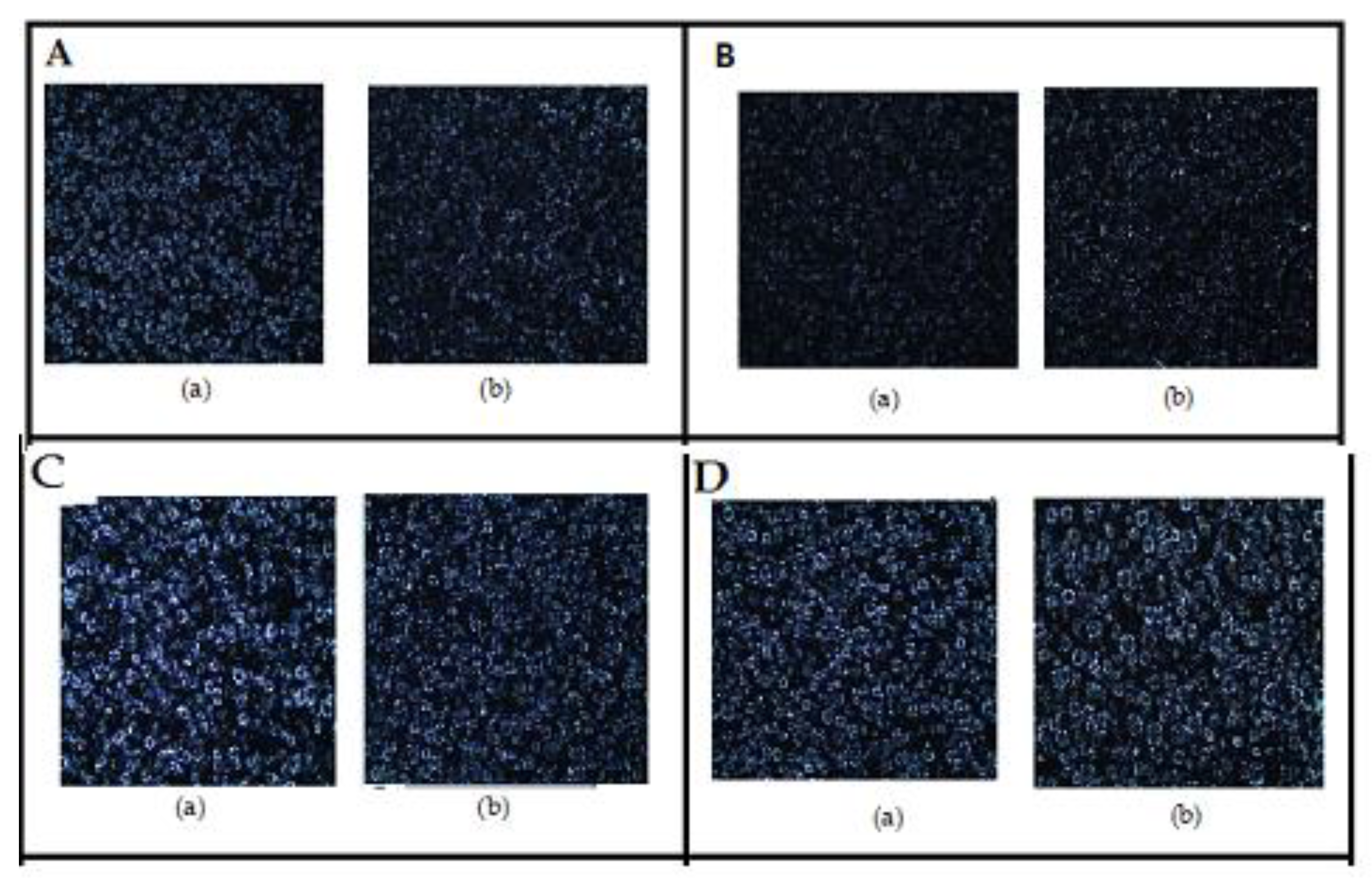
3.3. Microscopic Analysis of the Formulations
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Correa, M. Cosmetologia—Ciência e Técnica, 1st ed.; Medfarma: São Paulo, Brasil, 2012; p. 492. [Google Scholar]
- Tadros, T.F. Emulsion Formation and Stability, 1st ed.; Wiley-VCH: Alemanha, Germany, 2013; p. 252. [Google Scholar]
- Neto, A.M.F.; Salinas, S.R.A. The Phisics of Lyotropic Liquid Crystals: Phase Transitions and Structural Properties, 1st ed.; Oxford University Press: New York, NY, USA, 2005; p. 301. [Google Scholar]
- Silva, S.A.M.; Rigon, R.B.; Valarini, M.F.; Chorilli, M.; Leonardi, G.R. Análise da influência do agente umectante na estabilidade reológica e avaliação de cristais líquidos em formulações cosméticas. Rev. Bras. Farm. 2013, 3, 199–210. [Google Scholar]
- Baby, A.R.; Maciel, C.P.M.; Zague, V.; Kaneko, T.M.; Consiglieri, V.O.; Velasco, M.V.R. Estabilidade de produtos de aplicação tópica: Ensaios aplicados aos produtos cosméticos e dermatológicos emulsionados. Int. J. Pharm. Compd. 2004, 6, 130–139. [Google Scholar]
- Bacelar, V.C.F.; Vieira, M.E.S. Importância da vacuoterapia no fibro edema gelóide. Fisioter Bras. 2006, 7, 440–443. [Google Scholar]
- Draelos, Z.D. Cosmecêuticos, 2nd ed.; Elsevier: Rio de Janeiro, Brasil, 2009; p. 276. [Google Scholar]
- Leonardi, G.R.; Chorilli, M. Celulite—Prevenção e Tratamento, 1st ed.; Pharmabooks: São Paulo, Brasil, 2010; p. 118. [Google Scholar]
- Yarnell, E. Botanical Medicines for Dermatologic Conditions. Altern. Complement. Ther. 1999, 5, 106–109. [Google Scholar] [CrossRef]
- Ramos, M.F.D.S.; Santos, E.P.; Silva, A.B.; Leitão, A.C.; Dellamora-Ortiz, G.M. Avaliação fototoxica e screening mutagênico de extratos de propolis, Aloe spp. e Hamamelis virginiana. Rev. Ciênc. Farm. Basica Apl. 2005, 26, 105–111. [Google Scholar]
- Mukherjee, P.K.; Maity, N.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Dudeke-Makuch, M.; Studzinska-Sroka, E. Horse chestnut—Efficacy and safety in chronic venous insufficiency: An overview. Rev. Bras. Farmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef]
- Marina, A.M.; Man, Y.C.; Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 301–307. [Google Scholar] [CrossRef]
- Affonso, R.S.; Rennó, M.N.; Slana, G.B.C.A.; França, T.C.C. Aspectos Químicos e Biológicos do Óleo Essencial de Cravo da Índia. Rev. Virtual Quim. 2012, 4, 146–161. [Google Scholar]
- Toma, W.; Guimarães, L.L.; Brito, A.R.M.S.; Santos, A.R.; Cortez, F.S.; Pusceddu, F.H.; Cesar, A.; Júnior, L.S.; Pacheco, M.T.T.; Pereira, C.D.S. Safflower oil: An integrated assessment of phytochemistry, antiulcerogenic activity, and rodentand environmental toxicity. Rev. Bras. Famacogn. 2014, 24, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Ramos, B. Quebrando paradigmas da nanotecnologia. C&T 2015, 27, 50–52. [Google Scholar]
- The Brazilian Health Regulatory Agency (ANVISA). Guia de Estabilidade de Produtos Cosméticos; Ministério da Saúde, ANVISA: Brasília, Brazil, 2004. Available online: http://www.anvisa.gov.br/divulga/public/series/cosmeticos.pdf (accessed on 11 September 2016).
- The Brazilian Health Regulatory Agency (ANVISA). Guia de Controle de Qualidade de Produtos Cosméticos; Ministério da Saúde, ANVISA: Brasília, Brazil, 2008. Available online: http//www.anvisa.gov.br/cosméticos/material/guia_cosmetico.pdf (accessed on 9 August 2016).
- Carlton, R.A. Polarized light microscopy. In Pharmaceutical Microscopy, 1st ed.; Springer: New York, NY, USA, 2011; pp. 7–64. [Google Scholar]
- Silva, S.A.M.; Silva, M.M.; Leonardi, G.R.; Baillo, R.P.V.; Valarina, M.F.C. Cristais líquidos aplicados à cosmetologia. C&T 2015, 27, 52–57. [Google Scholar]
- Pedriali-Moraes, C.A.; Arêas, E.P.G.; Velasco, M.V.R. Assessment of functional stability of photoprotective formulations containing rutin succinate. Cosmetics 2017, 4, 1–14. [Google Scholar]
- Ansel, H.C.; Popovich, N.G.; Alen, L.V., Jr. Farmacotécnica: Formas Farmacêuticas & Sistemas de Liberação de Fármacos, 6th ed.; Premier: São Paulo, Brasil, 2000; p. 568. [Google Scholar]
- Iwai, H.; Fukasawa, J.; Suzuki, T. A liquid crystal application in skin care cosmetics. Int. J. Cosmet. Sci. 1998, 20, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.A.M.; Valarini, M.F.C.; Chorilli, M.; Venturini, A.; Leonardi, G.R. Atividade antioxidante do extrato seco de cacau orgânico (Theobroma cacao)—Estudo de estabilidade e teste de aceitação de cremes acrescidos deste extrato. Rev. Ciênc. Farm. Basica Apl. 2013, 34, 493–501. [Google Scholar]
| INCI NAME | Function |
|---|---|
| Formulations A, B, C, and D | |
| cetearyl alcohol, dicetyl phosphate, and ceteth-10 phosphate | emulsifying and conditioning system |
| behenyl alcohol | emulsion stabilizer and emollient |
| butilhydroxitoluene | Antioxidant |
| glycerin | Humectant |
| isopropyl palmitate | Emollient |
| aqua | Solvent |
| acrylates/C10-30 alkyl acrylate crosspolymer | viscosity increasing agent |
| propyleneglycol | Humectant |
| disodium EDTA | chelating agent |
| methylisothiazolinone and phenoxyethanol | antimicrobial agent |
| triethanolamine | pH buffer |
| coconut oil | antioxidant, emollient |
| Helianthus annus seed oil | Emollient |
| almond oil | Emollient |
| Actives | |
| water, Carthamus tictorius seed oil, Cocos nucifera oil, Eugenia caryophyllus bud oil, phenoxyethanol, methylisothiazolinone | anti-cellulite actives (Formulation B) |
| glycerin, alcohol, Centella asiatica leaf, Aesculus hippocastanum seed, Hamamelis virginiana leaf extract | anti-cellulite actives that prevent vasoconstriction and increase skin elasticity (Formulation C) |
| bioactives from Formulations B and C in association | Formulation D |
| Formulation | pH (t0 *) | pH (t30 *) | pH (t60 *) | pH (t90 *) | Stability |
|---|---|---|---|---|---|
| A | 6.1 | 6.3 | 6.1 | 6.3 | stable |
| B | 6.1 | 6.0 | 5.9 | 5.9 | stable |
| C | 6.1 | 6.1 | 6.2 | 6.2 | stable |
| D | 5.9 | 6.1 | 6.2 | 6.2 | stable |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Ueoka, A.; Pedriali Moraes, C.A. Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives. Cosmetics 2018, 5, 25. https://doi.org/10.3390/cosmetics5020025
Rodrigues Ueoka A, Pedriali Moraes CA. Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives. Cosmetics. 2018; 5(2):25. https://doi.org/10.3390/cosmetics5020025
Chicago/Turabian StyleRodrigues Ueoka, Andreza, and Carla Aparecida Pedriali Moraes. 2018. "Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives" Cosmetics 5, no. 2: 25. https://doi.org/10.3390/cosmetics5020025





